Abstract
A goal of interventions designed to increase reading speed is to reduce the practice of articulating words in an individual’s thoughts, or subvocalization. This practice may require redundant cognitive resources, slow reading speed, and detract from efficient transfer of written words to semantic understanding. It is unclear, however, whether exercises designed to promote faster reading speed generalize to cognitive function beyond the reading task itself. To investigate this possibility, we measured resting state functional connectivity in classical language regions before and after a course of cognitive exercise designed to increase reading speed in 9 healthy adolescent female volunteers. We found significantly decreased correlation between left Broca Area and right Broca Homologue and between right Broca Homologue and right Wernicke Homologue in the resting state after the training period compared to before training. Differences in functional connectivity after training to left Broca Area showed a spatial distribution reflecting decreased correlation to memory-associated brain regions and increased correlation to auditory regions, that might be consistent with a hypothesis that such training may decrease subvocalization associated with semantic memory function during the resting state.
Introduction
The ability to read is a high-level cognitive capacity supported by the functional convergence of multiple lower-level sensory processes. As such, reading ability is considered a model system for exploring the emergence of higher order cognitive processes from their more evolutionarily basal building blocks (Posner, Petersen et al., 1988; Schlaggar & McCandliss, 2007). The ability to engage written language (i.e., orthography) relies necessarily on basic visual processing systems that have evolved in the human brain for this purpose. Additionally, the refined primary sensory development of the human auditory system supports the phonographic engagement of spoken language. Successful integration of orthographic visual input and phonographic auditory data has been referred to as the sine qua non of the human ability to read (Share, 1995). Unsurprisingly, canonical language regions along the perisylvian fissure (e.g., Broca and Wernicke Areas) have demonstrated a crucial role specifically in the convergence of orthographic and phonological processing that support reading ability (Pugh, Mencl et al., 2001; Raij, Uutela et al., 2000; van Atteveldt, Formisano et al., 2004).
Multiple studies have explored the plasticity of brain networks involved in reading performance, and the ability of these brain regions to adapt under short-term training. In a study on pre-adolescent children with dyslexia, a two month interventional reading program showed fMRI-based activation changes that correlated with the degree of linguistic skill improvement (Temple, Deutsch et al., 2003). Similarly, a fourteen-day reading intervention program conducted for ten children with dyslexia demonstrated significant functional changes in regions associated with language tasks (Aylward, Richards et al., 2003). A number of topically-related studies have likewise reported changes in activational patterns in the brain during reading tasks after interventional programs conducted across weeks to months (Kujala, Karma et al., 2001; Shaywitz, Shaywitz et al., 2004; Simos, Fletcher et al., 2002).
In recent years, functional connectivity magnetic resonance imaging (fcMRI) has emerged within the neural imaging community as an effective metric of functional relationships in the human brain (Fox & Raichle, 2007). Research in fcMRI has given a framework for understanding the large scale architecture of human brain networks (Marcus, Harms et al., 2013; Yeo, Krienen et al., 2011). The majority of the studies published in the fcMRI literature take advantage of the fact that the strength of functional relationships across the brain are captured by the correlations in spontaneously occurring neural activity during a task-neutral state of wakeful rest. Because functional correlations are measured during a task-neutral resting state, they are considered to reflect the underlying structural connectivity (Honey, Sporns et al., 2009). Further, changes in the functional connectivity assessed during a task-neutral state are therefore used to mark alterations in synchronized co-activation of brain regions, resultant from plasticity and adaptation of neural systems to external conditioning.
Despite a series of publications looking at changes in neural activity from reading intervention, there is a dearth of published studies that have explored changes in the intrinsic connectivity in response to interventional reading training. Such studies are of interest, however, in their distinct contribution toward understanding brain plasticity from high level considerations of structural-functional adaptations to task-based conditioning. In contrast to task-based data which reflect regional adaptation and local neural efficiency, functional connectivity investigations provide an opportunity to explore changes in distributed connectivity that support learned behaviors, acquired skills, and habit formation.
As a test case for examining the effect of behavioral training on the functional architecture of networks involved in reading, we employed a speed reading training program aimed at altering the mechanisms for skilled reading. A core claim of speed reading proponents is that learned associations between orthographic and phonologic processing actually slow down the process of visual reading via subvocalization, the tendency of a reader to internally speak the words they are reading visually (Smith, Beckmann et al., 2013). In theory, such a tendency represents cognitive redundancy, in the sense that language content is transformed from visual cues into auditory cues by the reader, and then deciphered for meaning.
