Abstract
Background
In laboratory studies, exercise immediately before sexual stimuli improved sexual arousal of women taking antidepressants [1]. We evaluated if exercise improves sexual desire, orgasm, and global sexual functioning in women experiencing antidepressant-induced sexual side effects.
Methods
Fifty-two women who were reporting antidepressant sexual side effects were followed for 3 weeks of sexual activity only. They were randomized to complete either three weeks of exercise immediately before sexual activity (3×/week) or 3 weeks of exercise separate from sexual activity (3×/week). At the end of the first exercise arm, participants crossed to the other. We measured sexual functioning, sexual satisfaction, depression, and physical health.
Results
Exercise immediately prior to sexual activity significantly improved sexual desire and, for women with sexual dysfunction at baseline, global sexual function. Scheduling regular sexual activity significantly improved orgasm function; exercise did not increase this benefit. Neither regular sexual activity nor exercise significantly changed sexual satisfaction.
Conclusions
Scheduling regular sexual activity and exercise may be an effective tool for the behavioral management of sexual side effects of antidepressants.
Keywords: exercise, sexual function, antidepressants, women, sexual satisfaction, depression
INTRODUCTION
Antidepressants1 are the most common treatment for depression in the United States.[2] Most women taking antidepressants (96%) report sexual side effects.[3] In addition to diminishing patients’ quality of life,[4] sexual side effects significantly impede medication compliance.[5, 6] Despite this, there are few empirically validated interventions for sexual side effects proven better than placebo.[7] Often, adjunctive treatments interfere with the antidepressant function[8] or increase risk of additional side effects.
A promising behavioral intervention for sexual side effects is exercise. Exercise improves sexual function in depressed women not taking medication.[9] Also, moderately intense exercise prior to presentation of sexual stimuli increases sexual arousal in healthy women[10, 11] possibly because sympathetic nervous system activity facilitates female sexual arousal.[12] There is a curvilinear relationship between SNS activity and genital sexual arousal in women, with moderate SNS activation associated with higher sexual arousal than either high or low SNS activity.[13] Antidepressants have been shown to depress SNS activity.[14–18] Female sexual arousal and orgasm side effects of antidepressants may be linked to suppression of SNS activity in genital nerves,[1, 19, 20] particularly for women taking selective serotonin reuptake inhibitors (SSRIs [1]).
In a laboratory study, Lorenz and Meston[1] tested the effects of exercise in 47 women taking antidepressants. Participants ran on a treadmill for 20 min at a moderate to high intensity (70% of VO2max) and then watched a sexually explicit film while their genital arousal was recorded. On average, exercise prior to sexual stimuli increased genital arousal more than a noexercise control, and these effects were strongest in women reporting greater sexual dysfunction. While these findings supported exercise as a possible intervention for sexual side effects, there were a few limitations to the study. Laboratory-based psychophysiological measures of women’s sexual arousal may not map onto sexual function, and the link between objective measures of female genital arousal and women’s subjective ratings is modest.[21] Also, nonacute effects of exercise may influence sexual function, most notably through reduction of depressive symptoms.[22]
The present study attempted to address these limitations. We compared the effects of exercise immediately before sexual activity to the effects of exercise at the same duration and frequency but separate from sexual activity. This allowed us to compare the effects of SNS activation on sexual responding (e.g., increased genital blood flow associated with exercise) versus the general benefits of exercise on sexuality (e.g., improvements in depression).
We hypothesized that exercise would improve sexual functioning in women taking antidepressants. However, we also expected that sexual functioning would improve when exercise was timed before sexual activity above and beyond the effects of exercise in general. Finally, based on findings from laboratory studies, we expected that effects of exercise would be strongest in women with sexual dysfunction at baseline.
MATERIALS AND METHODS
RECRUITMENT
Participants were recruited from the community online advertisements on craigslist.org and the laboratory website. Advertisements provided information about selection criteria and time commitment, and were aimed at the broad population of women taking antidepressants. Interested participants were screened by telephone, and eligible participants were scheduled for an initial lab visit. The study protocol was approved by the University of Texas at Austin Institutional Review Board from 2010–2013 and the trial was registered on ClinicalTrials.gov (trial #NCT01188720). Power analyses based on effects sizes of the laboratory-based pilot study[1] indicated a sample of 35–50 women would be needed to achieve 80–90% power.
