Abstract
Chronic exposure to arsenic (As) in food and water is a significant public health problem. Person-specific aggregate exposure is difficult to collect, and modeling based on limited food As residue databases is of uncertain reliability. Two, cross-sectional, population exposure studies—the National Human Exposure Assessment Survey (NHEXAS)-Arizona and the Arizona Border Survey (ABS)— had a total of 252 subjects with diet, water, and urinary As data. Total As was measured in 24-hour duplicate diet samples and modeled using 24-hour diet diaries in conjunction with several published food surveys of As. Two-stage regression was used to assess the effects of dietary As on urinary total As (uAs): 1) generalized linear mixed models of uAs above versus below the limit of detection (LOD); and 2) restricted models limited to those subjects with uAs > LOD, using bootstrap sampling and mixed models adjusted for age, sex, BMI, ethnicity, current smoking, and As intake from drinking and cooking water. In restricted models, measured and modeled estimates were significant predictors of uAs. Modeled dietary As based on Total Diet Study mean residues greatly underestimated dietary intake. In households with tap water As ≤ 10 ppb, over 93% of total As exposure was attributable to diet.
Keywords: arsenic, water, urine, dietary exposure
Introduction
Inorganic arsenic (As) is associated with numerous adverse health effects and is a known human carcinogen, even at relatively low exposure levels (1–4). Until recently, drinking water was considered the primary source of exposure. However, food is now considered the only widespread source of exposure, as high concentrations of As in drinking water tend to be localized (5–8). Arsenic is regulated in drinking water in the U.S., but there are currently no regulatory standards for As in food (9). Arsenic enters the food chain primarily through natural contamination of groundwater and is taken up by plant roots, though some contamination is a result of historic use of arsenical pesticides and supplements in animal feed (10–12). According to results from the U.S. FDA Total Diet Study, approximately one-quarter of commonly consumed foods in the U.S. contains measureable As (13).
Modeling ingestion exposure is inherently complex. Collection of duplicate food samples is the most accurate method for assessing dietary exposures because it involves direct measurement of contaminants in duplicate portions of the foods consumed (14). These samples account for local and individual differences in agricultural practices, food processing and food preparation, including water used in preparation. However, because of the time and expense of collecting and analyzing food samples, especially in population studies, indirect methods of dietary assessment are often more expedient. Indirect methods generally utilize national databases of food consumption and residue concentrations to model contaminant exposure that may not represent individual, ethnic, and regional differences in diet and water intake and contaminant exposures (15,16).
Urinary Total As (uAs) measures organic, inorganic and methylated products of As metabolism. It is the most consistently used biomarker of exposure (7) and is thought to reflect aggregate As exposure over the previous few days (17,18). Over 50% of ingested inorganic As is readily absorbed and metabolized within 3–4 days (19). While some studies have found a high correlation between As concentration in urine and water (20), others report only modest correlations and have suggested that the relation may be confounded by other sources of exposure, such as food (4,7).
A small number of studies have specifically modeled the effects of dietary As on urinary As excretion, but most of these studies have focused on consumption of specific food items (21–24). In this analysis, we compared direct and indirect methods of modeling the relation of urinary total As to person-specific aggregate exposure measures, adjusted for confounders. Twenty-four hour duplicate diet samples and diet diaries from the National Human Exposure Assessment Survey (NHEXAS) Arizona and the Arizona Border Survey (ABS) (15,25–27) were used to compare measured and modeled dietary As intake. Diet diaries were compiled over the same period of time that the duplicate diet samples were collected, and then used to model ingestion of As from all foods and beverages consumed during the previous 24 hours. Food items listed were matched to the following residue databases:
Total Diet Study (TDS), 1991–2005 mean total As residues (28),
TDS maximum total As residues (28),
Schoof et al. mean total As concentrations in a market basket survey of foods that encompasses 90% of dietary inorganic As intake in the U.S. (29), and
Schoof et al. mean inorganic As (29).
Source-specific water As concentration and consumption were included in the estimates of the contribution of food, drinking water and cooking water to total urinary As and to total exposure. We stratified our analyses by household tap water As concentration above versus below the current EPA standard of 10 ppb and above versus below a more stringent threshold of 5 ppb to assess the potential impact of dietary As under different water standards while accounting for water consumption by source.
Methods
Study Populations
The National Human Exposure Assessment Survey (NHEXAS) –Arizona was conducted between 1995 and 1997. NHEXAS used a proportion-based sample of the total population in Arizona to evaluate multiple contaminant exposures via multiple pathways (25–27). The methods used for sampling and analysis of metals have been described in detail by O’Rourke et al., 1999 (30). Diet and time-activity diaries were completed and duplicate food samples, water sample from multiple sources, urine and other media were collected for 179 subjects.
From 1997 to 1998, the Arizona Border Survey (ABS) was conducted in 25 census tracts along the Arizona-Mexico border to test whether exposures in the Arizona border population (80% Hispanic) were higher than in the rest of the state. ABS was also a probability-based exposure survey and the methods used were the same as those used in NHEXAS. In ABS, 86 households were targeted for intensive sampling.
Dietary Methods
Participants in NHEXAS and ABS completed a consumption checklist, organized by food category, of TDS-coded foods plus a few additional food items commonly consumed by Mexican-Americans along the border (15). In addition, they kept a duplicate diary record of foods in the 24-hour duplicate diet sample. Solid and liquid foods/beverages were composited in separate sample bags. The samples were sent on ice to the FDA where they were assayed using inductively coupled plasma mass spectrometry (ICP-MS), which had a limit of detection between 1.9 and 2.7 μg/kg (26).