Consistent with this theoretical view, reduction or elimination of subvocalization in favor of direct semantic processing from visual cues, rather than semantically processing subvocalized phonological cues, would represent reduced cognitive load during the reading process, and allow reading to proceed at a faster rate. Because speed reading training provides a direct intervention for modulating a specific cognitive behavior, and additionally provides a framework for expected brain regions involved in cognitive adaptation, it is a suitable paradigm for examining the relationships between behavioral training and underlying changes to functional neural architecture. We attempted to determine whether a course of training involving speed reading practice would be associated with detectable changes in functional connectivity in brain regions associated with language that generalized to a resting state, and not associated with merely the act of reading alone.
Methods
Participant Sample
To minimize heterogeneity of the sample, all participants were typically developing right-handed young female adolescents, ages 14–22. A total of 9 participants completed both initial and followup scan after performing the cognitive training exercises. Participants were recruited by posted flyers at an area high school. Reading proficiency was assessed using the Gray Oral Reading Tests (GORT-4) (Wiederholt & Bryant, 2001) at enrollment into the study. All subjects consented to participate in the study following informed consent under guidelines agreed upon by the University of Utah Institutional Review Board.
| Mean | Standard deviation | |
|---|---|---|
| Age (n=9) | 18 | 2.3 |
| GORT-4 comprehension | 10.6 (63 percentile) | 1.7 |
| GORT-4 fluency | 13.2 (84 percentile) | 3.0 |
| Scan interval | 6 mo. | 2 mo. |
An additional sample of 26 typically developing male participants was selected from an ongoing longitudinal study involving functional MRI connectivity. These data were obtained on the same scanner with the same protocol, pulse sequence, and same instructions to participants for resting state scanning, and are included for public release in 2014 as part of the Consortium for Reliability and Reproducibility dataset from the International Neuroimaging Datasharing Initiative (http://fcon_1000.projects.nitrc.org/indi/IndiRetro.html). For these subjects, age range was from 8 to 39 (mean 20.2 +/− 8.3 yrs). Individuals were scanned twice, at least 2 years apart (mean 928 days +/− 105 days, range 733 – 1187 days).
Cognitive Exercise Training
Subjects were instructed to participate in a 6-week intervention consisting of internet-based training (EyeQ Advantage, Salt Lake City). Before a repeat MRI scan, subjects were required to complete 12 modules designed to facilitate progressively faster reading speed and increased comprehension. Each training exercise lasted approximately 10 minutes, and most of the participants performed many of the modules multiple times, with engagement in the training 3–5 times weekly. Modules consisted of practice reading passages at slow, medium, and fast presentation speeds, as well as following with their eyes the presentation of geometric images placed at progressively faster speeds around a computer screen as an exercise in shifting visual attention. Each module consists of similar exercises performed in the same order. For the initial scan, subjects were naïve to any training, and performed their first module as the final sequence obtained during the first scan.
fMRI Acquisition
Images were acquired on Siemens 3 Tesla Trio scanner. The scanning protocol consisted of initial 1 mm isotropic MPRAGE acquisition for an anatomic template. BOLD echoplanar images (TR= 2.0 s, TE = 28 ms, GRAPPA parallel acquisition with acceleration factor = 2, 40 slices at 3 mm slice thickness, 64 × 64 matrix) were obtained during the resting state, where subjects were instructed to “Keep your eyes open and relax. Remain awake and try to let thoughts pass through your mind without focusing on any particular mental activity.” Prospective motion correction was performed during BOLD imaging with PACE sequence (Siemens, Erlangen). An 8-minute scan (240 volumes) was obtained for each subject. On the initial scan an additional fMRI sequence (7-minutes, 210 volumes) was obtained during performance of the first cognitive training exercise module. For both scans, an additional task-based sequence (4-minutes, 125 volumes) was obtained during presentation of a sentence completion visual language task. Details of this task have been presented previously (J.S. Anderson, Lange et al., 2010). Briefly, a 20-second block paradigm alternated between periods of fixation on an isoluminant screen and periods where subjects read sentence fragments “He put the dishes in the _______” and covertly thought in their mind of a word to complete the sentence.
fMRI Preprocessing
Offline post-processing was performed in Matlab (Mathworks, Natick, MA) using SPM8 (Wellcome Trust, London) software. Post-processing pipeline has been previously reported (J.S. Anderson, Ferguson et al., 2011; J.S. Anderson, Nielsen et al., 2011). Initial slice timing correction was performed to adjust for interleaved slice acquisition. Field map sequence was acquired for each subject for distortion correction, and all images were motion corrected using realign and unwarp procedure. BOLD images were coregistered to MPRAGE anatomic image sequence for each subject. All images were normalized to MNI template brain (T1.nii in SPM8), with manual inspection of appropriate normalization in all subjects.