Inclusion criteria included: women 18 or older, stabilized on an antidepressant (SSRIs such as citalopram or sertraline, or SNRIs such as venlafaxine or duloxetine), currently sexual active with a partner or masturbation, and experiencing regular menstrual periods. Women were included if they reported a change in their sexual function after they started antidepressant; this included some women (N = 14) who were not distressed about these changes and thus would not be diagnosed with a sexual dysfunction as per the DSM-IV-TR. Exclusion criteria included: taking any psychoactive medication other than an antidepressant, factors that would put participant at risk of physical harm while exercising (e.g., significant cardiovascular illness2), factors associated with potential genital nerve damage (e.g., hysterectomy), severe untreated mental illness, distress related to a history of unwanted sexual contact, and use of medications (other than oral contraceptives) that have been shown to alter sexual function. Women were not included or excluded based on sedentary status; however, 71% of participants were sedentary at baseline (see Table 1).
TABLE 1.
Demographics and baseline characteristics
| Total sample (N = 52)
|
Sexual dysfunction only (N = 38)
|
|||
|---|---|---|---|---|
| N | % | N | % | |
| Race/ethnicity | ||||
| Non-Hispanic White | 38 | 73 | 27 | 71 |
| Hispanic | 10 | 19 | 7 | 18 |
| Other | 4 | 8 | 4 | 11 |
| Relationship type | ||||
| Dating | 11 | 21 | 9 | 24 |
| Committed relationship/married | 38 | 73 | 26 | 68 |
| Other | 3 | 6 | 3 | 8 |
| Highest education completed | ||||
| High school diploma | 3 | 8 | ||
| Some college/undergraduate degree | 41 | 79 | 31 | 82 |
| Advanced degree | 7 | 14 | 4 | 10 |
| M | SD | M | SD | |
| Age (years) | 32.44 | 8.68 | 32.82 | 9.44 |
| Length of relationship (years) | 5.01 | 6.34 | 4.84 | 6.78 |
| Sexual orientationa | 22.46 | 27.48 | 14.61 | 20.45 |
| n | % | n | % | |
| Reason for starting medication | ||||
| Depression | 22 | 42 | 16 | 42 |
| Anxiety | 14 | 27 | 11 | 29 |
| Mixed anxiety/depression | 13 | 25 | 10 | 29 |
| Other | 3 | 6 | 0 | 0 |
| Type of antidepressant | ||||
| SSRI | 22 | 42 | 17 | 45 |
| SNRI | 30 | 58 | 21 | 55 |
| M | SD | M | SD | |
| Body mass index | 27.34 | 6.99 | 25.99 | 6.05 |
| Waist-to-Hip ratio | 0.76 | 0.07 | 0.76 | 0.07 |
| Resting heart rate | 74.25 | 15.02 | 71.14 | 14.83 |
| Average daily METs in last 7 daysb | 253.91 | 26.12 | 253.37 | 25.23 |
| Pretrial frequency of sexual activity (events per week) | 1.37 | 1.31 | 1.43 | 1.71 |
| M | SD | M | SD | |
| Beck Depression Inventory | 12.17 | 8.58 | 12.97 | 8.75 |
| Beck Anxiety Inventory | 5.60 | 7.59 | 5.50 | 7.17 |
Sexual orientation ranged from 0 (completely heterosexual) to 100 (completely homosexual).
Includes both exercise and nonexercise physical activity.
PROCEDURE
Women reporting changes in sexual response attributable to antidepressants were entered into a 9-week trial. Participants completed a pretrial laboratory session in they were instructed in the study protocol and gave informed consent, had their physical health assessed, and practiced using a 30 min strength training and cardio exercise video with resistance bands. They were instructed to maintain 70–85% of their maximum heart rate (HR) by changing resistance during exercise. Participants were also given HR recorders that time stamped exercise sessions; this information was used to determine compliance (see below).
Following the laboratory session, participants completed a 3-week baseline arm in which they engaged in sexual activity 3×/week (defined as “sex with a partner or masturbation”). This was required to allow enough sexual events during the trial to observe effects. Prior to enrolling, women engaged in an average of 1.37 sexual events per week (SD = 1.31 events).