Diet diary data were sent to the Arizona Diet, Behavior and Quality of Life Assessment Lab at the Arizona Cancer Center for nutritional and arsenic assessment. Total energy and nutrient intake was modeled based on the diet diaries using Nutrition Data System for Research (NDSR) software (31). Dietary As intake was modeled from the “Total Diet Study Statistics on Element Results” based on the FDA 1991–2005 market basket surveys (28), and from the Schoof et al. market basket survey (29). TDS measured As using hydride generation atomic absorption spectrometry (HGAAS), and Schoof et al. used ICP-MS (29). The limit of detection (LOD) for total As was 0.01 mg/kg (10 μg/kg) in TDS (32) and 3.6 ng/g (3.6 μg/kg) in Schoof. LOD for inorganic As in Schoof was 2 μg/kg (29). Additional detail on these methods is presented elsewhere (33).
Water Arsenic
As concentrations in household water from all sources used for drinking and food preparation (“cooking”) were analyzed using inductively coupled plasma-mass spectroscopy (EPA Method 200.8, ICP-MS) (26). The minimum detection limit in water was 0.20 μg/L (26,30). Arsenic exposure via drinking water and water used for food preparation (generally tap water) were calculated separately, as the product of the concentration (μg/L) times the quantity consumed (L/day).
Urinary Arsenic
Urine samples were collected on the morning after completion of the duplicate diet sample and placed on ice for transport to the University of Arizona. Urinary total As was analyzed using inductively coupled plasma-mass spectroscopy (ICP-MS), with a minimum detection limit of 4.1 μg/L (26,30).
Statistical Analysis
Statistical analyses were performed using Stata version 11.2. Standard descriptive statistics were used to evaluate the frequency and distribution of all of the variables. Urinary, water and dietary As values that were below their respective limits of detection were assigned a value of one-half the LOD for that medium. Since 43% of values for uAs were below the limit of detection, uAs was treated as either a dichotomous variable (above versus below the LOD) or analyses were restricted to those subjects with uAs values > LOD. Arsenic values (food, water and urine) were log(10)-transformed for statistical analyses and means reported are geometric means (gmeans). Differences between ethnic groups and study populations were assessed using chi-squared, Kruskal-Wallis, and t-tests. Intraclass correlations between measured and modeled dietary As intake were estimated using maximum likelihood for variance components models; Fisher’s z-test was used to compare the equality of the intraclass correlation coefficients.
The crude and adjusted relations between uAs and each of the dietary As variables were assessed using two-stage mixed models in which “study population” was included as a random effect to account for intra-correlation within the studies. Covariates in the models included drinking and cooking water As (log(10)-transformed), age, BMI, sex, and indicator variables (yes/no) for Hispanic ethnicity, current smoking status, and seafood consumption in the previous 24 hours.
The first stage of the regression modeling used a generalized linear mixed model to assess the effect of dietary As on uAs above versus below the LOD. The second stage models were linear mixed models, restricted to subjects with uAs > LOD. These were run using bootstrap sampling techniques to obtain better estimates of the standard errors for the regression coefficients. Bootstrap sampling involved 500 simulations with replacement from the original dataset, including observations both below and above the LOD. Likelihood ratio tests were used to compare models with and without covariates. To assess potential interaction, these same models were stratified by: 1) Hispanic vs. non-Hispanic ethnicity, and 2) tap water As concentration, above vs. below 10 ppb and 5 ppb. Sensitivity analysis was performed to evaluate the effects of consumption of seafood on the relation between dietary and urinary total As by assessing the crude and adjusted relations between seafood consumption and urinary excretion and by excluding subjects who consumed seafood during the previous 24 hours and re-running the two-stage models. A two-sided significance level of 0.05 was assumed for all analyses except for likelihood ratio tests in which P < 0.10 was considered significant.
Results
The total number of participants in the study with complete data for specific analyses was 252. There were 166 people in NHEXAS and 85 in ABS with duplicate diet and uAs data, for a total of 251, and 163 people in NHEXAS and 83 in ABS with diet diary and uAs data, for a total of 246. Characteristics of Hispanics and non-Hispanics in the combined (total) population are shown in Table 1. Overall, 46% of the population was Hispanic and 64% female. There were no differences between Hispanics and non-Hispanics in mean age, proportion of females, current smokers or percent of subjects with uAs < LOD.
Table 1.
Population characteristics of Hispanics and non-Hispanics in the total population.
| TOTAL POPULATION | HISPANICS | NON-HISPANICS | |
|---|---|---|---|
| N (%) of subjects | 252 | 115 (45.6) | 137 (54.4) |
| N (%) female | 161 (63.9) | 78 (67.8) | 83 (60.6) |
| N (%) current smokers | 43 (17.1) | 15 (13.0) | 28 (20.4) |
| Females | 22 (13.7) | 6 (7.7) | 16 (19.3) |
| Males | 21 (23.1) | 9 (24.3) | 12 (22.2) |
| N (%) consumed seafood | 34 (13.5) | 17 (14.8) | 17 (12.4) |
| N (%) urinary As < LOD1 | 109 (43.3) | 53 (46.1) | 56 (40.9) |
| Age, years (mean±SD) | 43.9±19.1 | 44.9±17.2 | 43.20±20.6 |
| (min-max) | 6–83 | 10–78 | 6–83 |
LOD, limit of detection = 4.1 μg/L
Mean Arsenic
There were statistically significant differences in geometric mean values for water and dietary As intake between Hispanics and non-Hispanics in the population (Table 2). The gmean water intake for both drinking and cooking was significantly lower among Hispanics (P=0.002 and 0.027, respectively). While there was no difference in As concentration of cooking water (gmean 4.39, 95% CI 3.91–4.92 μg/L), mean As concentration of drinking water was 0.70 μg/L for Hispanics and 1.35 μg/L for non-Hispanics (P=0.004).
