Abstract
Scedosporium apiospermum is an emerging fungal pathogen that causes both localized and disseminated infections in immunocompromised patients. Glucosylceramides (CMH, GlcCer) are the main neutral glycosphingolipids expressed in fungal cells. In this study, glucosylceramides (GlcCer) were extracted and purified in several chromatographic steps. Using high-performance thin layer chromatography (HPTLC) and electrospray ionization mass spectrometry (ESI-MS), N-2′-hydroxyhexadecanoyl-1-β-D-glucopyranosyl-9-methyl-4,8-sphingadienine was identified as the main GlcCer in S. apiospermum. A monoclonal antibody (Mab) against this molecule was used for indirect immunofluorescence experiments, which revealed that this CMH is present on the surface of the mycelial and conidial forms of S. apiospermum. Treatment of S. apiospermum conidia with the Mab significantly reduced fungal growth. In addition, the Mab also enhanced the phagocytosis and killing of S. apiospermum by murine cells. In vitro assays were performed to evaluate the CMHs for their cytotoxic activities against the mammalian cell lines L.929 and RAW, and an inhibitory effect on cell proliferation was observed. Synergistic in vitro interactions were observed between the Mab against GlcCer and both amphotericin B (AmB) and itraconazole. Because Scedosporium species develop drug resistance, the number of available antifungal drugs is limited; our data indicate that combining immunotherapy with the available drugs might be a viable treatment option. These results suggest that in S. apiospermum, GlcCer are most likely cell wall components that are targeted by antifungal antibodies, which directly inhibit fungal development and enhance macrophage function; furthermore, these results suggest the combined use of monoclonal antibodies against GlcCer and antifungal drugs for antifungal immunotherapy.
Introduction
The opportunistic pathogen Scedosporium apiospermum is present worldwide in plant and soil residues and is involved in a wide range of human infections in both immunocompetent and immunocompromised hosts [1], [2]. In cystic fibrosis patients, S. apiospermum is the second most abundant filamentous fungus colonizing the respiratory tract; its colonization frequency ranges from 6.5–10% [3], [4]. Recently, Zouhair et al. (2013) [5] observed that two other species of the Pseudallescheria/Scedosporium complex, i.e., S. aurantiacum and P. minutispora can colonize the respiratory tract of cystic fibrosis (CF) patients. Despite the rising frequency of Scedosporium/P. boydii infections, the pathogenesis and mechanisms by which these fungi evade host pulmonary defenses and reach other organs are poorly understood [2]. The cell wall glycoconjugates of the Pseudallescheria/Scedosporium complex have been studied extensively to identify the structures that are critical for fungal physiology and pathogenesis. Elucidation of the primary structures of these glycoconjugates, specifically the monohexosylceramides (CMHs) that function as virulence determinants is important for understanding the mechanisms of fungal pathogenicity. Glucosylceramides (GlcCer) are the main neutral glycosphingolipids expressed in fungal pathogens. GlcCer are bioactive molecules in fungal cells and have several distinct roles. They are associated with fungal growth [6], [7] and morphological transitions in Cryptococcus neoformans, P. boydii, Candida albicans, Aspergillus fumigatus and Collectotrichum gloeosporioides [8]–[10]. These glycosylated molecules are present in the fungal cell wall and absent from most mammalian cells, and they are excellent targets for the design of new agents that inhibit fungal growth and the differentiation of pathogens. A Mab targeting a fungal glycosphingolipid (Mab to GlcCer) protects mice against lethal C. neoformans infection [11]. Because fungal cerebrosides are highly conserved and expressed in almost all known pathogenic species [8], [10], GlcCer-binding antibodies might also be useful for the control of other mycoses.
In this study, we characterized the CMHs in the mycelia of S. apiospermum. Structural analyses revealed the presence of the conserved CMHs that were identified in other fungal pathogens [8]. The major CMH is N′-hydroxyhexadecanoyl-1-β-D-glucopyranosyl-9-methyl-4,8-sphingadienine. A Mab against GlcCer was used for immunofluorescence analyses, which indicated that the GlcCer was mainly expressed on the surface of growing cells [12], [13]. The Mab to GlcCer inhibited the growth of S. apiospermum conidia and enhanced the antifungal function of murine macrophages. Furthermore, we evaluated the in vitro susceptibility of S. apiospermum to the Mab against GlcCer in combination with the conventional antifungal agents amphotericin B (AmB) and itraconazole.
Materials and Methods
Microorganisms and Growth Conditions
The S. apiospermum strain was kindly provided by Dr. J. Guarro from Unitat de Microbiologia, Facultat de Medicina e Institut d’Estudis Avançats, Réus, Spain. Cells were maintained on Sabouraud (SAB; 2% glucose, 1% peptone, 0.5% yeast extract) agar slants. Fresh cultures were inoculated in SAB liquid culture medium and incubated for 7 days at 25°C with orbital shaking. Conidia were grown at 30°C on Petri dishes containing SAB agar medium. After 7 days in culture, conidial cells were obtained by washing the plate surface with phosphate-buffered saline (pH 7.2) (PBS; 10 mM NaH2PO4, 10 mM Na2HPO4, 150 mM NaCl) and filtering them through gauze to remove hyphal fragments and debris. The conidia were washed three times in PBS (pH 7.2) and counted in a Neubauer chamber.
Reagents and Cell Lines
MTT [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyl tetrazolium bromide] and paraformaldehyde were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Peritoneal macrophages were obtained from male BALB/c mice (4–8 weeks) and maintained in RPMI 1640 medium containing 10% fetal calf serum (FCS). The RAW and A549 cells were maintained in DMEM. In all experiments, the cell counts and viability were determined by trypan blue vital dye exclusion using a hemocytometer. This method yielded conidia that had an initial viability of >95%, as confirmed by plating. Peptidorhamnomannan (PRM) was produced as described [14]. A goat anti-mouse (GAM) IgG was used as an isotype-matched control in all the experiments. For immunofluorescence experiments, an Alexa Fluor 546-conjugated donkey anti-mouse IgG (h4l) was used (Invitrogen Molecular Probes, Carlsbad, CA, USA). Nitric oxide levels were measured using a commercial Griess reagent kit (Promega, Madison, WI, USA).
Mice
Balb/C mice were obtained from the Universidade Federal do Rio de Janeiro Breeding Unit (Rio de Janeiro, Brazil). The animals were maintained at constant temperature (25°C) with free access to chow and water in a room with a 12-h light/dark cycle. The experiments were approved by the Institutional Animal Welfare Committee of the Federal University of Rio de Janeiro.
Extraction and Purification of GlcCer from S. apiospermum
Intact hyphae of S. apiospermum were successively extracted at room temperature using chloroform: methanol at 2∶1 and 1∶2 (v/v) ratios. The extracts were combined and dried, and the crude lipid extract was partitioned as described by Folch et al. (1957) [15]. The lipids recovered from the Folch lower layer were fractionated on a silica gel column and eluted sequentially with chloroform, acetone and methanol. The acetone and methanol fractions containing glycosphingolipids were then purified further by silica gel column chromatography. This column was sequentially eluted with chloroform/methanol containing increasing concentrations of methanol (95∶5, 9∶1, 8∶2, and 1∶1 v/v) and finally, with methanol. Fractions of 5 ml were collected and analyzed by thin-layer chromatography (TLC), and the plate was developed with CHCl3/CH3OH/2 M NH4OH 40∶10:1 (v/v). The spots were visualized with iodine and by spraying with orcinol/H2SO4. The chloroform/methanol 8∶2 (v/v) fraction was further purified on Iatrobeads RS 2060 (Macherey & Nagel, Düren, Germany) using the same elution system to obtain a purified glycosphingolipid fraction, as visualized by HPTLC.