For resting state scans of both the cognitive exercise subjects and 26 control subjects, we used a regression algorithm using time series from voxels in the facial soft tissues, CSF and white matter to correct for artifactual correlations in the BOLD data to correct for BOLD signal attributable to physiological noise such as heart rate and respiration, (Fox, Zhang et al., 2009). No global signal regression was performed to avoid introducing artifactual anticorrelations in the data (J. S. Anderson, Druzgal et al., 2011; Murphy, Birn et al., 2009).
Scalp and facial soft tissues, CSF and white matter regression was performed after automated gray matter, white matter, and CSF segmentation of each subject’s MPRAGE image using SPM8. These segmented images were manually inspected to confirm appropriate identification of tissue components. The CSF time series for each subject was measured from the lateral ventricles. This was obtained from selecting voxels from the CSF segmented image for each subject within the bounding box defined by MNI coordinates: −35 < x< 35, −60 < y < 30, 0 < z < 30. White matter time series for each subject were obtained from the mean time series of voxels within 2 regions of interest in the bilateral centrum semiovale (MNI coordinates: left: x=−27, y=−7, z=30; right: x=27, y=−7, z= 30, each ROI had 10-mm radius). Before extracting the white matter time series, an exclusive mask was performed with the gray matter segmented image from each subject to eliminate voxels containing gray matter. A soft tissue mask of the facial and scalp soft tissues was used as previously described (J. S. Anderson, Druzgal, et al., 2011). The mean soft tissue, CSF and white matter time series were then used as regressors in a general linear model (glmfit.m in Matlab Statistics Toolbox) for the BOLD time series at each voxel in the brain, and the best fit was subtracted from the voxel’s time series data, producing the signal-corrected time series images. Each voxel’s time series was bandpass filtered with a frequency window of 0.001 Hz to 0.1 Hz (Cordes, Haughton et al., 2001) and linearly detrended to correct for scanner drift. No spatial smoothing was performed. Each frame was then inspected for significant motion using procedure reported by Power et al (Power, Barnes et al., 2012), and frames with DVARS or root-mean-square motion parameters > 0.2 were removed prior to analysis of connectivity results.
ROI Selection
Because the primary outcome of interest was the effect of functional connectivity in classical language regions, a 5 mm radius ROI was selected centered at MNI coordinates from the literature for left Broca Area and right Broca Homologue in the inferior frontal gyrus (left: x=−45, y=23, z=−2; right: x=36, y=24, z=−4) and left Wernicke Area and right Wernicke Homolgoue in the posterior superior temporal sulcus (left: x=−54, y=−44, z=4; right: left:−63,−36,3; right: 50,−25,−2) (J.S. Anderson, Lange, et al., 2010). Functional connectivity was measured before and after cognitive training for left Broca Area vs. right Broca Homologue, left Wernicke Area vs. right Wernicke Homologue, left Broca Area vs. left Wernicke Area, and right Broca Homologue vs. right Wernicke Homologue. Identical ROI’s were extracted from each scan of the 26 longitudinal participants without cognitive exercise training.
Because differences in functional connectivity were found for Broca Area vs. Broca Homologue, and because an a priori hypothesis was formed that cognitive training would decrease subvocalization in participants, we performed an additional exploratory analysis of functional connectivity between left Broca Area and the rest of the brain’s gray matter. 7266 ROIs were selected to form a lattice covering the gray matter as previously described (J.S. Anderson, Nielsen, et al., 2011; Ferguson & Anderson, 2011). The ROIs averaged 4.9 +/− 1.3 s.d. voxels in size for 3 mm isotropic voxels. For each subject, the preprocessed BOLD time series was averaged from the voxels in each of the 7266 ROIs, and functional correlation with the time series from the ROI containing left Broca Area coordinates was performed.
To more effectively account for inter-individual differences in precise position of language regions, visual language task data was processed using standard general linear model using SPM8, with activation t-statistic maps averaged between the pre-treatment and post-treatment scans for each subject. Activated clusters for bilateral Broca Area and Homologue and bilateral Wernicke Area and Homologue were obtained for each subject by identifying all voxels within 2 cm of above literature-based coordinates exhibiting p<0.05 activation, uncorrected voxelwise, within the region. In two subjects, no right Broca Homologue voxels met this threshold, and in one subject, no right Wernicke Homologue voxels met this threshold. For these individuals, 10 mm ROI’s were selected surrounding the coordinates for right Broca Homologue or right Wernicke Homologue.