Participants then entered either a 3-week experimental exercise arm, or a 3-week control exercise arm; during both, participants engaged in exercise 3×/week and sexual activity 3×/week. In the experimental exercise arm, participants exercised to the 30-min exercise video and engaged in sexual activity immediately afterwards (defined as “as soon as possible but no more than 30 min after the exercise video ends”). In the control arm, they engaged in the same duration and intensity of exercise but did not engage in sexual activity for at least 6 hr after exercising. After completing the first exercise arm, participants crossed over into the other exercise type. Women who were engaging in regular physical activity were asked to add the three exercise sessions on top of their normal exercise routine.
Participants completed validated assessments of sexual functioning, satisfaction, and psychological health before and after each arm (see Fig. 1). They also recorded each sexual event in online diaries. Time stamps on diaries were used to determine compliance (see below).
Figure 1.
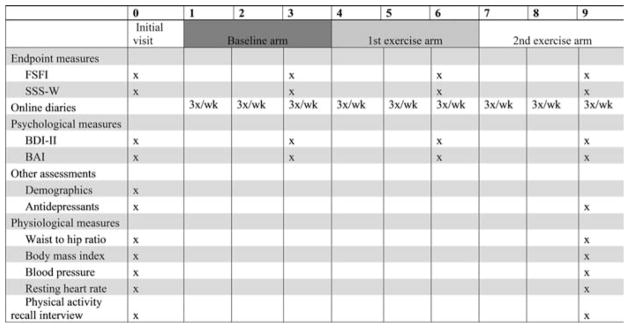
Assessment schedule.
At the completion of the second exercise arm, participants returned to the lab where they had their physical health reassessed, completed an exit survey, and were compensated and debriefed. No participants reported significant adverse events.
RANDOMIZATION AND BLINDING
Participants were randomized to complete either the experimental or control exercise arm first according to a predefined list of randomly generated 0 s and 1 s (constrained to equal numbers in each arm). If the participant dropped out, her randomization number was reassigned to a new participant. Participants were blinded to which arm was intended as active treatment. To test women’s expectancies, we asked women to guess the study hypotheses in the exit interview. Only one participant correctly identified the study hypotheses; most said the study was to examine the effects of exercise on sexuality without reference to the timing of exercise or medication.
MEASURES
Female Sexual Function Index (FSFI)[23]
Our primary endpoint was sexual functioning. The FSFI, an assessment of sexual function widely used in clinical trials, has 19 items subdivided into six domains supported by factor analysis: desire, arousal, lubrication, orgasm, satisfaction, and sexual pain. The FSFI has been shown to differentiate between women with and without sexual dysfunction.[23, 24] A score of 26.55 or below is considered within the clinical range.[25] We considered the total scale as well as the desire and orgasm subscales (as antidepressant sexual side effects are often noted these areas [26]).
Sexual Satisfaction Scale—Women (SSS-W)[27]
A secondary endpoint was sexual satisfaction. The 30 items of the SSS-W assess five domains of sexual satisfaction supported by factor analyses: comfort discussing sexual preferences, compatibility, contentment, personal distress concerning sexual problems, and relationship distress. The SSS-W has good internal consistency and reliably differentiates between participants with and without sexual.[27]
Beck Depression Inventory—II (BDI-II)[28]
This widely used 21-item depression questionnaire has been extensively validated.[29] The BDI-II measures both affective/cognitive symptoms (e.g., feelings of worthlessness) and physical symptoms (e.g., problems with sleep or appetite).
Beck Anxiety Inventory (BAI)[29]
The BAI has 21 items that describe common psychological and physiological symptoms of anxiety. Like the BDI-II, the BAI has been extensively validated.[30] We used the BDI-II and BAI to control for changes in mental health during the intervention.
Demographics
The type, dosage, and scheduling of each participant’s antidepressant was assessed before and after the intervention. We also collected data on participant’s age, socioeconomic status, level of education, sexual orientation, and length and type of current sexual relationship.
Physical Health
At the laboratory sessions (i.e., pre- and post-trial), we measured the following indices of physical health: resting HR and blood pressure (measured with a finger cuff blood pressure monitor), body mass index (BMI; calculated from height and weight measured in lab), and physical activity and exercise over the last 7 days as recalled by validated structured interview (the Physical Activity Recall[31]).
PLANNED ANALYSES
We replaced individual missing items from a subscale with the mean of the remaining data of that subscale; this technique has been shown equivalent to multiple imputation for datasets in which less than 10% of total data is missing.[32] Ninety-three cells (0.59% of total data) were imputed.