Table 2.
Geometric mean (g mean) values and range (minimum-maximum) for water and dietary (measured and modeled) As exposure and urinary arsenic excretion among Hispanics and Non-Hispanics and in the total population.
| TOTAL POPULATION g mean (min-max) |
HISPANICS g mean (min-max) |
NON-HISPANICS g mean (min-max) |
P-value | |
|---|---|---|---|---|
| Drinking water | ||||
| Quantity consumed, L/day | 1.48 (0–7.4) | 1.32 (0–4.1) | 1.64 (0–7.4) | 0.002 |
| As concentration, μg/L | 1.00 (0–37) | 0.70 (0–37) | 1.35 (0–32) | 0.004 |
| As intake, μg/day | 1.44 (0–140) | 0.90 (0–46) | 2.15 (0–140) | 0.001 |
| Water used in food preparation/cooking | ||||
| Quantity consumed, L/day | 0.40 (0–3.5) | 0.34 (0–2.1) | 0.47 (0–3.5) | 0.027 |
| As concentration, μg/L | 4.39 (0.3–286) | 4.41 (0.3–120) | 4.37 (0.6–286) | 0.825 |
| As intake, μg/day | 1.75 (0–200) | 1.46 (0–40) | 2.04 (0–200) | 0.176 |
| Dietary As | ||||
| Duplicate diet total, μg/day | 16.12 (0.3–2882) | 18.40 (0.3–720) | 14.43 (3.9–2882) | 0.047 |
| TDS mean total, μg/day | 7.39 (0.1–717) | 5.77 (0.1–255) | 9.08 (0.1–717) | 0.041 |
| TDS maximum total, μg/day | 46.66 (1.7–1395) | 38.35 (2.3–896) | 54.90 (1.7–1345) | 0.012 |
| Schoof mean total, μg/day | 57.02 (2.6–658) | 47.82 (2.6–658) | 65.97 (4.2–521) | 0.010 |
| Schoof mean inorganic, μg/day | 7.46 (0.4–81) | 6.03 (0.4–61) | 8.90 (1.1–81.4) | 0.001 |
| Urinary total As | ||||
| concentration, μg/L | 8.09 (<LOD–430) | 8.04 (2.3–341) | 8.13 (2.3–430) | 0.784 |
Geometric mean total As in 24-hour duplicate diet samples was 16.12 μg/day. In contrast, mean total As exposure based on TDS mean, TDS maximum, and Schoof total mean residue data was 7.39, 46.66 and 57.02 μg/day, respectively, and all of these estimates were statistically significantly different from As measured in the duplicate diet samples. Measured dietary As was higher among Hispanics than non-Hispanics (P=0.047), but modeled estimates, based on residue data, were significantly lower.
The intraclass correlations between measured and modeled dietary As were relatively low. Duplicate diet As was modestly correlated with estimates based on TDS means (rho=0.203±0.061, P=0.001), but showed no correlation with estimates based on TDS maximum or Schoof total As. However, Schoof total As was very highly correlated with TDS maximum total As estimates (rho=0.863±0.016, P<0.001).
Relation of Dietary As to Urinary As
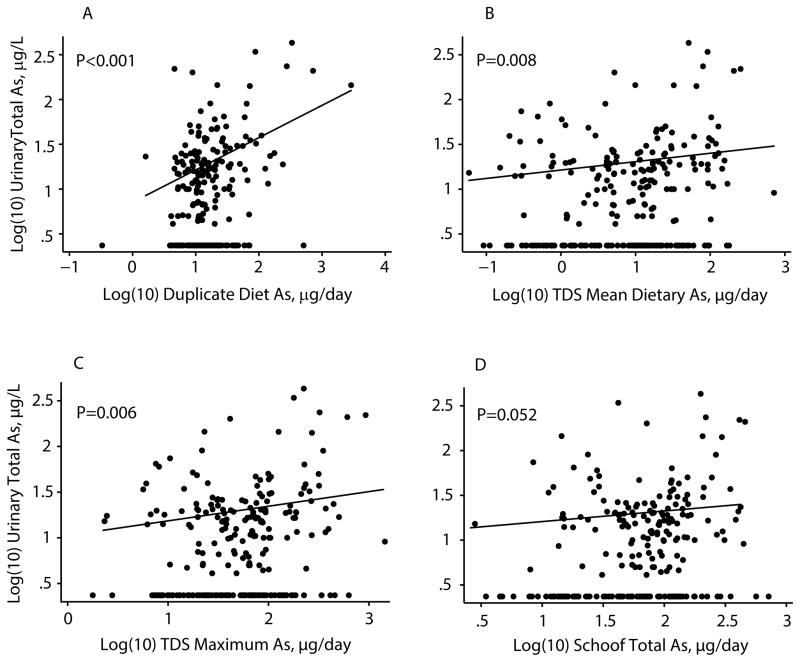
Two-stage adjusted models of uAs in the total population are shown in Table 3 and represented in Figure 1. In unrestricted models, a 10 fold increase in duplicate diet As was associated with 3.2 (1.45–7.14) times increased odds of uAs being above the LOD (P=0.004). Increased Schoof total and inorganic dietary As exposure were also associated with significantly increased odds of uAs > LOD (P=0.003 and 0.038, respectively). In the restricted analysis, log(10) duplicate diet, TDS mean, and TDS maximum dietary total As showed statistically significant linear relations with log(10) uAs in those subjects with uAs > LOD (all P-values < 0.009). Intake of inorganic As from food (29) was not a significant linear predictor of uAs in the restricted model, and inorganic As in either drinking or cooking water was not associated with uAs in any of the models. Age was a negative predictor of (log) uAs in the restricted models with dietary total As intake, but there was no confounding by any of the other covariates. Based on R-squared values, duplicate diet As accounted for approximately 12% of the total variance in the dependent variable (dichotomized uAs) in the stage 1 model, and 24% of the variance in the log(10) of uAs in the restricted, stage 2 model. The modeled estimates of dietary As explained <10% of the variance in both stage 1 and stage 2 models.