NeuroSynth Database
To assess the spatial distribution of functional connectivity differences associated with cognitive training, we attempted to determine whether changes in functional connectivity were associated with four cognitive domains: reading, memory (semantic), visual, and auditory function. For each case we obtained a mask of brain voxels associated with each function in the neuroimaging literature using the NeuroSynth database (www.neurosynth.org) (Yarkoni, Poldrack et al., 2011). For the search terms “reading,” “memory,” “auditory,” and “visual” we obtained reverse inference maps showing voxelwise specificity for the corresponding search terms in the literature, with false discovery rate q<0.05 for multiple comparison corrections. In these images, Z-scores were averaged for voxels in each of the 7266 ROIs for which they were nonzero, and spatial correlation was performed across regions between the Z-score for the search term and the T-statistic that functional connectivity of the region to left Broca Area differed between pre- and post-training scans. We were therefore testing whether differences in functional connectivity to left Broca Area were spatially localized to a particular cognitive domain.
Results
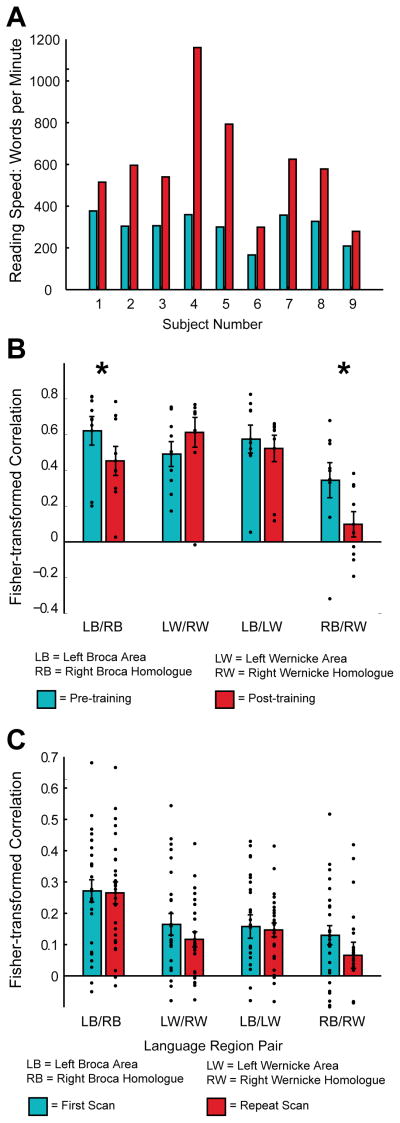
Resting state functional MRI images were obtained for each of 9 participants that completed cognitive training exercises before and after the training period. On the initial scan all subjects were naïve to the training software, and had not previously attempted a speed reading practice. On the followup scan, all participants had completed all 12 modules of the internet-based software at least one time, and most subjects had performed the modules multiple times. All 9 of the participants exhibited increase in reading speed measured by the training software over the course of the modules. These increases are shown for each subject in Figure 1A, and were significant (p=0.0021).
Figure 1.
A. Bar graphs show reading speed (words per minute) pre- versus post-training. (p=0.0021 for differences between reading speed pre- versus post-training). B. Bar graphs show mean functional connectivity across 9 participants, with error bars representing standard error of the mean. Results are shown for four region to region comparisons as indicated in the text. Functional connectivity from left Broca Area to right Broca Homologue was significantly lower (*) after cognitive training (p=0.036) as was connectivity between right Broca Homologue and right Wernicke homologue (p=0.046). C. Bar graphs show mean functional connectivity for healthy control subjects not participating in the reading training as indicated in the text, with error bars representing standard error of the mean. No significant differences are noted in functional connectivity across language areas indicated in the figure on baseline and repeat scans, separated by at least 2 years in 26 participants.
