Abstract
Background
Phosphatidylinositol-3-kinase delta (PI3Kδ) mediates B-cell receptor signaling and microenvironmental support signals that promote the growth and survival of malignant B lymphocytes. In a phase 1 study, idelalisib, an orally active selective PI3Kδ inhibitor, showed antitumor activity in patients with previously treated indolent non-Hodgkin's lymphomas.
Methods
In this single-group, open-label, phase 2 study, 125 patients with indolent non-Hodgkin's lymphomas who had not had a response to rituximab and an alkylating agent or had had a relapse within 6 months after receipt of those therapies were administered idelalisib, 150 mg twice daily, until the disease progressed or the patient withdrew from the study. The primary end point was the overall rate of response; secondary end points included the duration of response, progression-free survival, and safety.
Results
The median age of the patients was 64 years (range, 33 to 87); patients had received a median of four prior therapies (range, 2 to 12). Subtypes of indolent non-Hodgkin's lymphoma included follicular lymphoma (72 patients), small lymphocytic lymphoma (28), marginal-zone lymphoma (15), and lymphoplasmacytic lymphoma with or without Waldenström's macroglobulinemia (10). The response rate was 57% (71 of 125 patients), with 6% meeting the criteria for a complete response. The median time to a response was 1.9 months, the median duration of response was 12.5 months, and the median progression-free survival was 11 months. Similar response rates were observed across all subtypes of indolent non-Hodgkin's lymphoma, though the numbers were small for some categories. The most common adverse events of grade 3 or higher were neutropenia (in 27% of the patients), elevations in aminotransferase levels (in 13%), diarrhea (in 13%), and pneumonia (in 7%).
Conclusions
In this single-group study, idelalisib showed antitumor activity with an acceptable safety profile in patients with indolent non-Hodgkin's lymphoma who had received extensive prior treatment. (Funded by Gilead Sciences and others; ClinicalTrials.gov number, NCT01282424.)
Indolent non-hodgkin's lymphomas constitute approximately one third of all cases of non-Hodgkin's lymphoma and include follicular lymphoma, small lymphocytic lymphoma, marginal-zone lymphoma, and lymphoplasmacytic lymphoma with or without Waldenström's macroglobulinemia.1-3 It was estimated that approximately 20,000 people in the United States were diagnosed with indolent non-Hodgkin's lymphoma in 2012 and that approximately 7000 died of this disease.4,5
The mainstay of treatment for indolent non-Hodgkin's lymphoma is an anti-CD20 antibody (primarily rituximab) in combination with chemo-therapy consisting of alkylating agents, anthracyclines, antimitotic agents, or purine analogues. Although the current treatments for indolent non-Hodgkin's lymphomas are initially effective in inducing responses in most patients, they are not curative and show decreasing efficacy with repeated administrations. In addition, chemotherapy-based regimens are associated with long-term toxic effects, including cumulative myelosuppression, neuropathy, cardiac toxicity, and secondary cancers.6-9
The most recent chemotherapeutic agent that has been approved by the Food and Drug Administration for use in patients with rituximab- refractory indolent non-Hodgkin's lymphoma is the alkylating agent bendamustine,10 which has become an important therapeutic option, although it is not curative. Radioimmunotherapies,11 such as iodine-131 (131I)–labeled tositumomab12 and yttrium-90 (90Y)–labeled ibritumomab,13 can be active, but owing to the potential for hemato-logic toxic effects, their use has been limited to patients with adequate marrow function and limited marrow involvement by tumor. The use of these agents is further constrained by the complex procedures for their administration. For these reasons, 90Y-ibritumomab is used infrequently, and 131I-tositumomab has been withdrawn from the market.14 There is an unmet need for new treatments with novel mechanisms of action to offer therapeutic options for patients with rituximab- and chemotherapy-refractory disease.
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase that has a catalytic subunit with four different isoforms: α, β, γ, and δ. Activation of PI3K generates phospholipid second messengers at the cell membrane that recruit and activate multiple intracellular enzymes that are regulators of cell proliferation, survival, and motility.15,16 The α and β isoforms are widely expressed in many tissues, whereas the γ and δ isoforms are highly restricted to hematopoietic cells. In B lymphocytes, the δ isoform (PI3Kδ) plays a central role in normal B-cell development and function, transducing signals from the B-cell receptor as well as from receptors for various cytokines, chemokines, and integrins.17,18 PI3Kδ signaling pathways are frequently hyperactive in B-cell cancers,19-21 making inhibition of PI3Kδ a promising target for the therapy of indolent non-Hodgkin's lymphoma.