Sugar Analysis
CMH was hydrolyzed with 3 M trifluoroacetic acid at 100°C for 3 h. The resulting monosaccharide was characterized by HPTLC on a silica gel 60 plate developed with n-butanol: acetone: water at 4∶5:1 (v/v) and visualized using the orcinol-sulfuric acid reagent.
ESI-MS Analysis of S. apiospermum Glucosylceramides
The MS analysis was performed in a Quattro-LC electrospray ionization mass spectrometer (ESI-MS) (Waters, Milford, MA, USA) with a triple-quadrupole mass analyzer operating at atmospheric pressure ionization (API) and assisted by a syringe pump (KD Scientific) for sample infusion. Nitrogen was used as the nebulizing and desolvation gas, and the ionization energies were 50 V on the cone and 2 kV on the capillary when operating in the negative ionization mode or 80 V (cone) and 2.5 kV (capillary) when operating in the positive ionization mode. The second stage tandem-MS was obtained by collision-induced dissociation mass spectrometry (CID-MS) using argon as the collision gas and collision energies ranging between 35–60 eV. The samples were prepared in CH3OH at 1 mg/ml, then diluted to 0.1 mg/ml in CH3OH:H20 (7∶3 v/v) containing 1 mM LiCl for positive ion detection and directly infused into the ESI source at a flow rate of 10 µl/min.
Cytotoxic Assay of S. apiospermum GlcCer
L929 and RAW cells were plated in 96-well polystyrene tissue-culture plates and incubated for 24 h at 37°C and 5% CO2 prior to the addition of S. apiospermum GlcCer (200, 100, 50, 25, 12.5, 6.2 and 3.1 µg/ml). After a 48-h incubation, the cell viability was measured by the neutral-red dye uptake method [16].
Release of GlcCer from S. apiospermum in the Culture Supernatant
S. apiospermum was grown in Sabouraud liquid medium for seven days at room temperature under agitation. The mycelium was filtered out and the supernatant was concentrated and extracted at room temperature with chloroform:methanol at 2∶1 and 1∶2 (v/v) ratios. The crude lipid extract was partitioned as described by Folch et al. (1957) [15]. The upper layer was carefully removed, and the lipids recovered from the lower layer were concentrated and analyzed by thin-layer chromatography (TLC), and the plate was developed with chloroform: methanol: 2 M ammonium hydroxide 40∶10:1 (v/v). The spots were visualized with iodine and by spraying with orcinol/sulfuric acid.
Generation of Mabs against A. fumigatus GlcCer
Mabs were produced as previously described [9]. Briefly, six week-old female BALB/c mice were immunized intraperitoneally with 50 µg of GlcCer suspended in a 1∶1 (v/v) emulsion of complete Freund’s adjuvant. After 4 weeks, this procedure was repeated using incomplete Freund’s adjuvant. One week later, a final intraperitoneal injection without adjuvant was administered. Three days before fusion, the animals were boosted with an intrasplenic injection of 50 µg of GlcCer. For cellular fusion, the myeloma mouse cell line SP2/0 was mixed in a 1∶5 ratio with spleen cells. Polyethylene glycol (PEG 3000) was slowly added, and after settling for 15 min followed by centrifugation, the cells were resuspended in RPMI-HAT medium supplemented with bovine fetal serum (10%) and plated onto the wells of a flat–bottomed polystyrene microtiter plate. Ten days after the fusion, ELISA was used to select the positive hybridomas. The hybridoma culture whose supernatant contained the highest level of antibodies to GlcCer was expanded and cloned by limiting dilution over a feeder layer of BALB/c-derived macrophages in a 96-well microtiter plate. For preparation of antibodies in higher concentrations, the antibody-producing cells were injected into the peritoneal cavity of BALB/c mice. Antibodies to GlcCer were purified by protein G-affinity chromatography from ascetic fluids and isotyped as IgG2b using the Sigma ISO/2 kit.
Reactivity of Purified GlcCer with Antibodies to CMH
The reactivity of S. apiospermum GlcCer to anti-CMH Mabs was evaluated by ELISA as described by Nimrichter et al. (2005) [7]. Briefly, S. apiospermum CMH was dissolved in ethanol: methanol 1∶1 (v/v), and 1 µg/well was added to a flat-bottomed polystyrene microtiter plate (BD-Falcon, MD, USA). A. fumigatus CMH (at 1 µg/well) was used as positive control. The plate was dried and blocked with PBS containing 1% BSA (2 h, 37°C). Decreasing concentrations of the anti-CMH Mab and an unrelated IgG were added, and the plate was incubated at 37°C for 1 h. The plate was washed three times and then incubated with HRP-conjugated anti-mouse IgG (1∶1000 dilution) (Sigma-Aldrich) for 1 h at 37°C. The plate was again washed three times with PBS and the antigen-antibody complexes were detected with 0.04% ortho-phenylenediamine (OPD) in phosphate-citrate buffer at pH 5.0 containing 30 vol. H2O2. The signal at 490 nm was measured using a spectrophotometer.
Immunostaining of S. apiospermum CMH
CMHs from S. apiospermum and A. fumigatus were separated by HPTLC, and immunostaining was performed as described by Pinto et al. (2005) [17]. The CMHs were separated in chloroform: methanol: 2 M NH4OH 40∶10:1(v/v), and the plate was air-dried, soaked in 0.5% poly (isobutyl methacrylate) in chloroform: n-hexane (1∶10, v/v) and then blocked for 2 h with PBS-BSA 1% at 4°C. The plate was then incubated with anti-A. fumigatus CMH Mab for 18 h at 4°C followed by sequential incubation with a peroxidase-conjugated anti-mouse IgG (Sigma-Aldrich, 1∶1000 dilution and 0.05% 3,3′-diaminobenzidine (DAB) in PBS and H2O2 (30 vol.).
Immunofluorescence Analysis
Mycelial or conidial forms of S. apiospermum were fixed in 4% paraformaldehyde cacodylate buffer (0.1 M, pH 7.2) for 1 h at room temperature. The fixed cells were washed twice in PBS and then incubated in PBS containing 1% BSA for 1 h at 37°C. The conidia and mycelium were washed three times with PBS and incubated for 1 h at 37°C with either a Mab to CMH or an isotype-matched control, which was used at a concentration of 50 µg/ml in PBS containing 1% BSA. Cells were washed and incubated in 100 µl of Alexafluor 546 (at 1∶400 dilution) in PBS containing 1% BSA for 1 h at 37°C. After three washes, the cells were suspended in 50 µl of a mounting solution containing 0.01 M N-propyl gallate diluted in PBS: glycerol (1∶1, v/v). Ten microliters of the suspension was applied to a microscope slide and examined with an Olympus AX70 fluorescence microscope (Olympus America Inc., Center Valley, PA) using a 620-nm filter and a 100X magnification lens.
Germination Assay
The germination assay was performed as previously described but with minor modifications [18]. S. apiospermum conidia (1×105/ml) were incubated in RPMI 1640 in 24-well plates at 37°C with 50, 25, and 10 µg/ml of the Mab against A. fumigatus CMH or PBS. After 12 and 24 h of incubation, the wells were analyzed, and the germinated conidia were counted by optical microscopy. At least 100 conidia per field were counted, and the mean value of three independent counts was calculated. The percent germination was calculated as 100× the ratio of the number of germinated conidia to the total number of cells counted. For viability assays, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well and the plates were incubated at 37°C for 24 h. After incubation, the medium containing MTT was partially removed and DMSO was added to solubilize the MTT formazan product. The absorbance of each well was measured at 570 nm using a spectrophotometer. Both experiments were performed 15 and 24 h.