Regions of interest containing literature coordinates and subject-specific activated voxels in a neighborhood surrounding these coordinates for left Broca Area and Wernicke Area, and the corresponding right hemispheric homologues were extracted for each subject both before and after training. Functional connectivity measurements were obtained as the Fisher-transformed correlation coefficient between the preprocessed time series of (1) Left Broca Area to right Broca Homologue, (2) Left Wernicke Area to right Wernicke Homologue, (3) Left Broca Area to Left Wernicke Area, and (4) right Broca Homologue to right Wernicke Homologue. Results are shown as a bar graph in Figure 1B for the activation-map derived clusters. There was a significant decrease in functional connectivity between left and right Broca Area and Homologue consistent with our hypothesis that subvocalization would be decreased following training and that functional connectivity would be decreased between expressive language regions (p=0.017, one-tailed t-test for literature-based coordinates, p= 0.036 for activation-map derived clusters shown in Figure 1B, one-tailed t-test). For activation-map derived clusters, there was also decreased functional connectivity between right Broca Homologue and right Wernicke Homologue after cognitive training (p= 0.046, one tailed t-test). No significant differences were detected for the other 2 comparisons.
In this sample, we did not observe significant relationships between functional connectivity differences and changes in reading speed recorded by the software over the course of training (left Broca Area to right Broca Homologue: r=−0.036, p=0.93; right Broca Homologue to right Wernicke Homologue: r=−0.089, p=0.83). No significant effect was observed between age and connectivity (left Broca Area to right Broca Homologue: r=−0.48, p=0.19; right Broca Homologue to right Wernicke Homologue: r=−0.26, p=0.49). A negative correlation was seen between baseline reading skill (GORT) and left Broca Area to right Broca Homologue (r=−0.86, p=0.12) but this was not statistically significant, possibly given the small sample size.
To evaluate whether this effect might be due to cognitive training or simply normal developmental processes, we compared the same metrics in the longitudinal sample without cognitive exercise training, using literature-derived coordinates. None of the four comparisons showed significant changes between the first and second scan in this cohort (left Broca Area to right Broca Homologue: p=0.90; left Wernicke Area to right Wernicke Homologue: 0.28; left Broca Area to left Wernicke Area: p=0.82; right Broca Homologue to right Wernicke Homologue, p=0.13) despite an even longer interval between scans (2 years) and larger sample size. Similarly, no correlation was found between age of the subjects across all 52 scans and any of the four connectivity metrics in this sample. Individual subject values for each of these four connectivity metrics are shown in Figure 1C.
To further evaluate patterns of functional connectivity with left Broca Area, the expected primary locus for expressive language and putative region participating in subvocalization, we measured functional connectivity differences between this region and 7266 other ROIs covering the cortical and subcortical gray matter, and attempted to determine whether differences in functional connectivity to Broca Area aligned with a particular cognitive network as an exploratory analysis.
To accomplish this, we used the NeuroSynth database (Yarkoni, Poldrack, et al., 2011), consisting of inference maps to search terms in over 4000 studies in the neuroimaging literature. Specifically, we identified masks of brain regions significantly associated with the terms “auditory,” “visual,” “reading,” and “memory.” A significant reverse inference was indicative of a brain region specifically associated with these functions in the literature. For ROIs showing significant cognitive loading of each of the search terms, we compared T-statistics for differences in functional connectivity with left Broca Area before and after cognitive training, with the Z-score of loading for each of the search terms.
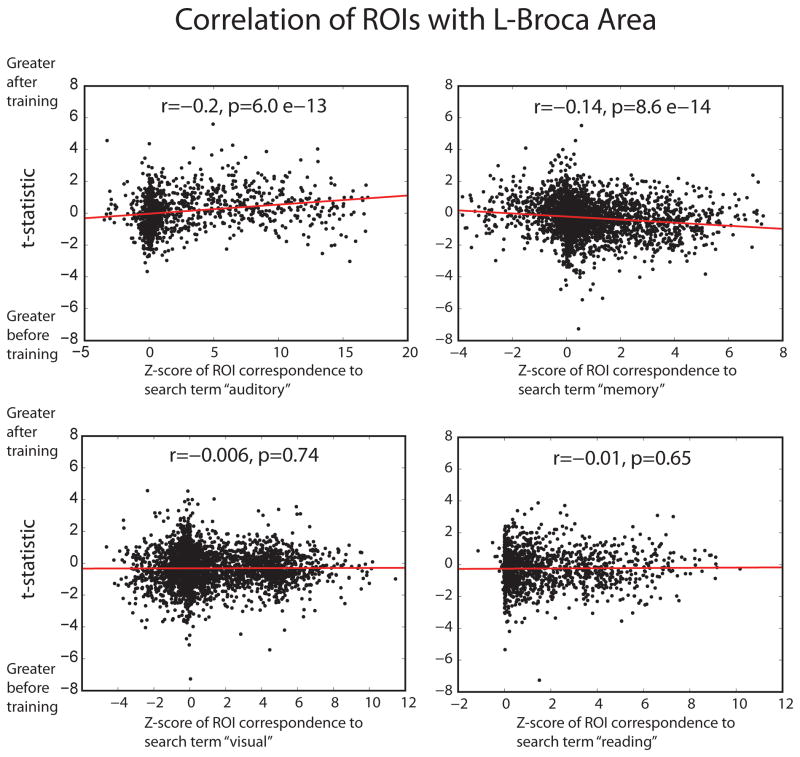
Results are shown in Figure 2. We found significant associations with two of the four search terms. For ROIs showing significant loading with the term “auditory” we found a correlation across ROIs that regions with higher loading of the term showed increased functional connectivity with left Broca Area after cognitive training (r=0.2, p=6.0 * 10−13). For the term “memory” we found a negative correlation between higher loading of the term and increased functional connectivity with left Broca Area after cognitive training across ROIs (r = −0.14, p=8.6 * 10−14). The other two search terms showed no correlation between loading for the search terms in the literature and changes in functional connectivity to left Broca Area (“visual”: r=−.0006, p=0.74; “reading”: r=−0.01, p=0.65).