Idelalisib is a potent, small-molecule inhibitor of PI3Kδ that is highly selective for the δ isoform, as compared with the α, β, and γ isoforms.19 In lymphoid cell lines and primary samples from patients, idelalisib blocked PI3Kδ-AKT signaling and promoted apoptosis.19-21 Phase 1 studies involving patients with hematologic cancers showed that idelalisib had an acceptable safety profile and promising antitumor activity in patients with indolent non-Hodgkin's lymphoma22 and chronic lymphocytic leukemia23 and established an idelalisib dose.
On the basis of these data, we hypothesized that continuous treatment with idelalisib could yield clinical benefit in patients with relapsed indolent non-Hodgkin's lymphoma. Our objectives in this multicenter, phase 2, interventional, single-group study were to characterize the clinical activity and safety of the drug in a group of patients with indolent non-Hodgkin's lymphomas who had received previous treatment with rituximab and an alkylating agent.
Methods
Patients
Eligible patients had a confirmed diagnosis of B-cell indolent non-Hodgkin's lymphoma without evidence of histologic transformation, according to the criteria in the World Health Organization 2008 classification. Histologic types included follicular lymphoma grade 1, 2, or 3a; small lymphocytic lymphoma; splenic, nodal, or extranodal marginal-zone lymphoma; or lymphoplasmacytic lymphoma with or without Waldenström's macroglobulinemia.3 In addition, eligible patients had radiographically measurable disease (defined as the presence of ≥1 lymph nodes with perpendicular dimensions measuring ≥2.0 × ≥1.0 cm) and had received at least two prior systemic therapies for indolent non-Hodgkin's lymphoma. Eligible patients met the criteria for refractoriness to both rituximab and an alkylating agent, whether administered together or in successive treatment regimens. Refractoriness was defined per protocol as less than a partial response or progression of disease within 6 months after completion of a prior therapy. Other major eligibility criteria included an age of at least 18 years, a Karnofsky performance score24 of 60 or higher (on a scale from 0 to 100, with 0 indicating death, 100 indicating the complete absence of symptoms, and lower numbers indicating greater tumor-related disability), the absence of active central nervous system lymphoma, no prior history of hepatic dysfunction, no active systemic infections (human immunodeficiency virus, hepatitis B virus, or hepatitis C virus), and an absolute neutrophil count of 1.0×109 per liter or higher and a platelet count of 50×109 per liter or higher.
Study Design
We conducted Study 101-09 (also known as DELTA), a single-group, open-label, phase 2 study, at 41 sites in the United States and Europe. Patients with previously treated indolent non-Hodgkin's lymphoma that was refractory (as defined above) to both rituximab and chemotherapy that included an alkylating agent received idelalisib, administered orally at a dose of 150 mg twice daily, until the disease progressed, unacceptable toxic effects developed, or the patient died. All patients provided written informed consent.
Study Oversight
The main sponsor of the study was Gilead Sciences. All the authors and the sponsors were responsible for designing the study protocol, amendments, and analysis plan. The authors, collaborators and their research teams collected all the data, and the sponsors confirmed the accuracy of the data and compiled the data for summation and analysis. The first draft of the manuscript was written by the first (academic) author and by an author who is employed by Gilead Sciences. All the authors had full access to the data and analyses for compilation of this report, reviewed and edited the manuscript, vouch for the completeness and accuracy of the data and analysis and for adherence of this report to the study protocol, and made the decision to submit the manuscript for publication. An institutional review board or independent ethics committee at each participating site approved the protocol (available with the full text of this article at NEJM.org). The study was conducted according to principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Assessments
The primary end point was the overall rate of response, with complete response and partial response assessed with the use of standard criteria for lymphoma25 and for Waldenström's macro-globulinemia.26 Responses were assessed by an independent review committee. Patients with inadequate data for an assessment of response were considered in the analysis of response rate as not having had a response to treatment. Secondary efficacy end points included the time to a response, the duration of a response (measured from the onset of response to disease progression), progression-free survival, and overall survival.
Adverse events and laboratory abnormalities that occurred during treatment were defined as those that began or worsened in the period from administration of the first dose of the study drug to 30 days after administration of the last dose. Events and abnormalities were graded according to the Common Terminology Criteria for Adverse Events, version 4.03.27
Patients were clinically evaluated at 2-week intervals during the first 12 weeks of treatment, at 4-week intervals from week 12 to week 24 of treatment, at 6-week intervals from week 24 to week 48 of treatment, and at 12-week intervals thereafter. All visits included evaluations of vital signs, adverse events, and concomitant medications. Tumor response and progression were evaluated by means of computed tomography, laboratory testing, and physical examination at screening and at weeks 8, 16, 24, 36, and 48 and every 12 weeks thereafter. Responses and disease progression were rigorously evaluated for each patient by an independent review committee that consisted of three board-certified radiologists (two readers and one adjudicator) and one board-certified oncologist–hematologist.