Phagocytosis Assays
Phagocytosis assays were performed as described previously [19]. Briefly, peritoneal macrophage cells from BALB/c mice were plated at a concentration of 105 cells per well in 24-well polystyrene cell-culture plates and grown overnight at 37°C in the presence of 5% CO2. S. apiospermum conidia were collected after 7 days of growth, washed three times with PBS, and 1×106 conidia were incubated with 50, 25, and 10 µg/ml of Mab to CMH for 1 h at 37°C. After washing, the fungal cells were added to the macrophages at a ratio of 5∶1 (conidia: macrophages), and the plates were incubated for 1 h at 37°C in the presence of 5% CO2. Samples were prepared in triplicate. The wells were washed with PBS and fixed with Bouin’s fixative solution. The numbers of macrophages and conidia were recorded for each field, and at least 200 macrophages were counted. The phagocytosis index was defined as the ratio of the number of intracellular conidia to the number of macrophages counted.
Macrophage Effector Functions
The growth of S. apiospermum conidia in the presence of Mabs was evaluated by incubating the fungus with either the Mab to CMH or PBS prior to co-culture with macrophages. Washed conidial cells were added to wells containing peritoneal macrophage cells at a ratio of 5∶1 and incubated for 2 h. The cultures were washed with ice-cold PBS, and the macrophages were lysed by adding sterile deionized water. Aliquots were plated on Sabouraud agar plates, and the plates were incubated at 30°C. The percentage of growth was determined as the ratio of the number of CFU for S. apiospermum pretreated with Mabs to the number of CFU for untreated conidia.
In vitro Susceptibility of S. apiospermum to Antifungals Alone and in Combination with the Anti-GlcCer Mab
S. apiospermum conidia were plated at 105 cells per well in 96-well polystyrene tissue-culture plates. Antifungal drugs (itraconazole and amphotericin B) were added in increasing concentrations (0.5 to 16 µg/ml), and 50 µg/ml of Mab to CMH was added. After 24-h incubation at 37°C, the plates were analyzed by measuring the absorbance at 490 nm using a spectrophotometer.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego CA). Unless otherwise noted, one-way analysis of variance using a Kruskal-Wallis nonparametric test was used to compare the differences between groups, and individual comparisons of groups were performed using a Bonferroni posttest. The t test was used to compare the number of CFU for different groups. The 90–95% confidence interval was determined in all experiments. Survival results were analyzed by a Kaplan-Meyer test to determine the differences between groups.
Results
Structural Analysis of S. apiospermum CMHs
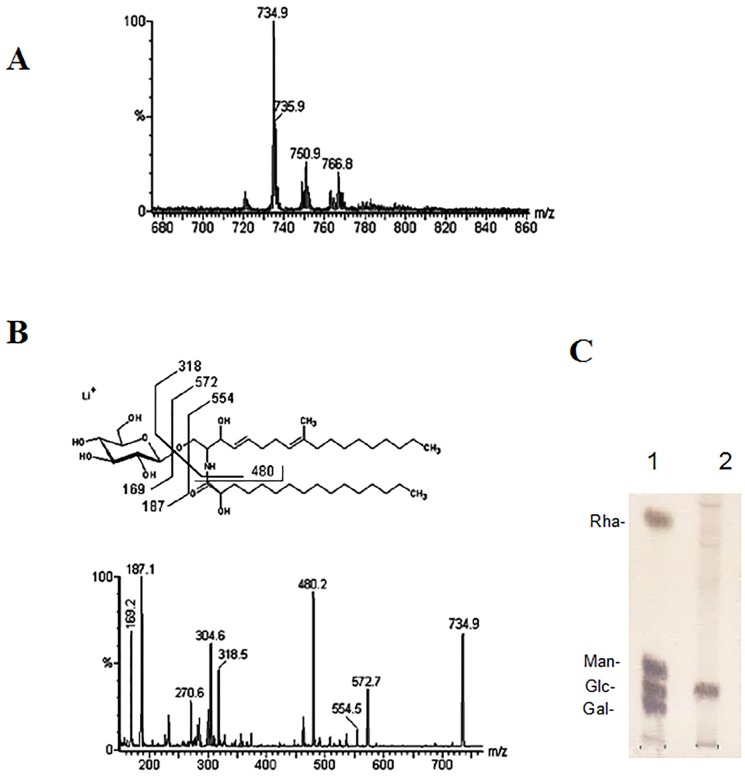
ESI-MS analysis was performed to elucidate the chemical structure of S. apiospermum CMHs. A major, lithiated, singly charged ion species at m/z 734.9 was observed at the MS1 spectrum ( Figure 1A ). When subjected to tandem (MS/MS) fragmentation, this ion species generated fragment ions consistent with the chemical structure of a CMH ( Figure 1B ). The loss of 162 units, common to all CMH analyzed and diagnostic of a monosaccharide unit, generated daughter ions at m/z 572 [M-hexose+ Li+] and m/z 554 [M-hexose-H2O+ Li+] corresponding to the ceramide monolithiated ion from the parental ion at m/z 734. The daughter ion at m/z 480 is consistent with the loss of a OH-C16 fatty acid. The fragments at m/z 187 and 169 confirmed the presence of a hexose. Based on these observations, we concluded that the glycosphingolipid structure consisted of a hexose, a long chain base (9-methyl-4,8-sphingadienine), and a hydroxylated C16∶0 fatty acid ( Figure 1B ). To determine the identity of the hexose in the structure, hydrolysis was performed using 3 M trifluoroacetic acid, which revealed that glucose was the sugar constituent of CMH ( Figure 1C ). Two other minor species were detected at m/z 750.9 and 766.8, and they most likely represent differences in hydroxylation and the lengths of the fatty acid chains ( Figure 1A ).
Figure 1. ESI-MS (positive ion mode, Li+ adducts) analysis of the GlcCer species of S. apiospermum.
(A) MS1 spectrum. (B) ESI-MS2 of the ions species with m/z 734.9 observed in (A) and proposed structures for the major GlcCer species in S. apiospermum. (C) HPTLC plate of monosaccharides from S. apiospermum CMH. 1. Galactose, glucose, mannose and rhamnose standards; 2. Glucose from CMH. The sugars were detected using the orcinol-sulfuric acid spray reagent.