Figure 2.
Scatterplots show spatial correlation of changes in functional connectivity to left Broca Area and loading to four specific terms in the neuroimaging literature. Masks were obtained for significant reverse inference to the terms “auditory,” “memory,” “visual,” and “reading” in the NeuroSynth database, and Z-score for significant loading to each of these terms is compared to T-statistic for change in functional connectivity to left Broca Area across gray matter regions within the mask.
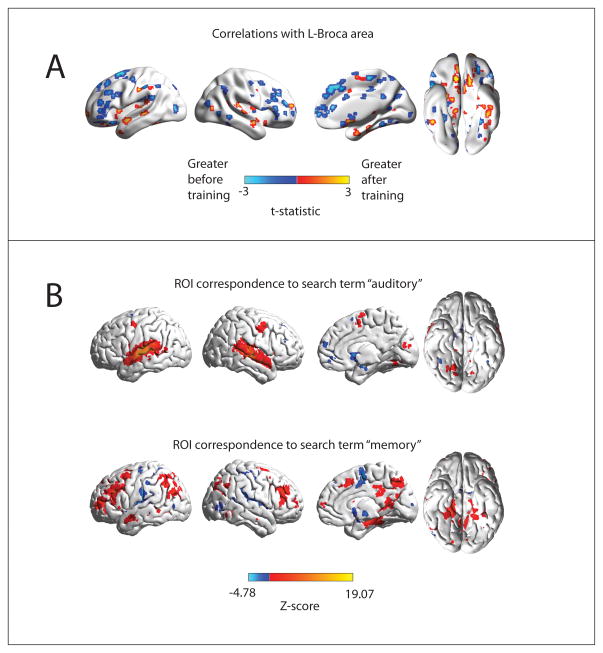
Images showing the spatial distribution of the “auditory” and “memory” masks as well as regions showing greatest changes in functional connectivity to left Broca Area before and after cognitive training are illustrated in Figure 3. The regions showing greatest differences in functional connectivity are closely aligned to “auditory” and “memory” networks, with greater functional connectivity to auditory regions after training, and relatively greater connectivity to memory regions before training. While the absolute differences in functional connectivity to left Broca Area do not survive multiple comparison corrections given the modest sample of 9 subjects (Figure 3A is thresholded at p<0.05, uncorrected for display), the spatial distribution of changes is correlated to specific cognitive networks.
Figure 3.
Spatial distribution of changes in functional connectivity to left Broca Area. A: Changes in functional connectivity after vs. before cognitive training of 7266 ROIs covering the gray matter to left Broca Area. Colored ROIs show regions that exhibited differential functional connectivity, thresholded at p<0.05, uncorrected, for display. B: Voxels showing significant reverse inference to the terms “auditory” and “memory” in the neuroimaging literature from the NeuroSynth database. Color scale represents Z-score for loading of the respective terms at each voxel, corrected for multiple comparisons with False Discovery Rate q<0.05.