Statistical Analysis
The study used Simon's two-stage design28 and with a sample of at least 100 patients had a power of more than 90% to test the hypothesis that the response rate would be 39% or higher against the null hypothesis that it would be 20% or lower, at a one-sided significance level of 0.005. Response rates, exact binomial 95% confidence intervals, and P values (based on the exact binomial test) were calculated. Secondary end points of the duration of response and progression-free survival were summarized with the use of the Kaplan– Meier method. The date of progression was the date on which progression was first identified objectively. Death occurring within 30 days after discontinuation of the study drug was considered to be an event in the calculations of response and progression-free survival. Data from patients with nonprogressing disease were censored on the date of the last tumor assessment.
Results
Patient Characteristics
Between April 2011 and October 2012, we enrolled 125 patients with relapsed indolent non-Hodgkin's lymphoma. The date of data cutoff was June 25, 2013. Table 1 summarizes the baseline characteristics of the patients. The median age of the patients was 64 years; 64% were male, and 89% were white. Most of the patients (89%) had stage III or IV indolent non-Hodgkin's lymphoma, 30% had elevated lactate dehydrogenase levels, 26% had lesions that were 7 cm or more in at least one dimension, 15% had a hemoglobin level of less than 10 g per deciliter, 14% had a neutrophil count of less than 1500 per cubic millimeter, and 8% had a platelet count of less than 75,000 per cubic millimeter. Of the patients with follicular lymphoma, 79% had scores on the follicular lymphoma International Prognostic Index that indicated intermediate risk or high risk, and 17% had grade 3a disease (follicular large-cleaved-cell lymphoma).
Table 1.
Baseline Characteristics of the Patients, Prior Therapy, and Treatment Disposition.*
| Characteristic | Patients (N = 125) |
|---|---|
| Age — yr | |
| Median | 64 |
| Range | 33–87 |
| Male sex — no. (%) | 80 (64) |
| Subtype of indolent non-Hodgkin's lymphoma — no. (%) | |
| Follicular lymphoma | 72 (58) |
| Small lymphocytic lymphoma | 28 (22) |
| Marginal-zone lymphoma | 15 (12) |
| Lymphoplasmacytic lymphoma with or without Waldenström's macroglobulinemia | 10 (8) |
| Disease status — no. (%) | |
| Stage III or IV | 111 (89) |
| Elevated LDH | 38 (30) |
| Bulky disease† | 33 (26) |
| Baseline cytopenia — no. (%)‡ | |
| Neutropenia | 17 (14) |
| Anemia | 19 (15) |
| Thrombocytopenia | 10 (8) |
| No. of prior regimens | |
| Median | 4 |
| Range | 2–12 |
| Prior therapy — no. (%) | |
| Rituximab | 125 (100) |
| Alkylating agent | 125 (100) |
| Combination of rituximab and alkylating agent | 114 (91) |
| Bendamustine | 81 (65) |
| Anthracycline | 79 (63) |
| Purine analogue | 42 (34) |
| Stem-cell transplantation | 14 (11) |
| Prior therapy to which the disease was refractory — no./total no. (%) | |
| Rituximab | 125/125 (100) |
| Alkylating agent | 124/125 (99)§ |
| Combined alkylator and rituximab | 108/114 (95) |
| Bendamustine–rituximab | 47/60 (78) |
| R-CHOP | 40/56 (71) |
| R-CVP | 29/36 (81) |
| Bendamustine | 61/81 (75) |
| Disease refractory to ≥2 regimens | 99/125 (79) |
| Disease refractory to most recent regimen | 112/125 (90) |
| Duration of idelalisib therapy — mo | |
| Median | 6.6 |
| Range | 0.6–23.9 |
| Treatment disposition — no. (%) | |
| Ongoing | 40 (32) |
| Discontinued | 85 (68) |
| Progressive disease | 41 (33) |
| Adverse event | 25 (20) |
| Death | 8 (6) |
| Investigator request | 7 (6) |
| Withdrew consent | 4 (3) |
LDH denotes lactate dehydrogenase, R-CHOP rituximab–cyclophosphamide–doxorubicin–prednisone, and R-CVP rituximab–cyclophosphamide–prednisone.
Bulky disease was defined as the presence of one or more nodes with at least one dimension of 7 cm or more.