Table 3.
Two-stage regression analysis of measured and modeled dietary As intake and urinary total As (uAs) excretion in the combined population, adjusted for potential confounders1: 1) Stage 1, unrestricted linearized mixed models of uAs above vs. below the limit of detection (LOD); 2) Stage 2, restricted linear mixed models using bootstrap sampling of log(10) uAs, restricted to subjects with uAs > LOD.
| 1) Stage 1 Models |
|
|||
|---|---|---|---|---|
| N | OR (95% CI) | P-value | R2 | |
| Duplicate diet total As | 247 | 3.21 (1.45–7.14) | 0.004 | 11.66% |
| TDS total mean As | 243 | 1.39 (0.91–2.12) | 0.130 | 8.97% |
| TDS total maximum As | 243 | 1.96 (0.83–4.66) | 0.127 | 8.90% |
| Schoof total As | 243 | 2.58 (1.37–4.84) | 0.003 | 9.44% |
| Schoof inorganic As | 243 | 2.12 (1.04–4.33) | 0.038 | 7.67% |
| 2) Stage 2 Models |
|
|||
|---|---|---|---|---|
| N | Coefficient ± s.e. | P-value | R2 | |
| Duplicate diet total As | 139 | 0.389±0.083 | 0.001 | 23.74% |
| TDS mean total As | 132 | 0.159±0.061 | 0.009 | 7.78% |
| TDS max total As | 132 | 0.258±0.092 | 0.005 | 9.10% |
| Schoof total As | 132 | 0.251±0.134 | 0.060 | 7.10% |
| Schoof inorganic As | 132 | 0.057±0.150 | 0.706 | 9.93% |
Models are adjusted for sex, Hispanic ethnicity, age, BMI, current smoking, drinking water As exposure, and (except in the duplicate diet model) As exposure from water used in food preparation.
Figure 1.
Relation of measured and modeled dietary total As to urinary total As (log(10)-transformed): a) Duplicate diet total As, b) Total Diet Study (TDS) mean total As, c) TDS maximum total As, and d) Schoof mean total As. Fitted line is restricted to subjects with urinary As above the limit of detection.
Stratified Models
Effect modification by Hispanic ethnicity was assessed using stratified models. In the first stage models, there was significant association between measured and modeled dietary total As and uAs among non-Hispanics, but dietary As was not a predictor of uAs among Hispanics. In the restricted linear models, measured duplicate diet As was a statistically significant predictor of uAs (P<0.001), regardless of stratification by ethnicity, but modeled estimates were not.
Models stratified by tap water As concentration above/below the U.S. EPA maximum contaminant level (MCL) of 10 ppb and above/below 5 ppb were also run to determine whether the effect of dietary As on excretion varied depending on the concentration of As in household tap water. There was a consistent and statistically significant relation between dietary As, modeled and measured, and uAs > LOD, but only among subjects who had tap water As concentrations ≤ 5 or 10 ppb (all P<0.05). Similarly, in the restricted models, modeled dietary arsenic was predictive of uAs only in households with tap water As concentrations ≤ 10 ppb. Measured log(10) total dietary As intake was linearly related to log(10) uAs concentration, regardless of stratification by tap water As, in the restricted models.
Sensitivity analysis
Several approaches were used to assess the potential confounding effects of seafood consumption on the relation between dietary and urinary total As. Only 34 subjects (13%) in the total study population reported eating seafood, and of them, 25 had concentrations of urinary As > LOD. Median values of dietary and urinary total As among seafood eaters fell in the upper 90th percentile of dietary As (measured and modeled) and 75th percentile of uAs of the total population.
In crude models, seafood consumption was a positive predictor of uAs (P=0.024 and 0.001, unrestricted and restricted models, respectively). When seafood consumption was added as a dichotomous covariate to the adjusted stage 1 models, it was not a significant confounder in the relation between measured or modeled dietary total As intake and uAs, but it was a significant confounder in the relation between dietary inorganic As intake and uAs (likelihood ratio test, P=0.052). In the adjusted stage 2 linear models restricted to subjects with urinary As > LOD, seafood consumption was a significant confounder in all of the models (all P<0.03).
Stratified analyses were run on subjects who had not consumed seafood in the previous 24 hours. Among subjects who had not consumed seafood, duplicate diet As was not a significant predictor of uAs in the stage 1 model (P=0.083), but both Schoof dietary total and inorganic As remained significant, regardless of stratification by seafood consumption (P=0.014 and P=0.032, respectively). In the stratified stage 2 models, measured duplicate diet As remained a significant linear predictor of uAs among non-seafood eaters, but modeled estimates of dietary As were no longer significant.