Binding of Mabs to S. apiospermum CMH
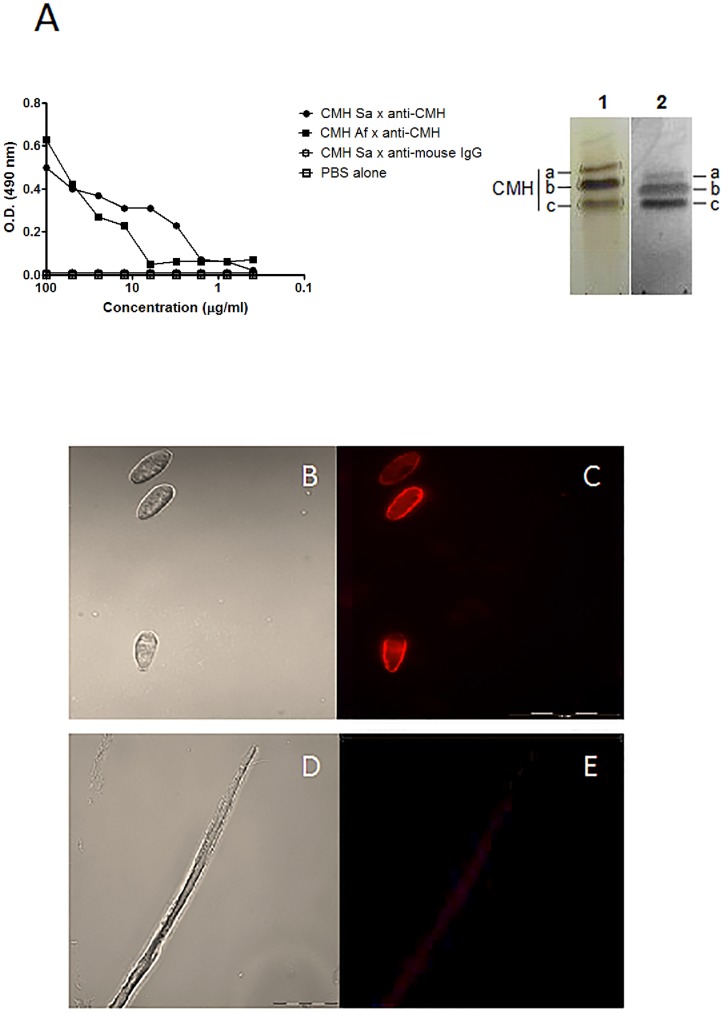
The specificity of the Mab to S. apiospermum CMH was analyzed using ELISA and indirect immunofluorescence and HPTLC immunostaining assays. Indirect ELISA was performed using A. fumigatus and S. apiospermum cells. The anti-A. fumigatus CMH Mab recognized the conidial forms of both fungi ( Figure 2A ), indicating the presence of conserved CMH structures on the cellular surfaces of these fungi. The binding of this antibody to the fungal cell surface was also analyzed by fluorescence microscopy. Immunofluorescence analysis of the S. apiospermum conidia and mycelium revealed the localization of CMH on the surface of the fungus. As shown in Figure 2 B–E, GlcCer are detectable on the surface of the conidial forms. Analysis of the mycelial forms revealed that the Mab weakly recognized mature hyphae. TLC immunostaining analysis revealed that the anti-A. fumigatus CMH Mab recognized bands comigrating with S. apiospermum CMHs (spots a, b and c), as revealed by the orcinol reagent ( Figure 2A , inset). These analyses indicate that CMHs are components of the fungal cell wall.
Figure 2. Reactivity of fungal CMH with the anti-CMH MAb.
(A) ELISA analysis of the binding of Mabs to S. apiospermum CMH. The amount of antibody bound to CMHs was determined by incubation with a rabbit anti-mouse IgG. CMH from S. apiospermum (closed circle); CMH from A. fumigatus (closed square); CMH from S. apiospermum + unrelated antibody (open circle); negative control (open square). Inset: HPTLC of S. apiospermum CMH (spots a,b,c), which was developed in CHCl3: MeOH: 2 M NH4OH (40:10:1 v/v). Lane 1: stained with orcinol/ H2SO4; Lane 2: immunostaining with the anti-CMH MAb. Indirect immunofluorescence analysis of conidial (B and C) and mycelial (D and E) forms of S. apiospermum using the anti-CMH MAb. B and D: phase contrast; C and E: fluorescence signal.
Cytotoxic Assay of S. apiospermum CMH
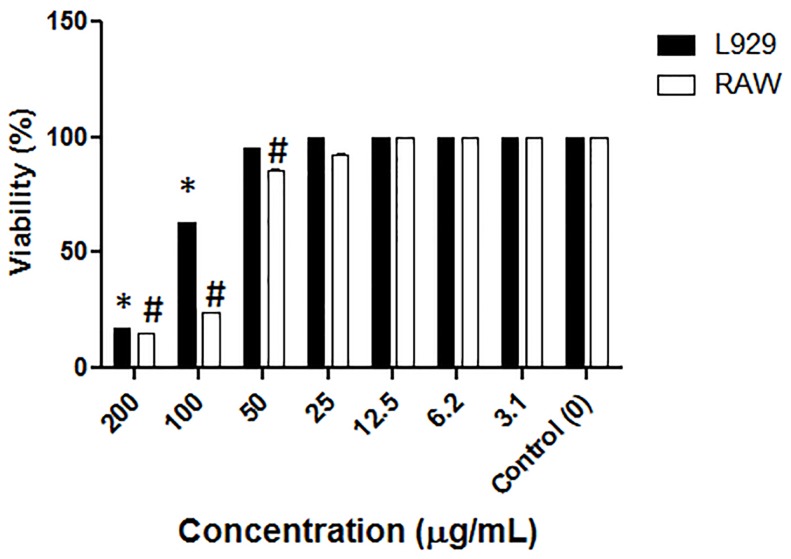
To study the cytotoxicity of the S. apiospermum CMH, a cellular cytotoxicity assay was performed using two mammalian cell lines (L.929 and RAW). As shown in Figure 3 , the CMH from S. apiospermum significantly inhibited the growth of both cell lines, and there was a dose-dependent inhibition with increasing concentrations of CMH, with the highest cytotoxic effects observed at 100 and 200 µg/ml of CMH. At 100 and 200 µg/ml of CMH, the viability of L.929 cells was less than 70% and 30%, respectively, compared to control cells. The cytotoxic effect was more pronounced for RAW cells because the cell viability was less than 30% at both 100 and 200 µg/ml of CMH ( Figure 3 ).
Figure 3. Cytotoxic activity of S. apiospermum CMH.
Cytotoxic assay of S. apiospermum CMH on L929 and RAW cells during 48 h of incubation. Cell viability was measured by adding a neutral-red solution and measuring the absorbance at 490 nm using a spectrophotometer. The presence of S. apiospermum CMH in the culture supernatant was analyzed using TLC.
Release of S. apiospermum CMH in the Culture Supernatant
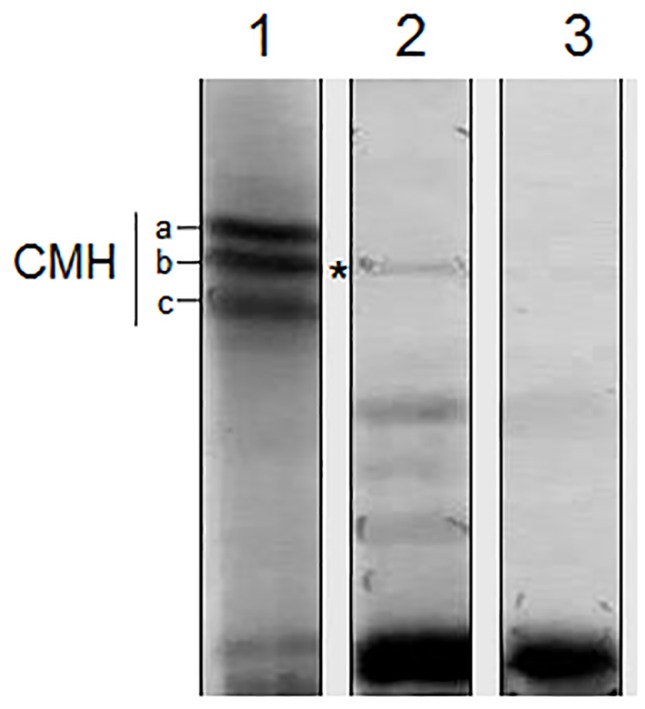
To evaluate the extracellular release of CMH, we tested whether CMH was present in the supernatant of S. apiospermum in the culture medium. TLC analysis confirmed the presence of CMH in the extracellular milieu, indicating the possible release of this molecule by the fungus ( Figure 4 ).
Figure 4. HPTLC analysis of CMH released by S. apiospermum in the culture supernatant.
Lane 1: Purified CMHs from S. apiospermum; Lane 2: CMH released in the supernatant; Lane 3: culture medium supernatant (control). The solvent used was chloroform: methanol: 2 M NH4OH (40:10:1 v/v). Detection was performed using iodine and the orcinol-sulfuric acid reagent.