For each endpoint (FSFI-total, FSFI-desire, FSI-orgasm, and SSS-total), we conducted a repeated measures mixed general linear model. In each case, we used time point as the repeated measures variable, a random slope by subject, and age, BMI, resting HR, and BDI-II and BAI as covariates. The mixed model allowed us to examine the entire dataset including data from dropouts, model the slope of each individual separately while still examining the main treatment effects, and to model covariates that (potentially) differed during each time point (e.g., depression). We conducted these models in the total sample as well as the subsample of women with clinically diagnosable sexual dysfunction at baseline (N = 38, Table 1). For significant repeated measures effects, we used post-hoc specific contrasts to examine the mean differences between time points.
We considered the above models the following predictors: randomization (control exercise arm before experimental vs. experimental exercise before control), medication type (SSRI, SNRI), baseline frequency of sexual activity, and percent masturbation (vs. partnered events; see Table 2) during each trial arm. However, these terms were nonsignificant in every model and dropping these terms did not change the significance nor direction of the remaining effects. Thus, we present here models without the medication group, randomization status, baseline sexual activity, or masturbation rate terms.
TABLE 2.
Percent sexual activity that was masturbation only during each trial arm
| % masturbation | Total sample (N = 52)
|
Sexual dysfunction only (N = 38)
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Baseline arm | 43.39 | 26.73 | 32.59 | 22.80 |
| Experimental exercise arm | 57.78 | 29.76 | 39.51 | 24.29 |
| Control exercise arm | 58.12 | 29.10 | 38.10 | 23.88 |
RESULTS
ENROLLMENT, DROPOUTS, AND BASELINE CHARACTERISTICS
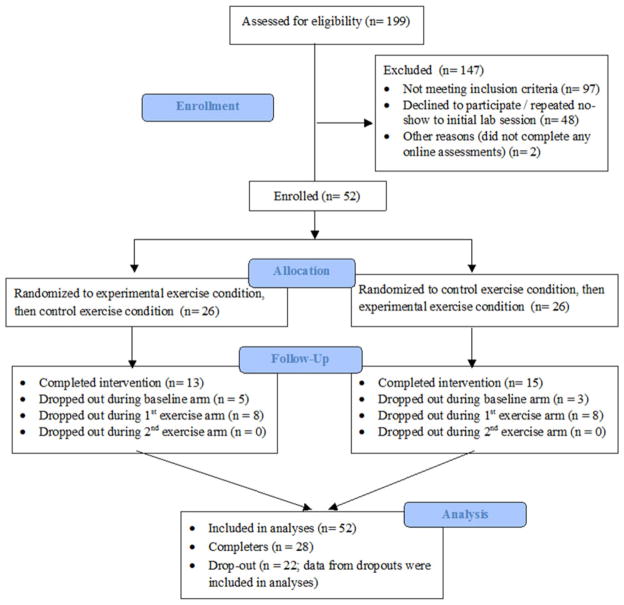
One hundred and ninety-nine women contacted the lab for more information on participating in the trial. Of these, 52 women were enrolled (see Fig. 2). Dropouts included 22 participants who formally withdrew from the study and two who had less than 50% compliance with the exercise interventions. Most women withdrawing cited time constraints; two had ended their sexual relationship, one discontinued her antidepressant, and one had a nonstudy-related injury. Dropouts did not differ from completers in terms of medication type (χ2(1) = .01, P = n.s.), age, body mass index, depression or sexual function at intake (F(1, 51) = 2.64, F(1, 51) = .04, F(1, 51) = 1.78, and F(1, 51) = 2.01, respectively, all ps = n.s.).
Figure 2.
Participant enrollment and flow.
Compliance was determined by comparing the timestamps on the HR monitors and online diary measures: compliant participants completed the diary within 3 h of exercise during the experimental arm and no less than 5 h after exercise during the control arm. The majority (75%) of exercise sessions were of the required duration, intensity, and timing relative to sexual activity.
The total sample (N = 52, Mage = 32.44) was predominately white (73%), in a committed relationship (73%) and with at least some college education (93%; see Table 1). The majority (60%) reported no to mild depression (BDI-II < 13). Three women reported no current sexual partner at intake; rerunning analyses with these women excluded did not significantly change results. There were no significant differences between the first and final assessments in BMI or resting HR (F(1, 50) = 1.79; F(1, 50) = 1.07, respectively; both Ps nonsignificant).