Contribution of Food to Total Ingestion Exposure
Estimates of total and inorganic arsenic intake, stratified by tap water As concentration, and the relative proportion ingested from food, drinking water and cooking water are shown in Table 4. These estimates include only subjects who did not report having eaten seafood in the previous 24 hours. Mean total As exposure in households with tap water As ≤ 10 ppb was 13.8 μg/day based on duplicate diet samples, and 48.5 μg/day based on Schoof residue data, and approximately 93–95% of total exposure was from food. In households with tap water As > 10 ppb, total As intake was 25.0 μg/day and 70.7 μg/day, based on duplicate diet and Schoof residues, respectively. In these homes, 60–74% of total intake was from food, and 26–39% was from water. Total inorganic As intake was 9.4 μg/day in subjects with tap water As below and 26.1 μg/day in subjects with tap water As above 10 ppb. In subjects with tap water As below 10 ppb, 75% of the total inorganic As ingested was contributed by food, while in subjects with tap water As above the MCL, food contributed just 30%.
Table 4.
Geometric mean (%) arsenic intake from food and water, μg/day, non-seafood-eaters only, stratified by tap water As concentration above versus below the maximum contaminant limit (MCL) of 10 ppb.
| Tap water As ≤ 10 ppb | Tap water As > 10 ppb | |||
|---|---|---|---|---|
|
| ||||
| Duplicate Diet Sample | Diet diary and Schoof et al. (1999) | Duplicate Diet Sample | Diet diary and Schoof et al. (1999) | |
| N | 181 | 176 | 35 | 35 |
| Dietary total As | 12.87 (93.0%) | 46.20 (95.2%) | 15.23 (60.9%) | 52.58 (74.3%) |
| Drinking water As | 0.97 (7.0%) | 0.97 (2.0%) | 9.78 (39.1%) | 9.78 (13.8%) |
| Cooking water As | § | 1.34 (2.8%) | § | 8.38 (11.8%) |
|
|
||||
| Total As intake | 13.84 | 48.51 | 25.01 | 70.74 |
| Dietary inorganic As | 7.09 (75.4%) | 7.97 (30.5%) | ||
| Drinking water As | 0.97 (10.3%) | 9.78 (37.4%) | ||
| Cooking water As | 1.34 (14.3%) | 8.38 (32.1%) | ||
|
|
||||
| Total Inorganic As intake | 9.40 | 26.13 | ||
Duplicate food samples contain water used in preparation and cooking.
Discussion
Archived data on 251 participants in the Arizona Border Survey and the National Health Exposure Assessment Survey-Arizona were used to evaluate different methods of estimating dietary As exposure and the contribution of dietary As to total exposure and urinary As excretion. Our models explicitly reflect subject-specific data on intake of food, use of multiple sources of water for drinking and cooking, and concentrations of total As in urine, cooking and drinking water, and food samples. We found that dietary As intake, measured and modeled, is a significant predictor of urinary total As, regardless of whether household tap water As concentration was above or below the EPA maximum contaminant level (MCL) of 10 ppb. However, duplicate diet As explained a greater proportion of the variance in urinary As excretion than any of the modeled estimates of dietary As exposure, and neither drinking nor cooking water As exposure was significantly associated with urinary total As.
Most studies that have evaluated dietary As intake have used national databases to extrapolate average intake in the general population and subpopulations (8,13,16). These indirect methods of estimating dietary intake have shown only modest correlation with duplicate diet residue data (15,16,34). Limitations of these studies include inadequate accounting of regional, demographic, and person-specific differences in dietary (15) and water consumption patterns (16).
In our study, geometric mean total As measured in duplicate diet samples was more than two times higher than dietary total As intake estimated from TDS mean residue data (16 and 7 μg/day, respectively), and the difference between duplicate diet and TDS mean As among Hispanics was greater than three-fold. The low As exposure estimates based on TDS means may be attributable to the high LOD (0.40 mg/kg) for As in the Total Diet Study and/or the averaging in of zeroes for food values below the LOD (32). In contrast, the dietary total As estimates based on TDS maximum and Schoof (29) residue data were two-three times higher than measured As in duplicate diet. These higher estimates reflect an emphasis on the maximum As level in foods (28) and specifically on foods with high As (29), but were comparable to dietary exposures reported by Tao and Bolger (1999) (28–92 μg/day) and MacIntosh et al. (1996) (40 μg/day in females and 46 μg/day in males) (8,13).
Various researchers have used NHEXAS-AZ data to model potential dietary As exposure. In our study, the geometric mean total dietary exposure based on duplicate diet samples was 0.239 μg/kg BW/day and 0.846 μg/kg BW/day based on Schoof residue data and the diet diaries. Moschandreas et al. (2002) estimated an average As exposure of 0.65 μg/kg BW/day using Dietary Exposure Potential Modeling with NHEXAS duplicate diet data (15). Xue et al. (2010), using Stochastic Human Exposure and Dose Simulation (SHEDS)-Dietary models on duplicate diet data from NHEXAS-AZ, estimated a median total As intake from food of 0.095 μg/kg/day (mean±SD = 0.185±0.3 (16), markedly lower estimates than from either of the other studies. The contribution of dietary intake to total As exposure, based on measured duplicate diet and water samples, was over nine times greater than that of drinking water. Our estimate is similar to that of Xue et al., who estimated total As exposure from food at 14 times that from water in the national population using SHEDS models (16).
Among subjects in our study who had household tapwater As > MCL and who did not consume seafood, over 60% of the total As ingested was from food, as compare with over 93% among subjects with water As ≤ MCL. This same trend with high/low drinking water As was observed in Bangladesh. There, in homes at the median or lower end of the distribution of drinking water As, the contribution from food was greater than that from water (35,36). These results suggest that regulation of water may reduce exposure, especially via water used for food preparation, but is not sufficient for protection of public health, given the high proportion of As exposure that comes from food.