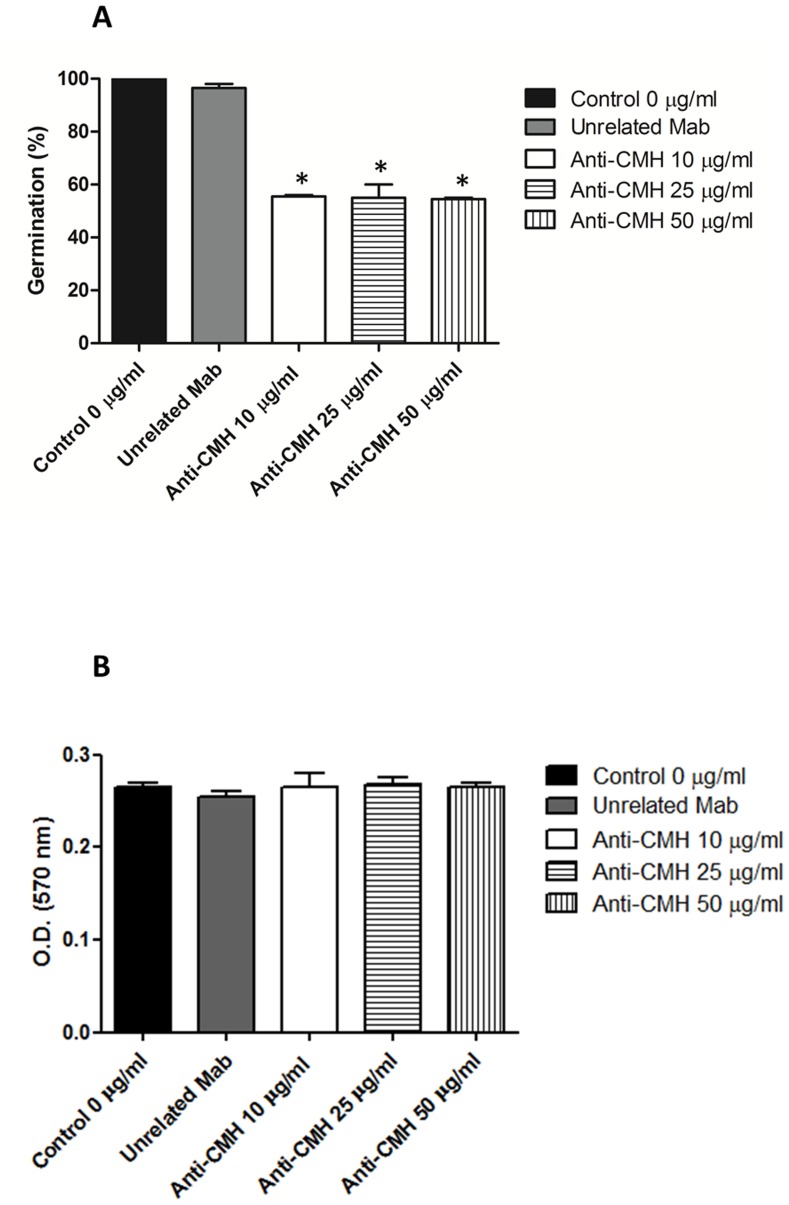
Influence of Mabs to CMH on S. apiospermum Germination and Viability
MAbs to CMH were used to evaluate the potential of CMHs as targets to inhibit the differentiation of S. apiospermum. Conidia were incubated with these antibodies and the process of mycelial differentiation was evaluated at different time points. The germination of S. apiospermum conidia was significantly altered by all tested concentrations (50, 25, and 10 µg/ml) of the Mabs to CMH. The conidia-mycelium transition decreased by approximately 50% after 15-h incubation (data not shown) and by 40% after 24-h incubation ( Figure 5A ), compared to the controls, which were not treated with the antibodies (p<0.05). These results indicate that binding of anti-CMH Mabs to conidial cells impedes the conidia-mycelium transition. However, viability analyses revealed that the decreased germination is not followed by an alteration in cell viability. After 15 (data not shown) and 24 h of incubation ( Figure 5B ), no significant change in cell viability was observed compared to the controls (p>0.05). These results suggest that the reduction of germination is caused by a fungistatic process rather than by cell death.
Figure 5. Effect of the anti-CMH Mab on the germination and viability of S. apiospermum conidial cells.
(A) Germinated conidia in the presence of different concentrations of anti-CMH Mab over 24 h were counted by optical microscopy. At least 100 conidia per field were counted, and the mean value of three independent counts was calculated. (B) Viability assays were performed using S. apiospermum conidia and the anti-CMH Mab over 24 h and evaluated using MTT. The absorbance at 570 nm was measured using a spectrophotometer.
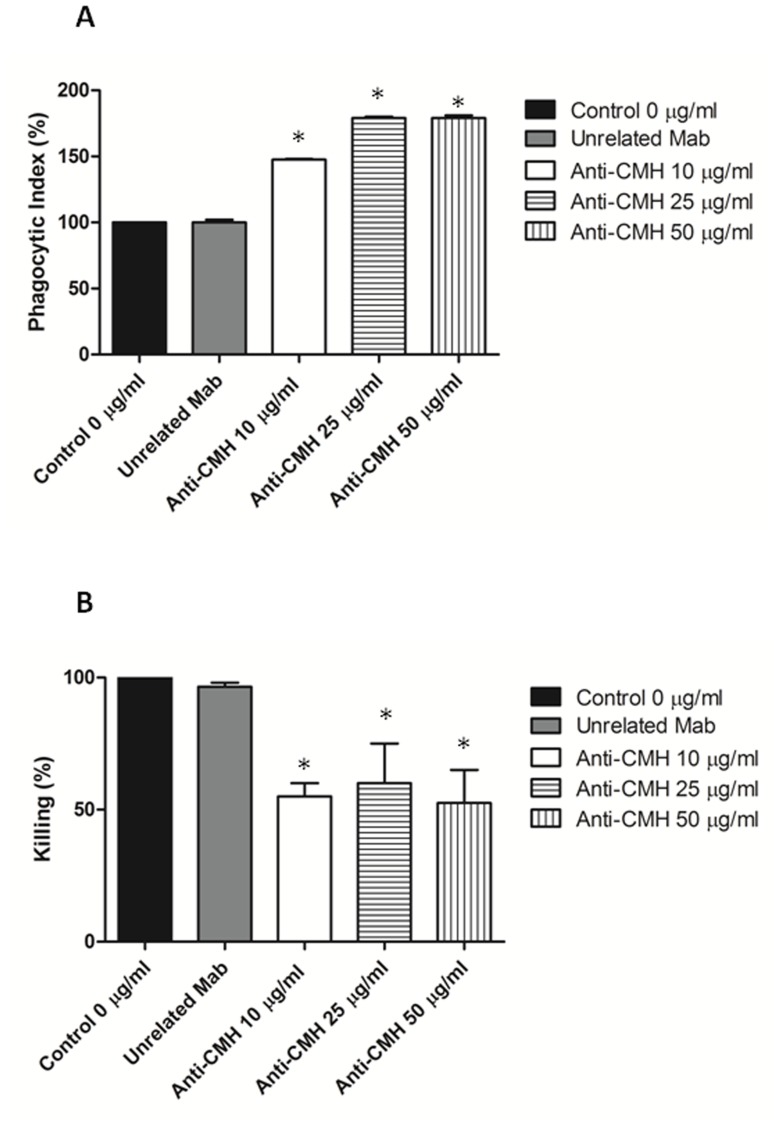
Influence of the Anti-CMH Mab on the Interaction between S. apiospermum Conidia and Peritoneal Macrophages
To evaluate whether the anti-CMH Mab influences the interaction between fungal cells and the host immune cells, fungal cells were treated with the anti-CMH Mab before interaction with macrophages. Treatment of fungal cells with the antibody to CMH resulted in a significant increase of phagocytosis by peritoneal macrophages compared to the controls (p<0.05), as shown in Figure 6A . In addition, fungal cells coated with the Mab against CMH were more efficiently killed by macrophages than untreated conidia ( Figure 6B ); these data indicate that apart from its direct antifungal action, the anti-CMH Mab also enhances the antimicrobial activity of host macrophages.