TREATMENT EFFICACY IN TOTAL SAMPLE
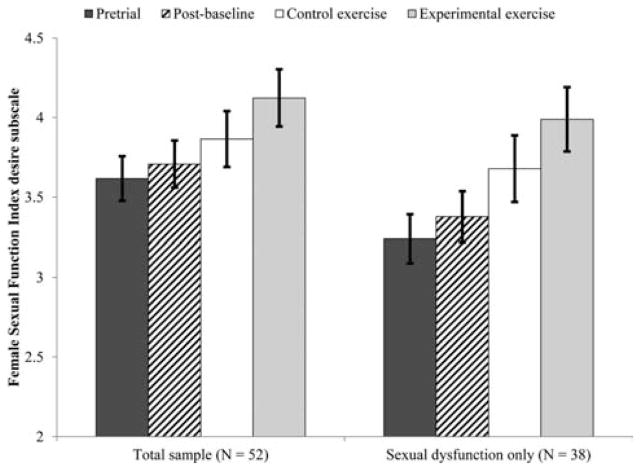
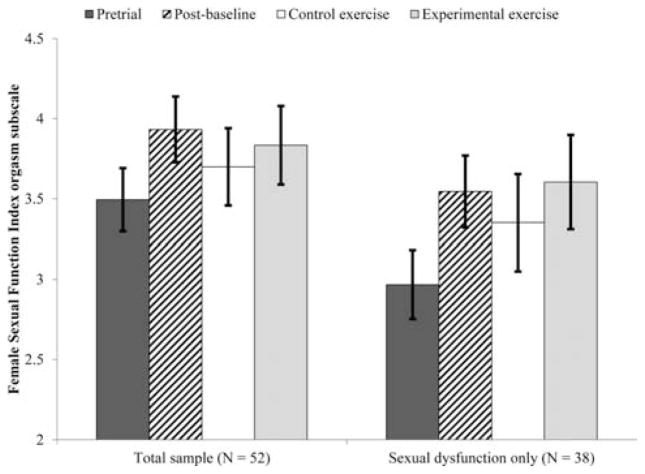
We first considered the effect of exercise on sexual function and satisfaction in all women who enrolled in the study (n = 52). There was a significant effect of time on sexual desire (F(3, 77.31) = 3.36, P = .023, see Fig. 3) such that women’s sexual desire during the experimental exercise arm was significantly higher than at pretrial (M diff =.51,σx̄=.16, P =.003) and postbaseline (M diff =.416,σx̄=.17, P =.014). The difference between experimental and control exercise arms was nonsignificant (M diff =.259,σx̄=.18, P =.155). There was a marginally significant effect of time on orgasm function (F(3, 80.64) = 2.61, P = .057, see Fig. 4). In this case, women’s orgasm function at the postbaseline time point was significantly higher than at pretrial (M diff =.44,σx̄=.16, P =.008) but there were no other significant contrasts.
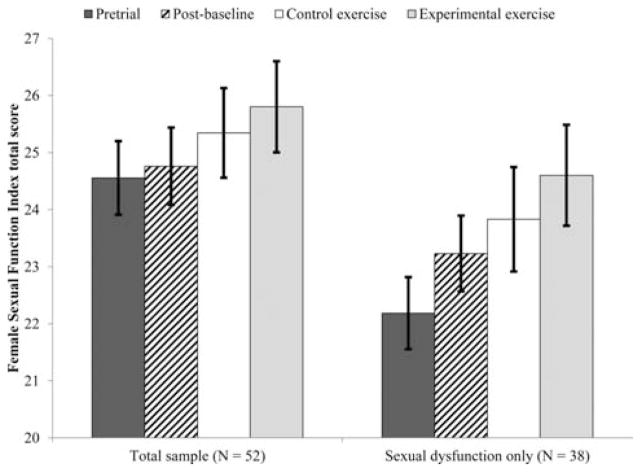
Figure 3.
Effect of exercise on global sexual function.
Figure 4.
Effect of exercise on sexual desire function.
Women’s FSFI desire and orgasm subscale scores following the experimental exercise arm were within one standard deviation of the mean for healthy controls and more than one standard deviation from the mean of women with Hypoactive Sexual Desire Disorder or Female Orgasmic Disorder (taken from validation studies[23, 24]), suggesting a clinically significant change in these domains.[33]
The effect of time on global sexual function was not significant in the total sample, F(3, 82.67) = 1.23, P = .305. Similarly, the effect of time on sexual satisfaction was not significant, F(3, 84.19) = .61, P = .610.