Neutropenia was defined as an absolute neutrophil count of less than 1500 per cubic millimeter, anemia as a hemoglobin level of less than 10 g per deciliter, and thrombocytopenia as a platelet count of less than 75,000 per cubic millimeter.
Refractoriness to two cycles was required to meet the criteria for alkylator-refractory disease. One patient received only one cycle of chemotherapy, with no response after that cycle.
Patients had received a median of 4 prior regimens (range, 2 to 12), with 73 patients (58%) having received 4 or more prior regimens. Among alkylating agents, cyclophosphamide had been administered to 111 patients (89%) and bendamustine to 81 patients (65%). The most common prior regimens included bendamustine–rituximab (60 patients [48%]), rituximab–cyclophosphamide–doxorubicin–prednisone (R-CHOP) (56 patients [45%]), rituximab monotherapy (50 patients [40%]), and rituximab–cyclophosphamide–prednisone (R-CVP) (36 patients [29%]). Fourteen patients (11%) had received prior high-dose chemo-therapy and had undergone autologous stem-cell transplantation.
All the patients had disease that was refractory (as defined above) to rituximab, and 124 patients (99%) had disease that was refractory to an alkylating agent; in 108 patients (86%), the disease was refractory to combination therapy with an alkylating agent and rituximab. A total of 99 patients (79%) had disease that was refractory to two or more prior regimens. Among patients who had received prior regimens consisting of bendamustine plus rituximab, R-CHOP, or R-CVP, 78%, 71%, and 81%, respectively, had disease that was refractory to those therapies. A total of 112 patients (90%) had disease that was refractory to the last therapy they had received immediately before idelalisib.
Efficacy
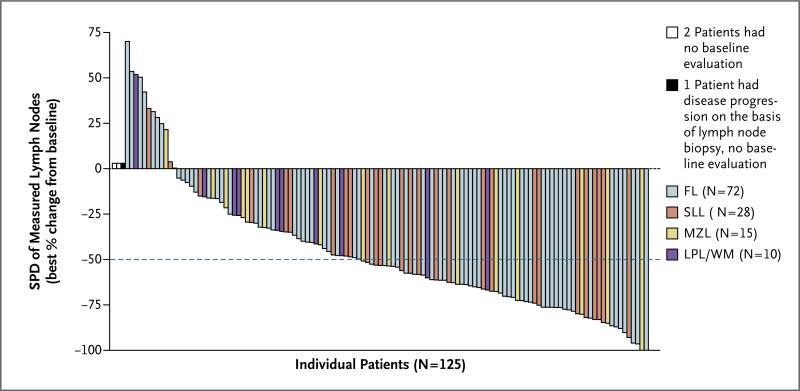
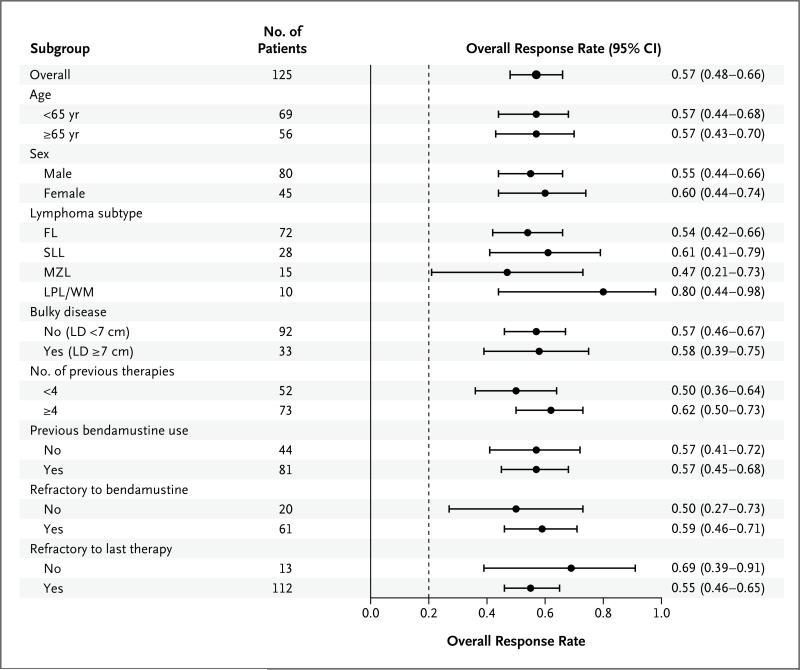
A waterfall plot of the best overall response with respect to tumor size shows that 110 of 122 patients who could be evaluated (90%) had a reduction in the size of lymph nodes during treatment (Fig. 1). On the basis of assessment by the independent review committee, the response rate was 57% (95% confidence interval, 48 to 66), with 71 responses in 125 patients. A total of 7 patients (6%) had a complete response, 63 patients (50%) had a partial response, and 1 patient (1%) with Waldenström's macroglobulinemia had a minor response. There was a high degree of concordance between the assessments of response by the independent review committee and the assessments by the investigators, with 85% agreement with respect to overall response (Table S1 in the Supplementary Appendix, available at NEJM.org). The rates of response were consistent across subgroups, with favorable response rates observed regardless of the number of prior regimens, refractoriness of the disease to the most recent prior therapy, refractoriness to bendamustine, disease subtype, bulky disease status, age, and sex (Fig. 2). The response rates ranged from 47 to 80% in various subgroups. There was no correlation of efficacy with pharmacokinetic measures (Fig. S2 in the Supplementary Appendix).