Discussion
We demonstrate in a small cohort of adolescent female volunteers that a brief period of cognitive speed reading training results in resting state functional connectivity changes between left Broca Area and right Broca Homologue, independent of any task being performed. This might be consistent with, though not definitive for, a decrease in subvocalization during the resting state paradigm. Additionally, subtle changes in functional connectivity to left Broca Area show close alignment with cognitive networks underlying auditory and semantic memory function, with trends toward relatively decreased functional connectivity between Broca Area and memory-associated brain regions after training, and relatively increased connectivity between Broca Area and auditory-associated brain regions after training. This would be consistent with a hypothesis that function of Broca Area becomes more synchronized or associated with auditory function and less synchronized with memory function after training, possibly representing decreased subvocalization of semantic content during the resting state.
Although decreasing subvocalization is a common goal of reading proficiency training, the literature regarding brain activation changes associated with subvocalization is relatively sparse. In one early study, a task-based design required subjects to subvocalize words in a block design, with associated activation of left Broca Area (Bullmore, Rabe-Hesketh et al., 1996). A unifying concept underlying function in Broca Area has been the association with speech or word generation and articulatory planning (Hinke, Hu et al., 1993). Data from transcranial magnetic stimulation experiments has also found that Broca Area was associated with excitability of the motor system underlying speech production (Watkins & Paus, 2004). Inferior frontal gyrus, but also posterior cingulate and superior frontal gyrus activation have also been demonstrated in ERP and fMRI studies of mechanisms of subvocalization and semantic selection (Dien & O’Hare, 2008), Functional MRI during subvocal auditory rehearsal activated predominantly Broca Area in the left lateralized inferior frontal gyrus (Hickok, Buchsbaum et al., 2003). It is likely that subvocalization may represent a process that spans from speech generation in inferior frontal gyrus to premotor areas and ultimately motor cortex with progressive vocalization of sounds.
How subvocalization might be reflected within functional connectivity is much less clear. Yet recent developments in brain network architecture have reinforced that synchrony of brain regions is a reliable metric of co-activation and co-localized function (Fox & Raichle, 2007). In language processing in particular, engagement of progressively more difficult language tasks involves increased recruitment of right hemispheric language region homologues, and left dominance decreases systematically with activation threshold (Ruff, Petrovich Brennan et al., 2008). Increased connectivity of bilateral Broca Area and between right Broca Area and Broca Homologue seen in our results with reading speed training designed to decrease subvocalization may indicate that increased synchrony and recruitment of inferior frontal gyri may be associated with changing patterns of subvocalization or engagement of the speech generation system during wakeful rest.
We hypothesized that Broca Area might be more active or more integrated with other language regions when individuals were articulating words they were reading. If activity in Broca’s area became more synchronized with auditory cortex and less synchronized with semantic memory regions with training, this would suggest reduced coactivation of articulatory planning and expressive language construction with reading comprehension and memory retrieval. In other words, Broca Area would become more aligned with auditory processing and less aligned or synchronized with memory and semantic processing regions engaged in comprehension. Although the changes in connectivity to left Broca Area are small, it is striking the degree to which these changes recapitulate precisely the spatial distribution of known auditory vs. memory networks. Future studies are needed to explore whether other quantification techniques of subvocalization (such as electromyography) will confirm activation of speech generation and premotor regions and whether functional connectivity may serve as a metric of subvocalization for following reading practice interventions.
A wealth of functional imaging literature from the past twenty years has pieced together a relatively fine-grained portrait of orthographic language processing. Most basically, the processes by which an individual learns to translate visual cues into semantic meaning involve functionally integrating visual attention regions and high-order language areas clustered around the left temporal lobe. Coactivation of homologous regions from the right hemisphere are observed in cognitively demanding language conditions or altered prosody or emotional content. This corresponds to a pattern observed across numerous tasks wherein contra-lateral recruitment of regions are observed for neurally demanding functions (Friederici, 2011; Friederici, Brauer et al., 2011; C. J. Price, 2010). The bottom-up construction of primary visual cues into linguistic content is complemented by top-down phonological responses. This top-down contribution reinforces the end goal of appropriate semantic interpretation (Cai, Paulignan et al.; Hellyer, Woodhead et al.). In skilled readers, the top-down phonological contributions to semantic processing are automated (Borowsky, Esopenko et al., 2007; Guo, Zheng et al.).
A specific goal of the speed reading intervention applied in this study is to disassociate the visual input of orthographic word representations from internalized voicing and subvocalization of the text while it is being read. This hypothesis is consistent with our neuroimaging results, in that expressive language regions most associated with subvocalization appear to show more focused correlation to auditory regions associated with spoken language, but not with higher-order semantic or memory function associated with internal narrative or dialogue (Binder, Desai et al., 2009).