Figure 6. MAb to CMH affect phagocytosis.
Influence of the Mab to CMH on the phagocytosis of S. apiospermum conidia cells by peritoneal macrophages (A) and on phagocyte antimicrobial activity. Fungal cells treated with the MAb against GlcCer were more efficiently internalized, as demonstrated by the phagocytic indices. (B) Microbial killing by macrophages was enhanced after treatment with antibodies to CMH.
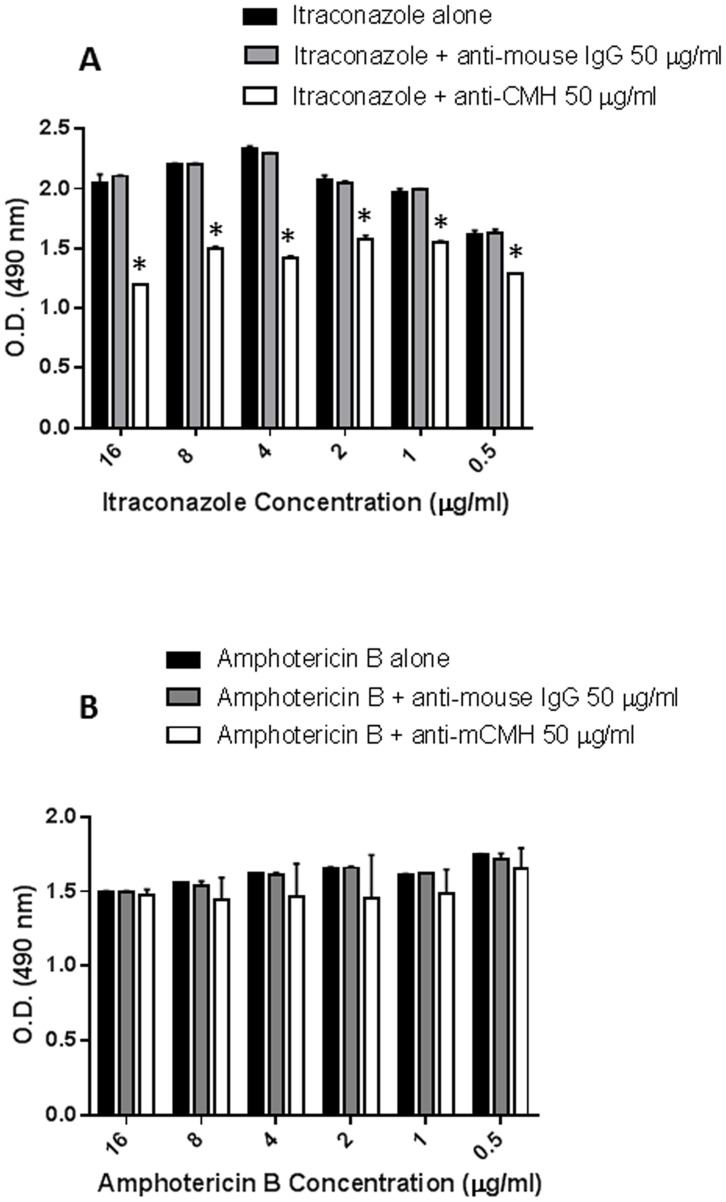
In vitro Susceptibilities of S. apiospermum to Itraconazole and AmB Alone and in Combination with Anti-CMH Mabs
The in vitro antifungal effect of 0.5–16 µg/ml of itraconazole and AmB on S. apiospermum conidia was tested either alone or in combination with the anti-CMH Mab. The anti-CMH MAb increased the viability inhibition of all concentrations of itraconazole ( Figure 7A ). No synergistic effect of the antibody was observed on Amphotericin B activity ( Figure 7B ).
Figure 7. Synergistic effect of combining the anti-CMH Mab with antifungal drugs.
The viability of S. apiospermum conidia was analyzed in the presence of the anti-CMH Mab (50 µg/ml) and different concentrations (0.5 -16 µg/ml) of itraconazole (A) or amphotericin B (B). The values shown are means of three independent experiments.
Discussion
Scedosporium species are involved in a wide range of human infections, especially in immunocompromised patients [20]. In these diseases, cerebral abscesses are relatively frequent [21], reflecting the neurotropic character of these fungi. A typical manifestation of these fungal diseases is the near-drowning syndrome [21], when patients develop cerebral abscesses weeks to months after the near-drowning incident [22]. In cystic fibrosis patients, Scedosporium species are among the most common fungal colonizers of the respiratory tract but rarely become invasive [3]. The majority of Scedosporium isolates display multiple antifungal resistance patterns. Because Scedosporium species do not have a normal MIC/MEC distribution, prediction of the antifungal susceptibility of a single strain is difficult, but the species have differing trends of susceptibilities to antifungal compounds [23]. The increasing frequency and high mortality rates of invasive infections caused by Scedosporium species necessitate the search for new treatment strategies [24]. Combination therapy might be an alternative to monotherapy for patients with invasive infections that are difficult to treat, such as those caused by the majority of Scedosporium isolates, which have multiple antifungal resistance patterns; combination therapy might also be useful for those patients who fail to respond to standard treatment [25]–[27]. It is important to identify new alternatives for the prevention or treatment of fungal infections. The study of additional targets of antifungal agents is extremely relevant, especially for targets associated with the fungal cell wall, which is a vital structure for fungal growth and differentiation. Therefore, cerebrosides might be promising targets for antifungal agents because they are vital for fungal viability [6], [9], [12], [28] and are highly conserved among fungal pathogens [8].
In this study, glycosphingolipids were isolated from the mycelia of S. apiospermum and purified to homogeneity using Iatrobeads chromatography. The structures of the glycosphingolipids were determined by chemical and spectrometric methods. The major species of glycosphingolipids produced by S. apiospermum was identified as N-2′-hydroxyhexadecanoyl-1-β-D-glucopyranosyl-9-methyl-4,8-sphingadienine (Figure 1). We previously described this structure for molecules in Fusarium sp [29], P. boydii [28] and the conidial and mycelial forms of Fonsecaea pedrosoi [7], [12]. We also detected a minor species appearing at m/z 750 [M+ Li+]. Similar results were obtained in the analysis of glycosphingolipids from Cladosporium resinae [30], a saprophytic species commonly found as a contaminant in fuel oil storage tanks [30], [31]. Sclerotic cells of F. pedrosoi also express the ion at m/z 750, which is most likely generated from a ceramide with an LCB containing one or more hydroxyl groups [7], [12].
The immunofluorescence experiments using a monoclonal anti-CMH antibody suggested that the GlcCer have different distributions in the mycelial and conidial forms. The immunofluorescence staining pattern of the conidial forms with the anti-CMH Mab used in this study is consistent with the model that GlcCer are distributed evenly throughout the fungal surface and are accessible to the anti-CMH Mab, whereas in the mycelial forms, the GlcCer localization does not permit interaction with this antibody.
Weak staining of the mycelial forms of P. brasiliensis and hyphae of A. fumigatus with an IgG2a monoclonal anti-glucosylceramide antibody (MEST-2) was previously observed [32]. The lower reactivity of the mycelial surface with these antibodies could be because mycelial cells might express lower amounts of GlcCer, or these cells might have a different type of cell wall assembly in which the conserved GlcCer are less accessible to antibodies.