Figure 1. Best Overall Response.
The best response with respect to tumor size during idelalisib treatment, according to assessment by an independent review committee, is shown for the 125 patients in the study. Among the 122 patients with measurable lesions both at baseline and after baseline, 110 patients (90%) had improvements in lymphadenopathy, as assessed by changes in the sums of the products of the perpendicular dimensions (SPD) of index lesions. The dashed line shows the percentage change that represents the criterion for lymphadenopathy response, according to Cheson et al.25 FL denotes follicular lymphoma, LPL/WM lymphoplasmacytic lymphoma with or without Waldenström's macro-globulinemia, MZL marginal-zone lymphoma, and SLL small lymphocytic lymphoma.
Figure 2. Forest Plot of Overall Response Rate.
A forest plot is shown of the overall response rate, in the total cohort and according to subgroups, among patients with refractory indolent non-Hodgkin's lymphoma. The response rate was assessed by an independent review committee. The dashed line shows the null hypothesis response rate of 20%. LD denotes longest diameter.
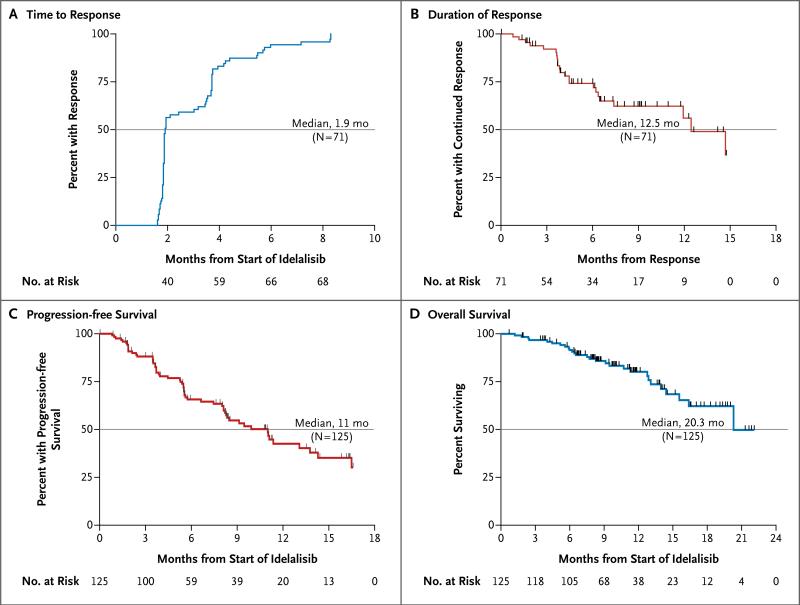
Responses were rapid and durable with continued administration of idelalisib. The median time to a response was 1.9 months (range, 1.6 to 8.3) (Fig. 3A). The median duration of response was 12.5 months (range, 0.03 to 14.8) (Fig. 3B), exceeding the median duration of response (5.9 months) in the group of 28 patients who had had a response to the most recent therapy before idelalisib. The median progression-free survival was 11.0 months (range, 0.03 to 16.6) (Fig. 3C), with 47% of the patients remaining progression-free at 48 weeks. At the time of data cutoff, the median overall survival was 20.3 months (range, 0.7 to 22.0) (Fig. 3D), and overall survival at 1 year was estimated to be 80%. The median follow-up time was 9.7 months.
Figure 3. Kaplan–Meier Curves for Secondary End Points.
Kaplan–Meier curves are shown for the secondary end points of the time to response (Panel A), the duration of response (Panel B), progression-free survival (Panel C), and overall survival (Panel D) among patients with refractory indolent non-Hodgkin's lymphoma who were treated with idelalisib (intention-to-treat population). The end points were assessed by an independent review committee.