TREATMENT EFFICACY IN WOMEN WITH SEXUAL DYSFUNCTION AT BASELINE
As not all women who entered the trial were distressed about the change in sexual function they experienced after starting an antidepressant, not all would be considered “sexually dysfunctional” as defined by the DSM-IV-TR.[34] To examine treatment efficacy in women with clinically relevant sexual dysfunction, we selected data from participants (n = 38) who reported distress and had an FSFI score less than 26.55 (the clinical cutoff[25]) at baseline.
In this group, the effect of time on global sexual function was significant (F(3, 58.74) = 2.86, P = .045, see Fig. 5), such that sexual function during the experimental exercise arm was significantly higher than at pretrial (M diff = 2.41,σx̄=.88, P =.008). The effect of time on sexual desire was also significant (F(3, 45.90) = 5.59, P = .002, see Fig. 3), such that women’s sexual desire during the experimental exercise arm was significantly higher than at pretrial (M diff =.75,σx̄=.19, P =.0002) and postbaseline (M diff =.61,σx̄=.19, P =.003). Finally, orgasm function changed significant across time points (F(3, 55.69) = 3.34, P = .026, see Fig. 4), such that women’s orgasm function was significantly higher postbaseline (M diff =.58,σx̄=.20, P =.006) and during the experimental exercise arm (M diff = − .64,σx̄=.28, P =.027) than at pretrial. In all three cases, the contrasts between control and experimental exercise were not significant (Mdiff = .77, .31, and .19, respectively).
Figure 5.
Effect of exercise on sexual orgasm function.
The effects of exercise appeared to be clinically significant in that participant’s scores during the experimental exercise arm were within the range of healthy controls and out of the range for women with sexual desire or orgasm dysfunction.
As with the total sample, the effects of time point on sexual satisfaction were not significant, F(3, 58.71) = .80, P = .499.
DISCUSSION
In addition to the positive effects of exercise on psychological and physical health of women taking antidepressants, it appears that there may be benefits for sexual health as well. Exercise improved sexual desire and, for women experiencing clinically relevant sexual dysfunction at baseline, global sexual function. In addition, scheduling regular sexual activity improved orgasm function. The beneficial effects of exercise and sexual activity on women’s sexual function observed were above and beyond any improvements in mental and physical health.
These data suggest that as well as being an effective treatment for depression, exercise may also be moderately effective in treating antidepressant-induced sexual dysfunction. Improvements between pretrial and the experimental exercise arm were modest but consistent and clinically relevant. As there were no reported adverse effects of exercise, high patient acceptability, and no additional cost burden of care, even moderate efficacy is promising. Considering the wide prevalence of antidepressant sexual side effects and the dearth of treatment options for those experiencing these distressing effects, this is an important step in treating iatrogenic sexual dysfunction.
There was some evidence to support the hypothesis that exercise immediately before sexual activity had a greater benefit than exercise in general. The specific contrasts between control and experimental exercise arms were not significant for any of the outcome variables measured. However, participants did not have a sufficient response to the control exercise arm to separate from the pretrial or postbaseline scores. In other words, although the experimental exercise arm increased sexual function significantly (and the control exercise arm did not), the differences between these schedules of exercise was not as great as the difference between exercising versus not exercising.
Interestingly, simply committing to regular sexual activity improved orgasm function in all participants. It has long been known that a systematic sequence of masturbation-related activities, known as “directed masturbation,” can improve orgasm function in women with primary anorgasmia;[35] however, relatively less is known about orgasm problems secondary to medication use. Similarly, although behavioral activation (BA)—a key component of which is scheduling pleasant activity—has been well validated as a primary treatment for depression,[36] the effects of BA on sexual function are unknown. The findings from the present study suggest engaging in sex may be sufficient to reduce antidepressant-related orgasm problems. It may be that committing to regular sexual activity breaks a pattern of avoidance established earlier in the antidepressant regimen, when side effects were more severe.[37] Alternatively, regular sexual activity may have a feed-forward physiologic effect, for example by increasing levels of testosterone.[38]
On the whole, these findings support the prescription of exercise for sexual side effects of antidepressants. For women with diagnosable sexual dysfunction, a regimen of 30 min of vigorous exercise 3×/week was sufficient to produce clinically relevant improvements in sexual function, particularly sexual desire. For maximal benefit, exercise should be scheduled to occur immediately prior to sexual activity. However, some benefit may be seen with exercise in general.