The surface distribution of GlcCer in S. apiospermum is suggestive of an involvement of these molecules in fungal growth. We showed that incubation of conidia with the monoclonal antibody to CMH significantly reduced cell growth. However, the decreased germination was not followed by an alteration in cell viability. These results suggest that the reduction of germination is caused a fungistatic effect rather than by cell death. Our previous studies using C. albicans, C. neoformans, C. gloeosporioides, P. boydii and F. pedrosoi are consistent with other results [6], [9], [12], [28]. The mechanism by which anti-CMH antibodies inhibit fungal growth and/or differentiation are yet unclear; however, it is possible that CMHs are associated with enzymes involved in the hydrolysis and synthesis of the fungal cell wall during differentiation and division [33].
In this study, conidial cells treated with the anti-CMH antibody were more effectively phagocytosed than the control PBS- or anti-mouse IgG-treated conidia. The phagocytic indices of conidial cells treated with the anti-CMH antibody were approximately 50% or higher than those of the PBS- or anti-mouse IgG- treated fungi; the phagocytic index increased in a dose-dependent manner with the three antibody concentrations used. In addition, macrophages killed fungal cells coated with the anti-CMH Mab more efficiently than untreated conidia; this effect was observed at the three antibody concentrations used. Therefore, apart from its direct antifungal action, the anti-CMH Mab also enhances the antimicrobial activity of host macrophages.
Previously, we obtained similar results using F. pedrosoi, the most frequent etiological agent of chromoblastomycosis. Nimrichter et al. (2004) [12] demonstrated for the first time that anti-CMH antibodies have direct antifungal action and help host cells to eliminate the internalized F. pedrosoi conidial cells. However, pretreatment of conidia with the anti-CMH Mab increased the phagocytic index of F. pedrosoi by murine macrophages and increased the antifungal activity of these phagocytes by six-fold. This observation indicates that CMHs are accessible to opsonizing antibodies at the cell wall; this might be an additional mechanism by which these antibodies promote host defense during infection by F. pedrosoi and other fungal pathogens such as S. apiospermum, which express CMHs. A similar mechanism was proposed to explain the protective ability of antibodies against glucuronoxylomannan, a capsule polysaccharide in C. neoformans [34].
In this study, we provide the first evidence for the in vitro cytotoxicity of a GlcCer from S. apiospermum. The GlcCer (at 100 to 200 µg/ml) had a dose-dependent inhibitory effect on the viability of both tested mammalian cell lines L.929 (mouse fibroblasts) and RAW (macrophage-like cells). Recently, four glucocerebrosides were isolated from the sea cucumber Cucumaria frondosa, and the in vitro cytotoxic effect of the molecules on Caco-2 colon cancer cells was evaluated [35]. An inhibitory effect on cell viability (27–32%) was observed when the cells were treated with 400 µg/ml of cerebrosides. In this study, we also observed the spontaneous release of GlcCer in the culture. However, because the amount of GlcCer was very low, it could not be quantified to estimate its physiological concentration. The release of CMH in vivo from S. apiospermum might influence the interaction of this fungus with the invaded host organism. Previous studies have shown that GlcCer is required for the survival of C. neoformans in the extracellular environment of the host [36].
Scedosporium species have low in vitro and in vivo susceptibilities to traditional antifungal drugs [23], [37], [38]. Several studies have shown that antifungal drugs such as amphotericin B, nystatin, fluconazole, ketoconazole, itraconazole and terbinafine have very weak effects on S. apiospermum in vitro [22]. Some studies have shown a low level of antifungal activity by itraconazole [21], [23], [37], [38]. However, because of the emergence of itraconazole-resistant Scedosporium strains, voriconazole has emerged as a potent antifungal drug used to treat disseminated Scedosporiosis [39]. The new triazoles such as voriconazole, ravuconazole and posaconazole have some in vitro activities against P. boydii; however, not all strains of this fungus responded equally to voriconazole. The limited choice of antifungal drugs and the increased resistance of Scedosporium species to the commonly used antifungal agents indicate the necessity for other therapeutic strategies. However, there are limited data on the clinical efficacy of combination therapy for Scedosporium infections [22], [40], [41].
Because fungi of the Pseudallescheria/Scedosporium complex have low susceptibilities to antifungal drugs, several studies have analyzed the combination of different drugs. The combination of amphotericin B with either fluconazole or micafungin had a strong synergistic effect [22], [26], [27], [42]; however, the mechanism of this synergy is unclear. The antifungal activity of micafungin is through inhibition of (1,3)-β-d-glucan synthase and the subsequent disruption of fungal cell wall synthesis. This activity may enhance the action of other, less-active antifungals such as amphotericin B or itraconazole and supports the combined use of micafungin with azoles in future in vitro and in vivo studies [22].
Cuenca-Estrella et al. (2008) [43] showed that Amphotericin B alone inhibited Scedosporium strains poorly, but synergistic effects have been observed in vitro with the combined use of Amphotericin B and various azoles and other agents.
Some studies have also observed a synergy between certain antifungal agents and antibodies. For instance, the combination of caspofungin and anti-β-glucan intensifies the damage caused to hyphae by polymorphonuclear cells [44].
Therefore, combination therapy using an antifungal drug with anti-GlcCer antibodies instead of other chemical drugs might be an alternative treatment method.
The anti-GlcCer Mab used in this study protects mice against lethal C. neoformans infection [13]. This protective activity is associated with enhanced phagocytosis and killing of the fungi [6], [12]. The antifungal activity of antibodies to GlcCer and the conserved distribution of these glycolipids in fungal pathogens suggest that the GlcCers might be promising targets for the design of vaccines that elicit the production of protective antibodies.
The MAb that targets β-1,7mannotriose, which is a component of the C. albicans phosphomannan, is protective against disseminated forms of the disease, and the antifungal activity is associated with enhanced phagocytosis and killing of the fungus [45]. The combined use of this MAb with AmB enhances its therapeutic efficacy against disseminated Candidiasis. The combination has a synergistic effect, and the use of a reduced AmB concentration might be beneficial because this would decrease the severe side effects of the antibiotic [46].
In this study, we evaluated the in vitro susceptibility of S. apiospermum to AmB and itraconazole alone and in combination with the anti-GlcCer Mab. The best results were obtained by combining itraconazole with the anti-GlcCer Mab. However, no synergistic effect was observed between the anti-CMH Mab and AmB. This synergistic effect might be specific because an anti-mouse IgG (unrelated antibody) did not have a protective effect. Itraconazole is more effective than Amphotericin B in cases of Scedosporium infections. But recently we are having some problems because of the emergence of Itraconazole-resistant Scedosporium strains. It can be a possible explanation for a synergy between CMH antibody and Itraconazole, but not Amphotericin B. Many species in the Pseudallescheria/Scedosporium complex show intrinsic resistance to Amphotericin B. Therefore, these studies indicate that combining itraconazole with the anti-CMH Mab might represent a solution to the problem of limited antifungal drug choices because of the drug resistance of Scedosporium species. To further analyze the potential use of this drug and anti-CMH Mab combination, in vivo studies of scedosporiosis are necessary. Such studies will elucidate which in vitro results are relevant to in vivo effects.