Lower water As and higher dietary As exposure among Hispanics as compared to non-Hispanics was observed in this study. Specific foods that are relatively high in inorganic As, such as rice, tortillas and beans, may be preferentially eaten by Arizona Hispanics. The lower water As exposure among Hispanics appears to be a result of both consumption of smaller quantities of drinking water and low As content of the water sources used for drinking. Roberge and colleagues (2012) reported a similar phenomenon in a binational study, in which they report that subjects in Mexico, as compared to those living in southern Arizona, consumed significantly less fluid (37).
Despite low numbers of seafood eaters in this study, seafood consumption was associated with greatly increased dietary and urinary total As. While most of the As in seafood is organic and thought to be of low toxicity, 20–30% may be inorganic or methylated forms (38), and would be expected to undergo metabolism in the liver (38,39). Other foods, too, are composed of various proportions of As forms (23). Because our focus was total As intake and excretion, we chose to address seafood consumption in a sensitivity analysis. In that analysis, seafood was a confounder in the relationship between dietary and urinary total As, and in models in which seafood-eaters were excluded, there was no association. In future studies, analysis of As species in both duplicate food samples and in urine might enable segregation of the effects of ingestion of organic, inorganic and methylated As forms in seafood and other foods on urinary excretion of As species.
Most (84%) of the subjects in NHEXAS/ABS were exposed to levels of arsenic in tapwater that were below the current MCL of 10 ppb, 60% were exposed to concentrations below 5 ppb, and only two subjects had concentrations greater than 50 ppb. Hence, it is not entirely surprising that arsenic intake from cooking and drinking water did not predict urinary As. A number of studies have reported modest correlations between As in drinking water and total urinary As, but this relationship appears to be somewhat variable, and other factors are undoubtedly involved (40–42). There have been no studies, to our knowledge, that have compared measured and modeled dietary As intake in a general population and parsed the relation between arsenic exposure from food and water and urinary As excretion.
In NHEXAS-AZ and ABS, dietary inorganic As was not measured in duplicate diet samples. We found a relation between modeled dietary inorganic As and urinary total As in the stage 1 models predicting urinary As above vs. below the LOD, but not in the models restricted to subjects with urinary As above the LOD. Exposure to inorganic As from drinking and cooking water constituted approximately 25% of total exposure to inorganic As in households with tapwater concentrations below the MCL, and almost 70% of exposure in households with tapwater As above the MCL.
Despite use of similar study designs and methods in both NHEXAS and ABS, there were differences in demographic characteristics and in the distribution of dietary, water and urinary As between study populations. These differences could not be accounted for by discrepancies in data collection protocols, nor were they related to differences in consumption of seafood, ethnicity, sex, total caloric intake, body weight, body mass index (BMI) or age. Mixed models were used to adjust for correlation within and discrepancies between study populations in dietary reporting.
There are many difficulties inherent to modeling ingestion exposure, including inaccurate dietary reporting (43–47). While duplicate diet samples allow direct measurement of contaminants in food, its accuracy depends on subjects’ willingness or ability to collect a replicate sample of each food and beverage consumed over the course of 24 hours (15). According to a study on these methods by Thomas et al. (1997), laboratory measured caloric content of duplicate diet samples was, on average, 16% lower than estimated energy expenditure and 12% lower than food diary records (14). In our study, food samples collected weighed on average half as much as reported for the same day in the food diaries. Forgetting, food insecurity, embarrassment in social situations (14), or psychological issues related to “throwing good food out,” despite reimbursement, may have prevented subjects from collecting the normal amounts of food eaten. Although the measured As in the foods provided the best fit in models of urinary As, the total measured dietary exposure is likely to be an underestimate of the true exposure.
In the NHEXAS/ABS diet diaries, over 22% of subjects reported consumption of over 150% of expected energy intake, based on sex, age, weight, height and sedentary lifestyle, and 19% reported consumption of less than 75% of energy requirements, based on gender and age-specific prediction equations (48,49), suggesting a high rate of misreporting. We compared models with and without adjustment of dietary arsenic intake for implausible caloric intake and observed minimal effect on the fit of the models. Because of too many uncertainties regarding actual consumption and no way of knowing whether our correction for apparent extreme under- and over-reporting improved the accuracy of our estimated exposures, we chose to present the unadjusted estimates.
Another uncertainty in modeling dietary exposure is the scarcity of data on As residues in food. Recent studies have documented extensive variability in total and speciated As content of foods, even within brands (11,23,50,51). It is clear that global sources of food production and processing, as well as regional and/or individual differences in food preparation, are important sources of heterogeneity and need greater consideration for accurate determination of As residue content of foods. In particular, data on arsenic species present in foods and better assessment of the variability of As concentrations and speciation of foods from different locales would be invaluable for assessing potential impact of specific foods on total exposure.
To summarize, duplicate diet samples analyzed for arsenic directly measure intake, assuming that all food items eaten are included in amounts that accurately reflected consumption and that sample preparation and laboratory methods are appropriate. Modeling of dietary exposure is presumed to be less accurate, but provides a less expensive correlative approach to estimating exposure. In this regional study, we found that the Total Diet Study mean residue results (1991–2005) appear to grossly underestimate dietary exposure, as compared with measured samples. In households with water As concentrations below the EPA maximum contaminant level of 10 ppb, over 93% of total As exposure was attributable to diet. Both measured and modeled dietary As were predictive of total urinary As excretion, but drinking and cooking water As were not, and Hispanics had higher exposure to As in food and lower exposure to As in water than non-Hispanics. Despite problems with reporting bias in both direct and indirect methods of dietary assessment and the accuracy of determining exposure from a residue database, both methods can be used to model urinary total As excretion. In this Arizona study, exposure to As in food is a better predictor of urinary As than exposure to As in water.