Safety Profile
The median duration of treatment with idelalisib was 6.6 months (range, 0.6 to 23.9), and the mean (±SD) duration was 8.1±5.7 months. At the time of the data cutoff, 108 patients (86%) had received idelalisib at a dose of 150 mg twice daily for at least 2 months, and 68 patients (54%) had received the drug for at least 6 months; treatment was ongoing for 40 patients (32%). The incidence rates of adverse events occurring during treatment in 10% or more of the patients are listed in Table 2. The following events (all grades) occurred in more than 20% of patients: diarrhea (in 43%), fatigue (in 30%), nausea (in 30%), cough (in 29%), and pyrexia (in 28%). The most frequently reported adverse events of grade 3 or higher were diarrhea (in 13% of the patients), pneumonia (in 7%), and dyspnea (in 3%). The most common laboratory abnormalities of grade 3 or higher that occurred during treatment (Table 2) included neutropenia (in 27% of the patients) and elevations in levels of serum alanine or aspartate aminotransferase (in 13%). Other cytopenias of grade 3 or higher included thrombocytopenia (in 6% of the patients) and anemia (in 2%). Changes in blood counts during therapy are described in Table S1 in the Supplementary Appendix. There were no significant changes in immunoglobulin levels or T-lymphocyte subsets.
Table 2.
Adverse Events during Treatment.*
| Event or Abnormality | Grade | |
|---|---|---|
| Any | ≥3 | |
| no. (%) | ||
| Adverse event | 103 (82) | 68 (54) |
| Diarrhea | 54 (43) | 16 (13) |
| Nausea | 37 (30) | 2 (2) |
| Fatigue | 37 (30) | 2 (2) |
| Cough | 36 (29) | 0 |
| Pyrexia | 35 (28) | 2 (2) |
| Decreased appetite | 22 (18) | 1 (1) |
| Dyspnea | 22 (18) | 4 (3) |
| Abdominal pain | 20 (16) | 3 (2) |
| Vomiting | 19 (15) | 3 (2) |
| Upper respiratory tract infection | 18 (14) | 0 |
| Weight decreased | 17 (14) | 0 |
| Rash | 16 (13) | 2 (2) |
| Asthenia | 14 (11) | 3 (2) |
| Night sweats | 14 (11) | 0 |
| Pneumonia | 14 (11) | 9 (7) |
| Peripheral edema | 13 (10) | 3 (2) |
| Headache | 13 (10) | 1 (1) |
| Hematopoietic laboratory abnormality | ||
| Decreased neutrophils | 70 (56) | 34 (27) |
| Decreased hemoglobin | 35 (28) | 2 (2) |
| Decreased platelets | 32 (26) | 8 (6) |
| Chemical laboratory abnormality | ||
| Increased ALT | 59 (47) | 16 (13) |
| Increased AST | 44 (35) | 10 (8) |
| Increased alkaline phosphatase | 28 (22) | 0 |
| Increased bilirubin | 13 (10) | 0 |
Included are adverse events and selected laboratory abnormalities that occurred during treatment in 10% or more of the 125 patients in the study, regardless of whether the event was related to the study drug. Adverse events that occurred during treatment are classified according to the preferred term in the Medical Dictionary for Regulatory Activities (MedDRA), version 15.1. Patients who had multiple events within the same preferred-term category were counted once in that category. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
Adverse events led to discontinuation of idelalisib in 25 patients. These adverse events included elevations in levels of serum alanine or aspartate aminotransferase in 5 patients (4%), colitis in 4 patients (3%), pneumonia and pneumonitis in 3 patients each (2%), and diarrhea and neutropenia in 2 patients each (2%). The initial dose of 150 mg twice daily was reduced to 100 mg twice daily or to 75 mg twice daily in 42 patients (34%). The adverse events that led most frequently to dose reduction were elevations in levels of serum alanine or aspartate aminotransferase, followed by diarrhea and neutropenia. The most common serious adverse events included pyrexia in 13 patients (10%), pneumonia and diarrhea in 9 patients each (7%), colitis in 5 patients (4%), dehydration and febrile neutropenia in 4 patients each (3%), and acute renal failure and pneumonitis in 3 patients each (2%).
Grade 3 or higher diarrhea, colitis, or both developed in 20 patients (16%), at a median of 6 months after the initiation of treatment (range, 1 to 13); of these, 6 cases resolved spontaneously or after dose reduction, 6 cases (2 in patients with progressive disease) led to permanent discontinuation of idelalisib, and 8 cases resolved with a temporary interruption of the drug. Of the 8 patients in whom the drug was temporarily interrupted, 5 were able to resume therapy without a recurrence of toxic effects.