The greatest limitation to this study was a high dropout. The dropout rate (approximately 46%) was not out of context for exercise intervention trials,[39–41] particularly in premenopausal depressed women.[42] As with all clinical trials, there was a trade-off between restricting sample variance and generalizability. We excluded women taking more than one psychoactive medication, which constitutes approximately one third of the general population of women taking antidepressants.[43] Also, most of the women in the current study were Caucasian, non-Hispanic, and college educated: generalizing these results to women of color, or to women with less education, should be done with caution. Future trials will be needed to examine effectiveness in a clinical setting (as opposed to a carefully controlled laboratory-based trial).
CONCLUSIONS
The results of the present study suggest that exercise improves sexual function in women experiencing sexual arousal side effects of antidepressants, with modest evidence of a specific benefit in exercising immediately before sexual activity. These findings have important implications for public health, as exercise as a treatment for sexual side effects is accessible, cheap, and does not add to burden of care.
Acknowledgments
Grant sponsor: National Institute of Mental Health; grant number F31MH085416 (to Tierney Lorenz); grant sponsor: NICHD; grant number RO1 HD051676 (to Cindy Meston) and grant number T32HD049336 (supporting Tierney Lorenz).
This study was completed with support from grant number 1F31MH085416 from the National Institute of Mental Health and grant number T32HD049336 from the National Institute of Child Health and Human Development (NICHD) to Tierney Lorenz, and from grant number RO1 HD051676 from the NICHD to Cindy Meston. The authors would like to thank Drs. Juan Dominguez, Timothy Schallert, Caryn Carlson, and John Bartholomew for their helpful comments on an earlier version of this manuscript.
Footnotes
As about 85% of antidepressants prescribed in the US are selective serotonin reuptake inhibitors (SSRIs) or selective serotonin and norepinephrine reuptake inhibitors (SNRIs) (Paulose-Ram, Safran, Jonas, Gu, & Orwig, 2007), we use the term “antidepressant” to refer to these two classes of drugs, unless noted otherwise.
Additionally, women were to be excluded if they were found to have any of the following as measured during the fitness assessment of the first session: a body mass index (BMI) over 40, waist circumference over 40 inches, waist-to-hip ratio (WHR) of greater than .9, systolic blood pressure greater than 150 or diastolic pressure over 95, or resting heart rate (HR) over 90.
Data from this manuscript was also presented in part as a poster at the annual meeting of the Society for Behavioral Medicine, March 2013.
References
- 1.Lorenz T, Meston CM. Acute exercise improves physical sexual arousal in women taking antidepressants. Ann Behav Med. 2012;43:352–361. doi: 10.1007/s12160-011-9338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olfson M, Marcus S, Druss B, et al. National trends in the outpatient treatment of depression. JAMA. 2002;287(2):203–209. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Clayton A, Segraves R, Leiblum S, et al. Reliability and validity of the Sexual Interest and Desire Inventory-Female (SIDI-F), a scale designed to measure severity of female hypoactive sexual desire disorder. J Sex Marital Ther. 2006;32(2):115–135. doi: 10.1080/00926230500442300. [DOI] [PubMed] [Google Scholar]
- 4.McElroy SL, Keck PE, Friedman LM. Minimizing and managing antidepressant side effects. J Clin Psychiatry. 1995;56:49–55. [PubMed] [Google Scholar]
- 5.Maddox J, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48–52. doi: 10.1177/026988119400800108. [DOI] [PubMed] [Google Scholar]
- 6.Masand P. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289–2304. doi: 10.1016/s0149-2918(03)80220-5. [DOI] [PubMed] [Google Scholar]
- 7.Taylor M, Rudkin L, Hawton K. Strategies for managing antidepressant-induced sexual dysfunction: systematic review of randomised controlled trials. J Affect Disord. 2005;88(3):241–254. doi: 10.1016/j.jad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504–1509. doi: 10.1176/ajp.2006.163.9.1504. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman BM, Babyak MA, Sherwood A, et al. Effects of aerobic exercise on sexual functioning in depressed adults. Ment Health Phys Activity. 2009;2(1):23–28. [Google Scholar]
- 10.Meston CM, Gorzalka BB. The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behav Res Ther. 1996;34(2):143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 11.Meston CM, Gorzalka BB. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. J Abnorm Psychol. 1996;105:582–591. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- 12.Bradford A, Meston CM. The Psychophysiology of Sex. Bloomington, Indiana: Indiana University Press; 2007. Autonomic Nervous System Influences: The Role of the Sympathetic Nervous System in Female Sexual Arousal; pp. 66–82. [Google Scholar]