Acknowledgments
This project was funded by EPA Science to Achieve Results (STAR) grants #R825813 to The University of Arizona. We would also like to acknowledge the integral role of Seumas Rogan in coordination of data collection and database management, and numerous students for their work in field and lab.
This study was completed in partial fulfillment of the requirements of the PhD program in Epidemiology at the University of Arizona, Mel & Enid Zuckerman College of Public Health. Funding for this research was provided by the U.S. EPA Star Grant # R83399201-0.
Footnotes
None of the coauthors have any competing financial interests to declare.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, et al. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7(2):117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Nunez Z, Meliker JR, Meeker JD, Slotnick MJ, Nriagu JO. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. J Expo Sci Environ Epidemiol. 2012;22(2):182–190. doi: 10.1038/jes.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KG, Ross GL. Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul Toxicol Pharmacol. 2002;36(2):162–174. doi: 10.1006/rtph.2002.1573. [DOI] [PubMed] [Google Scholar]
- 6.Meacher DM, Menzel DB, Dillencourt MD, Bic LF, Schoof RA, Yost LJ, et al. Estimation of Multimedia Inorganic Arsenic Intake in the U.S. Population. Human and Ecological Risk Assessment. 2002;8(7):1697–1721. [Google Scholar]
- 7.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIntosh DL, Spengler JD, Ozkaynak H, Tsai L, Ryan PB. Dietary exposures to selected metals and pesticides. Environ Health Perspect. 1996;104(2):202–209. doi: 10.1289/ehp.96104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NRC. Arsenic in Drinking Water: 2001 Update. Free Executive Summary. 2001 Retrieved from http://www.nap.edu/catalog/10194.html.
- 10.Meharg A, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytologist. 2002;154(1):29–43. [Google Scholar]
- 11.Meharg A, Raab A. Getting to the bottom of arsenic standards and guidelines. Environ Sci Technol. 2010;44(12):4395–4399. doi: 10.1021/es9034304. [DOI] [PubMed] [Google Scholar]
- 12.Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989–2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112(1):18–21. doi: 10.1289/ehp.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao SS, Bolger PM. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991–December 1996. Food Addit Contam. 1999;16(11):465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- 14.Thomas KW, Sheldon LS, Pellizzari ED, Handy RW, Roberds JM, Berry MR. Testing duplicate diet sample collection methods for measuring personal dietary exposures to chemical contaminants. J Expo Anal Environ Epidemiol. 1997;7(1):17–36. [PubMed] [Google Scholar]
- 15.Moschandreas DJ, Karuchit S, Berry MR, O’Rourke MK, Lo D, Lebowitz MD, et al. Exposure apportionment: ranking food items by their contribution to dietary exposure. J Expo Anal Environ Epidemiol. 2002;12(4):233–243. doi: 10.1038/sj.jea.7500230. [DOI] [PubMed] [Google Scholar]
- 16.Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic Modeling of Dietary Arsenic Exposure and Dose and Evaluation with 2003–2004 NHANES Data. Environ Health Perspect. 2010;118(3):345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera-Nunez Z, Meliker JR, Linder AM, Nriagu JO. Reliability of spot urine samples in assessing arsenic exposure. Int J Hyg Environ Health. 2010;213(4):259–264. doi: 10.1016/j.ijheh.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellizzari ED, Clayton CA. Assessing the measurement precision of various arsenic forms and arsenic exposure in the National Human Exposure Assessment Survey (NHEXAS) Environ Health Perspect. 2006;114(2):220–227. doi: 10.1289/ehp.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal BK, Ogra Y, Anzai K, Suzuki KT. Speciation of arsenic in biological samples. Toxicol Appl Pharmacol. 2004;198(3):307–318. doi: 10.1016/j.taap.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999;107(8):663–667. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soleo L, Lovreglio P, Iavicoli S, Antelmi A, Drago I, Basso A, et al. Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere. 2008;73(3):291–299. doi: 10.1016/j.chemosphere.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Cleland B, Tsuchiya A, Kalman DA, Dills R, Burbacher TM, White JW, et al. Arsenic exposure within the Korean community (United States) based on dietary behavior and arsenic levels in hair, urine, air, and water. Environ Health Perspect. 2009;117(4):632–638. doi: 10.1289/ehp.11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascio C, Raab A, Jenkins RO, Feldmann J, Meharg AA, Haris PI. The impact of a rice based diet on urinary arsenic. J Environ Monit. 2011;13(2):257–265. doi: 10.1039/c0em00482k. [DOI] [PubMed] [Google Scholar]
- 24.Lovreglio P, D’Errico MN, Gilberti ME, Drago I, Basso A, Apostoli P, et al. The influence of diet on intra and inter-individual variability of urinary excretion of arsenic species in Italian healthy individuals. Chemosphere. 2012;86(9):898–905. doi: 10.1016/j.chemosphere.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 25.Lebowitz MD, O’Rourke MK, Gordon S, Moschandreas DJ, Buckley T, Nishioka M. Population-based exposure measurements in Arizona: a phase I field study in support of the National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1995;5(3):297–325. [PubMed] [Google Scholar]