Acknowledgments
We are grateful to Igor de Almeida from the Department of Biological Sciences, University of Texas at El Paso for helpful discussions and for comments on the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding Statement
This work was supported by Grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Conselho de Pesquisa da UFRJ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ and Programa de Núcleos de Excelência (PRONEX). LCLL and EBB are supported by CAPES/FAPERJ and CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tadros TS, Workowski KA, Siegel RJ, Hunter S, Schwartz DA (1998) Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum Pathol 29: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 2. Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, et al. (2008) Infections caused by Scedosporium spp. Clin Microbiol Rev 21: 157–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cimon B, Carrere J, Vinatier JF, Chazalette JP, Chabasse D, et al. (2000) Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 19: 53–56. [DOI] [PubMed] [Google Scholar]
- 4. Blyth CC, Middleton PG, Harun A, Sorrell TC, Meyer W, et al. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med Mycol 48 Suppl 1S37–44. [DOI] [PubMed] [Google Scholar]
- 5. Zouhair R, Rougeron A, Razafimandimby B, Kobi A, Bouchara JP, et al. Distribution of the different species of the Pseudallescheria boydii/Scedosporium apiospermum complex in French patients with cystic fibrosis. Med Mycol 51: 603–613. [DOI] [PubMed] [Google Scholar]
- 6. Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, et al. (2000) Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 68: 7049–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nimrichter L, Cerqueira MD, Leitao EA, Miranda K, Nakayasu ES, et al. (2005) Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect Immun 73: 7860–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barreto-Bergter E, Sassaki GL, de Souza LM (2011) Structural analysis of fungal cerebrosides. Front Microbiol 2: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Silva AF, Rodrigues ML, Farias SE, Almeida IC, Pinto MR, et al. (2004) Glucosylceramides in Colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett 561: 137–143. [DOI] [PubMed] [Google Scholar]
- 10. Barreto-Bergter E, Pinto MR, Rodrigues ML (2004) Structure and biological functions of fungal cerebrosides. An Acad Bras Cienc 76: 67–84. [DOI] [PubMed] [Google Scholar]
- 11. Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, et al. (2007) Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nimrichter L, Barreto-Bergter E, Mendonca-Filho RR, Kneipp LF, Mazzi MT, et al. (2004) A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microbes Infect 6: 657–665. [DOI] [PubMed] [Google Scholar]
- 13. Rodrigues ML, Shi L, Barreto-Bergter E, Nimrichter L, Farias SE, et al. (2007) Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol 14: 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopes LC, Rollin-Pinheiro R, Guimaraes AJ, Bittencourt VC, Martinez LR, et al. Monoclonal antibodies against peptidorhamnomannans of Scedosporium apiospermum enhance the pathogenicity of the fungus. PLoS Negl Trop Dis 4: e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 16. Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24: 119–124. [DOI] [PubMed] [Google Scholar]
- 17. Pinto MR, Gorin PA, Wait R, Mulloy B, Barreto-Bergter E (2005) Structures of the O-linked oligosaccharides of a complex glycoconjugate from Pseudallescheria boydii. Glycobiology 15: 895–904. [DOI] [PubMed] [Google Scholar]
- 19. Guimaraes AJ, Frases S, Gomez FJ, Zancope-Oliveira RM, Nosanchuk JD (2009) Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum. Infect Immun 77: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamaris GA, Chamilos G, Lewis RE, Safdar A, Raad, II, et al (2006) Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin Infect Dis 43: 1580–1584. [DOI] [PubMed] [Google Scholar]
- 21. Lackner M, Klaassen CH, Meis JF, van den Ende AH, de Hoog GS (2012) Molecular identification tools for sibling species of Scedosporium and Pseudallescheria. Med Mycol 50: 497–508. [DOI] [PubMed] [Google Scholar]
- 22. Guarro J, Kantarcioglu AS, Horre R, Rodriguez-Tudela JL, Cuenca Estrella M, et al. (2006) Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 44: 295–327. [DOI] [PubMed] [Google Scholar]
- 23. Lackner M, de Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 56: 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiederhold NP, Lewis RE (2009) Antifungal activity against Scedosporium species and novel assays to assess antifungal pharmacodynamics against filamentous fungi. Med Mycol 47: 422–432. [DOI] [PubMed] [Google Scholar]
- 25. Kontoyiannis DP, Hachem R, Lewis RE, Rivero GA, Torres HA, et al. (2003) Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98: 292–299. [DOI] [PubMed] [Google Scholar]
- 26. Walsh TJ, Hiemenz JW, Seibel NL, Perfect JR, Horwith G, et al. (1998) Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis 26: 1383–1396. [DOI] [PubMed] [Google Scholar]
- 27. Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, et al. (2002) Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 346: 225–234. [DOI] [PubMed] [Google Scholar]
- 28. Pinto MR, Rodrigues ML, Travassos LR, Haido RM, Wait R, et al. (2002) Characterization of glucosylceramides in Pseudallescheria boydii and their involvement in fungal differentiation. Glycobiology 12: 251–260. [DOI] [PubMed] [Google Scholar]
- 29. Duarte RS, Polycarpo CR, Wait R, Hartmann R, Bergter EB (1998) Structural characterization of neutral glycosphingolipids from Fusarium species. Biochim Biophys Acta 1390: 186–196. [DOI] [PubMed] [Google Scholar]
- 30. Calixto R, Mattos B, Bittencourt V, Lopes L, Souza L, et al. (2010) beta-Galactofuranose-containing structures present in the cell wall of the saprophytic fungus Cladosporium (Hormoconis) resinae. Res Microbiol 161: 720–728. [DOI] [PubMed] [Google Scholar]
- 31. Seifert T, Giacomini G, Kapeller P, Fazekas F (2002) MR brain changes following terpentine oil ingestion. J Neurol 249: 1109–1110. [DOI] [PubMed] [Google Scholar]
- 32. Toledo MS, Suzuki E, Levery SB, Straus AH, Takahashi HK (2001) Characterization of monoclonal antibody MEST-2 specific to glucosylceramide of fungi and plants. Glycobiology 11: 105–112. [DOI] [PubMed] [Google Scholar]
- 33. Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, et al. (2008) Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, et al. (1998) Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 42: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu J, Go ML, Lim LY (2003) Modulation of digoxin transport across Caco-2 cell monolayers by citrus fruit juices: lime, lemon, grapefruit, and pummelo. Pharm Res 20: 169–176. [DOI] [PubMed] [Google Scholar]
- 36. Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH Jr, Hennig M, et al. (2006) Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116: 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lackner M, Rezusta A, Villuendas MC, Palacian MP, Meis JF, et al. (2011) Infection and colonisation due to Scedosporium in Northern Spain. An in vitro antifungal susceptibility and molecular epidemiology study of 60 isolates. Mycoses 54 Suppl 312–21. [DOI] [PubMed] [Google Scholar]
- 38. Gilgado F, Serena C, Cano J, Gene J, Guarro J (2006) Antifungal susceptibilities of the species of the Pseudallescheria boydii complex. Antimicrob Agents Chemother 50: 4211–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanafani ZA, Comair Y, Kanj SS (2004) Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur J Clin Microbiol Infect Dis 23: 836–840. [DOI] [PubMed] [Google Scholar]
- 40. Steinbach WJ, Perfect JR (2003) Scedosporium species infections and treatments. J Chemother 15 Suppl 216–27. [DOI] [PubMed] [Google Scholar]
- 41. Steinbach WJ, Schell WA, Miller JL, Perfect JR (2003) Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J Clin Microbiol 41: 3981–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh TJ, Groll AH (1999) Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl Infect Dis 1: 247–261. [DOI] [PubMed] [Google Scholar]
- 43. Cuenca-Estrella M, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Gomez-Lopez A, et al. (2008) In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob Agents Chemother 52: 1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, et al. (2008) Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 198: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caesar-TonThat TC, Cutler JE (1997) A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun 65: 5354–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han Y (2010) Efficacy of combination immunotherapy of IgM MAb B6.1 and amphotericin B against disseminated candidiasis. Int Immunopharmacol 10: 1526–1531. [DOI] [PubMed] [Google Scholar]