In all patients with grade 1 or 2 elevations in levels of serum alanine or aspartate aminotransferase, the levels returned to values within normal ranges despite the fact that the patients continued taking the drug. Grade 3 or higher elevations of serum aminotransferase levels developed in 16 patients (13%), at a median of 6.3 weeks after the initiation of treatment (range, 4 to 11). These cases were asymptomatic and after interruption of idelalisib therapy, all resolved to grade 1 or less within a median of 3.9 weeks (range, 1 to 6). Fourteen patients were rechallenged, 10 of whom (71%) were successfully able to continue therapy with a temporary dose reduction and subsequent reescalation.
A total of 28 deaths (22%) were reported. Eleven deaths occurred while the patient was receiving the study drug or within 30 days after the last dose. The causes of death were progressive disease (3 patients), pneumonia (3 patients), and cardiac arrest, cardiac failure, splenic infarction, septic shock, and pneumonitis (1 patient each). The remaining 17 deaths, due predominantly to progressive disease, occurred during long-term follow-up. No evidence of cumulative toxic effects was documented.
Discussion
In this noncomparative trial, idelalisib showed antitumor activity in patients with indolent non-Hodgkin's lymphoma that had become refractory to both rituximab and alkylating agents. Idelalisib monotherapy resulted in tumor reductions in 90% of the patients, with 57% meeting the criteria for an objective tumor response. Disease control could be protracted: the median duration of response was 12.5 months, and the median progression-free survival was 11.0 months. Objective responses were observed in patients with follicular lymphoma and small lymphocytic lymphoma, as well as in patients with the rarer subsets of indolent non-Hodgkin's lymphoma — marginal-zone lymphoma and lymphoplasmacytic lymphoma with or without Waldenström's macroglobulinemia. Thus, therapeutic targeting of the PI3Kδ pathway may be clinically relevant in all these B-cell cancers.
The value of this regimen included a favorable toxicity profile, with low rates of discontinuation due to toxic effects and a low incidence of severe adverse events. Though serum elevations in hepatic aminotransferase levels were observed in 47% of patients, elevations of grade 3 or higher occurred in 13% of patients, were reversible in all patients, and resulted in discontinuation of treatment in only 5 patients (4%). Grade 3 or higher diarrhea or colitis occurred in 13% and 4% of the patients, respectively, and could often be managed with interruptions in drug treatment or adjustments of the dose.
Clinically significant hematologic toxic effects were also uncommon. Since we designed the trial for a patient population that had received extensive prior treatment, we specifically allowed the use of red-cell and platelet transfusions as well as neutrophil growth factors to meet the protocol-required baseline eligibility thresholds for patients with impaired marrow reserve or extensive tumor infiltration. In this context, the 27% rate of neutropenia of grade 3 or higher was not considered to be excessive, and febrile neutropenia was a rare event, occurring in 4 patients (3%).
The results with idelalisib are similar to those observed in evaluations of other cytotoxic and noncytotoxic agents in previously treated patients. 131I-tositumomab and 90Y-ibritumomab, when studied in rituximab-refractory follicular lymphoma, yielded response rates of 62 to 74% and progression-free survival of 6.8 to 8.8 months.29,30 The alkylating agent bendamustine was approved for the treatment of indolent non-Hodgkin's lymphoma that is refractory to rituximab, on the basis of a response rate of 75% and a median duration of response of 9.2 months in a cohort of patients who had received a median of two prior regimens.31 A 30-patient subgroup from the bendamustine trial,31 comprising patients with alkylator-refractory disease, had a response rate of 64% and median progression-free survival of 7.5 months. The majority of patients treated with idelalisib in the current trial had received prior therapy with bendamustine. Other noncytotoxic agents, such as bortezomib,32 fostamatinib,33 ibrutinib,34 and lenalidomide,35 have been associated with response rates of 12 to 52% in populations that received less extensive prior therapy than did those taking idelalisib in this study.
In conclusion, in this uncontrolled trial, idela lisib targets the PI3Kδ pathway and appears to provide effective oral monotherapy in patients with previously treated indolent non-Hodgkin's lymphoma. The toxicity profile does not generally overlap with that of most other active agents and may allow the development of more highly active combination regimens.