- 26.O’Rourke MK, Van de Water PK, Jin S, Rogan SP, Weiss AD, Gordon SM, et al. Evaluations of primary metals from NHEXAS Arizona: distributions and preliminary exposures. National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1999a;9(5):435–445. doi: 10.1038/sj.jea.7500049. [DOI] [PubMed] [Google Scholar]
- 27.Robertson GL, Lebowitz MD, O’Rourke MK, Gordon S, Moschandreas D. The National Human Exposure Assessment Survey (NHEXAS) study in Arizona--introduction and preliminary results. J Expo Anal Environ Epidemiol. 1999;9(5):427–434. doi: 10.1038/sj.jea.7500053. [DOI] [PubMed] [Google Scholar]
- 28.FDA; U.S. Food and Drug Administration H. a. H. S, editor Total Diet Study Statistics on Element Results Vol. Revision 4.1, 1991–3 through 2005–4. 2007. [Google Scholar]
- 29.Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, et al. A market basket survey of inorganic arsenic in food. Food Chem Toxicol. 1999;37(8):839–846. doi: 10.1016/s0278-6915(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.O’Rourke MK, Rogan SP, Jin S, Robertson GL. Spatial distributions of arsenic exposure and mining communities from NHEXAS Arizona. National Human Exposure Assessment Survey. J Expo Anal Environ Epidemiol. 1999b;9(5):446–455. doi: 10.1038/sj.jea.7500050. [DOI] [PubMed] [Google Scholar]
- 31.NDSR. Nutrition Data System for Research Software Version 2009. Minneapolis, MN: Nutrition Coordinating Center (NCC), University of Minnesota; 2009. Retrieved from http://www.ncc.umn.edu/products/database.html. [Google Scholar]
- 32.Egan SK, Tao SS, Pennington JA, Bolger PM. US Food and Drug Administration’s Total Diet Study: intake of nutritional and toxic elements, 1991–96. Food Addit Contam. 2002;19(2):103–125. doi: 10.1080/02652030110071354. [DOI] [PubMed] [Google Scholar]
- 33.Kurzius-Spencer M. PhD. University of Arizona; Tucson, AZ: 2012. Modeling the effects of dietary arsenic and nutrient intake on urinary arsenic biomarkers. [Google Scholar]
- 34.Ryan PB, Scanlon KA, MacIntosh DL. Analysis of dietary intake of selected metals in the NHEXAS-Maryland investigation. Environ Health Perspect. 2001;109(2):121–128. doi: 10.1289/ehp.01109121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kile ML, Houseman EA, Breton CV, Quamruzzaman Q, Rahman M, Mahiuddin G, et al. Association between total ingested arsenic and toenail arsenic concentrations. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(12):1827–1834. doi: 10.1080/10934520701566819. [DOI] [PubMed] [Google Scholar]
- 36.Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M, et al. Dietary arsenic exposure in Bangladesh. Environ Health Perspect. 2007;115(6):889–893. doi: 10.1289/ehp.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberge J, O’Rourke MK, Meza-Montenegro MM, Gutierrez-Millan LE, Burgess JL, Harris RB. Binational Arsenic Exposure Survey: Methodology and Exploration of the Relationship between Estimated Arsenic Intake from Drinking Water and Urinary Arsenic Concentrations. Int J Environ Res Public Health. 2012;9:1051–1067. doi: 10.3390/ijerph9041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ATSDR. Agency for Toxic Substances and Disease Registry Toxicological Profile for Arsenic (Update) 2007. [PubMed] [Google Scholar]
- 39.Lorenzana R, Yeow A, Colman J, Chappell L, Choudhury H. Arsenic in Seafood: Speciation Issues for Human Health Risk Assessment. Human and Ecological Risk Assessment: An International Journal. 2009;15(1):185–200. [Google Scholar]
- 40.Hopenhayn-Rich C, Biggs ML, Kalman DA, Moore LE, Smith AH. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ Health Perspect. 1996;104(11):1200–1207. doi: 10.1289/ehp.961041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ Res. 2004;96(2):119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Josyula AB, McClellen H, Hysong TA, Kurzius-Spencer M, Poplin GS, Sturup S, et al. Reduction in urinary arsenic with bottled-water intervention. J Health Popul Nutr. 2006;24(3):298–304. [PMC free article] [PubMed] [Google Scholar]
- 43.Macdiarmid J, Blundell J. Assessing dietary intake: Who, what and why of under-reporting. Nutr Res Rev. 1998;11(2):231–253. doi: 10.1079/NRR19980017. [DOI] [PubMed] [Google Scholar]
- 44.Johansson L, Solvoll K, Bjorneboe GE, Drevon CA. Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr. 1998;68(2):266–274. doi: 10.1093/ajcn/68.2.266. [DOI] [PubMed] [Google Scholar]
- 45.McCrory MA, Hajduk CL, Roberts SB. Procedures for screening out inaccurate reports of dietary energy intake. Public Health Nutr. 2002;5(6A):873–882. doi: 10.1079/PHN2002387. [DOI] [PubMed] [Google Scholar]
- 46.Huang TT, Roberts SB, Howarth NC, McCrory MA. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13(7):1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- 47.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(Suppl 2):S73–85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 48.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 49.James WPT, Schofield C Food and Agriculture Organization of the United Nations. Human energy requirements : a manual for planners and nutritionists. Oxford; New York: Published by arrangement with the Food and Agriculture Organization of the United Nations by Oxford University Press; 1990. [Google Scholar]
- 50.Yost LJ, Schoof RA, Aucoin R. Intake of inorganic arsenic in the North American diet. Human and Ecological Risk Assessment. 1998;4(1):137–152. [Google Scholar]
- 51.Roberge J, Abalos A, Skinner J, Kopplin M, Harris RB. Presence of arsenic in commercial beverages. American Journal of Environmental Sciences. 2009;5(6):688–694. [Google Scholar]