- 13.Lorenz T, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology. 2012;49(1):111–117. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balogh S, Fitzpatrick D, Hendricks S, Paige S. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacol Bull. 1993;29(2):201–206. [PubMed] [Google Scholar]
- 15.Barton D, Dawood T, Lambert E, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25(10):2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 16.Khaykin Y, Dorian P, Baker B, et al. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Can J Psychiatry. 1998;43(2):183–186. doi: 10.1177/070674379804300209. [DOI] [PubMed] [Google Scholar]
- 17.McFarlane A, Kamath M, Fallen E, et al. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J. 2001;142(4):617–623. doi: 10.1067/mhj.2001.116766. [DOI] [PubMed] [Google Scholar]
- 18.Shores M, Pascualy M, Lewis N, et al. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrino. 2001;26(4):433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Montejo AL, Rico-Villademoros F. Psychometric properties of the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX) in patients with schizophrenia and other psychotic disorders. J Sex Marital Ther. 2008;34(3):227–239. doi: 10.1080/00926230701866125. [DOI] [PubMed] [Google Scholar]
- 20.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 21.Chivers ML, Seto MC, Lalumière ML, et al. Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Arch Sex Behav. 2010;39(1):5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 23.Rosen RC, Brown C, Heiman JR, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 24.Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003;29(1):39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiegel M, Meston CM, Rosen RC. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 26.Clayton A, Kornstein S, Prakash A, et al. Changes in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorder. J Sex Med. 2007;4(4):917–929. doi: 10.1111/j.1743-6109.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Meston CM, Trapnell P. Development and validation of a five-factor sexual satisfaction and distress scale for women: the Sexual Satisfaction Scale for Women (SSS-W) J Sex Med. 2005;2(1):66–81. doi: 10.1111/j.1743-6109.2005.20107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- 29.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 30.Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord. 1992;6(1):55–61. [Google Scholar]
- 31.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 32.Shrive F, Stuart H, Quan H, Ghali W. Dealing with missing data in a multi-question depression scale: a comparison of imputation methods. BMC Med Res Methodol. 2006;6(57) doi: 10.1186/1471-2288-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Author; 2000. [Google Scholar]
- 35.Meston CM, Hull E, Levin RJ, Sipski M. Disorders of orgasm in women. J Sex Med. 2004;1(1):66–68. doi: 10.1111/j.1743-6109.2004.10110.x. [DOI] [PubMed] [Google Scholar]
- 36.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 37.Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. J Clin Psychiatry. 2001;62:10–21. [PubMed] [Google Scholar]
- 38.Dabbs JM, Mohammed S. Male and female salivary testosterone concentrations before and after sexual activity. Physiol Behav. 1992;52(1):195–197. doi: 10.1016/0031-9384(92)90453-9. [DOI] [PubMed] [Google Scholar]
- 39.Herman S, Blumenthal JA, Babyak M, et al. Exercise therapy for depression in middle-aged and older adults: predictors of early dropout and treatment failure. Health Psychol. 2002;21(6):553–563. [PubMed] [Google Scholar]
- 40.Oldridge NB, Donner AP, Buck CW. Predictors of dropout from cardiac exercise rehabilitation: ontario exercise-heart collaborative study. Am J Cardiol. 1983;51(1):70–74. doi: 10.1016/s0002-9149(83)80013-7. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt JA, Gruman C, King MB, Wolfson LI. Attrition in an exercise intervention: a comparison of early and later dropouts. J Am Geriatr Soc. 2000;48(8):952–960. doi: 10.1111/j.1532-5415.2000.tb06894.x. [DOI] [PubMed] [Google Scholar]
- 42.Moroshko I, Brennan L, O’Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obesity Rev. 2011;12(11):912–934. doi: 10.1111/j.1467-789X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 43.Ohayon MM, Caulet M, Priest RG, Guilleminault C. Psychotropic medication consumption patterns in the UK general population. J Clin Epidemiol. 1998;51(3):273–283. doi: 10.1016/s0895-4356(97)00238-2. [DOI] [PubMed] [Google Scholar]