Supplementary Material
Acknowledgments
Sponsored by Gilead Sciences and Calistoga Pharmaceuticals (which was acquired by Gilead Sciences in 2011), by a Fred Hutchinson Cancer Research Center–University of Washington Cancer Consortium Cancer Center Support Grant of the National Institutes of Health (P30 CA015704), and by philanthropic gifts from Frank and Betty Vandermeer. Dr. Gopal is a Scholar in Clinical Research for the Leukemia and Lymphoma Society.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients for their dedication to this clinical trial and the clinical personnel at each of the study sites for their diligence in caring for patients and collecting study data, as well as the study team members at INC Research, Raleigh, NC, for overseeing the administration of the trial.
References
- 1.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–57. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin's lymphomas. Semin Oncol. 1993;20(Suppl 5):75–88. [PubMed] [Google Scholar]
- 3.Swerdlow S, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. IARC Press; Lyon, France: 2008. [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute . Estimated new cancer causes and deaths for 2013. National Institutes of Health; Bethesda, MD: 2013. ( http://seer.cancer.gov/csr/1975_2010/results_single/sect_01_table.01.pdf) [Google Scholar]
- 6.Lunning MA, Vose JM. Management of indolent lymphoma: where are we now and where are we going. Blood Rev. 2012;26:279–88. doi: 10.1016/j.blre.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gribben JG. How I treat indolent lymphoma. Blood. 2007;109:4617–26. doi: 10.1182/blood-2006-10-041863. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Net work. Treatment guidelines for lymphoma. 2013 ( http://www.nccn.org/professionals/physician_gls/f_guidelines.asp)
- 9.Leonard JP, Gregory SA, Maloney DG, Vose JM, Younes A, Zelenetz AD. Optimizing the treatment of patients with rituximab-pretreated recurrent indolent non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2008;6:437–45. [PubMed] [Google Scholar]
- 10.Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. J Clin Oncol. 2009;27:1492–501. doi: 10.1200/JCO.2008.18.7252. [Erratum, J Clin Oncol 2009;27:2892.] [DOI] [PubMed] [Google Scholar]
- 11.Tomblyn M. Radioimmunotherapy for B-cell non-Hodgkin lymphomas. Cancer Control. 2012;19:196–203. doi: 10.1177/107327481201900304. [DOI] [PubMed] [Google Scholar]
- 12.William BM, Bierman PJ. I-131 tositumomab. Expert Opin Biol Ther. 2010;10:1271–8. doi: 10.1517/14712598.2010.504707. [DOI] [PubMed] [Google Scholar]
- 13.Johnston PB, Bondly C, Micallef IN. Ibritumomab tiuxetan for non-Hodgkin's lymphoma. Expert Rev Anticancer Ther. 2006;6:861–9. doi: 10.1586/14737140.6.6.861. [DOI] [PubMed] [Google Scholar]
- 14.GSK press release of Glaxo-SmithKline. Vol. 7. Brentford, Middlesex, United Kingdom: Aug, GSK to discontinue manufacture and sale of the BEXXAR therapeutic regimen (tositumomab and iodine I 131 tositumomab). p. 2013. [Google Scholar]
- 15.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 17.Durand CA, Hartvigsen K, Fogel-strand L, et al. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673–84. doi: 10.4049/jimmunol.0900432. [DOI] [PubMed] [Google Scholar]
- 18.Bilancio A, Okkenhaug K, Camps M, et al. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–50. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 19.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman SE, Lapalombella R, Gordon AL, et al. The role of phosphatidylinositol 3-kinase-δ in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117:4323–7. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson DM, Kahl BS, Furman RR, et al. Final results of a phase I study of idelalisib, a selective inhibitor of PI3Kδ, in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL). J Clin Oncol. 2013;31(Suppl):8526. abstract. [Google Scholar]
- 23.Brown JR, Furman RR, Flinn I, et al. Final results of a phase I study of idela lisib (GSE-1101) a selective inhibitor of PI3Kδ, in patients with relapsed or refractory CLL. J Clin Oncol. 2013;31(Suppl):7003. abstract. [Google Scholar]
- 24.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–6. doi: 10.1111/bjh.12102. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Therapy Evaluation Program, common terminology for adverse events, version 30. National Cancer Institute; Bethesda, MD: 2009. Common Terminology Criteria for Adverse Events v4.0 (CTCAE). ( http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf) [Google Scholar]
- 28.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 29.Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:3262–9. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Horning SJ, Younes A, Jain V, et al. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J Clin Oncol. 2005;23:712–9. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a multicenter study. Cancer. 2010;116:106–14. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Bella N, Taetle R, Kolibaba K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–80. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 33.Friedberg JW, Sharman J, Sweeten-ham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol. 2009;27:5404–9. doi: 10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.