At the basic sensory level, the neural processes wherein visual input is converted to phonology can be parsed into at least three distinct pathways: sublexical, lexical, and semantic pathways (S. E. Price, Kinsella et al.). As such, the cognitive strategies for completing the same gross task (i.e., converting written language cues into phonology) demonstrate a significant amount of intersubject variability, since multiple strategic pathways are capable of accomplishing the same outcome. In spite of variability in cognitive strategy and neural pathways involved in reading, the commonality of the training task translates into a common focal point for the intervention: disassociating subvocalization of phonemes from their orthographic representations. As such, the observed reduction of functional correlation strength between regions involved in expressive speech fit into an expected neurological model for changes due to the interventional training.
While the results indicating that functional connectivity changes can be seen even in a resting state may indicate generalization of training beyond reading function, several study limitations suggest additional study is warranted to understand the effects of cognitive exercise training. First, the study sample is modest, limiting statistical power of the functional connectivity changes that can be discriminated. The spatial relationship with the auditory and memory networks indicates only that to the extent functional connectivity changes are present, they are closely aligned with these networks. Nevertheless, a definitive whole-brain characterization of functional connectivity alterations that could survive rigorous multiple comparison corrections would likely require a much larger cohort. Additionally, there must remain tentativity about the interpretation of functional connectivity changes involving Broca Area and subvocalization, given uncertainty about the precise neural mechanisms of subvocalization and alternate possible hypotheses about changes in functional connectivity, given that functional connectivity does not produce unambiguous interpretations of neural processes. The observed differences in functional connectivity, however, are encouraging in their consistency with hypothesized language function and the anticipated effects of cognitive training.
It is possible that functional connectivity changes seen could represent developmental effects rather than cognitive training. To test this possibility, we included longitudinal data from an additional cohort spanning the age range of our cognitive exercise subjects. No significant changes were seen in functional connectivity of classical language regions for this cohort, nor was there any relationship between age and connectivity for these regions. Nevertheless, these subjects were male, and the cognitive exercise subjects were female, so a gender-specific effect is not tested. Moreover, if developmental effects were specific to a very narrow age range in late adolescence, this might not be seen in the cohort spanning a larger age range (8–39).
Additional work would be required to demonstrate the durability and temporal evolution of changes in functional connectivity during reading interventions. It is unknown how much training is required to effect such changes, or how this might correspond to reading or cognitive performance. It has also been observed that Broca Area exhibits heterogeneity of function with overlapping regions processing phonology, syntax, and semantics, and a more detailed portrait of the functional connectivity of Broca Area will likely require higher-resolution discrimination of Broca complex subregions (Xiang, Fonteijn et al., 2010).
These results may inform future studies of reading interventions by focusing on the role of subvocalization and associated changes in functional connectivity with expressive language areas. Additional measurement strategies for quantifying subvocalization that are amenable to the MRI environment would be helpful for confirming the role of subvocalization in cognitive training. Further work will also be needed to establish the cognitive consequences of reduced subvocalization beyond reading and whether such training may contribute to enhanced cognitive function in attention, memory, or other domains.
Acknowledgments
MRI scanning costs were funded by the Flamm Family Foundation and Morrell Family Foundation. Jeff Flamm is CEO of EyeQ Advantage, which provided the software used in cognitive training free of charge for the study. The study authors have no financial interest in the EyeQ software or EyeQ Advantage company. Additional support was provided by NIH grant K08MH092697 (JSA).
References
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32(6):919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity Gradients Between the Default Mode and Attention Control Networks. Brain Connectivity. 2011;1(2):147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray M, Druzgal T, Froimowitz M, et al. Decreased Left Posterior Insular Activity During Auditory Langauge in Autism. AJNR Am J Neuroradiol. 2010;31:131–139. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(12):3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, et al. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61(2):212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, Esopenko C, Cummine J, Sarty GE. Neural representations of visual words and objects: a functional MRI study on the modularity of reading and object processing. Brain Topogr. 2007;20(2):89–96. doi: 10.1007/s10548-007-0034-1. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Rabe-Hesketh S, Morris RG, Williams SC, Gregory L, Gray JA, et al. Functional magnetic resonance image analysis of a large-scale neurocognitive network. NeuroImage. 1996;4(1):16–33. doi: 10.1006/nimg.1996.0026. [DOI] [PubMed] [Google Scholar]
- Cai Q, Paulignan Y, Brysbaert M, Ibarrola D, Nazir TA. The left ventral occipito-temporal response to words depends on language lateralization but not on visual familiarity. Cereb Cortex. 20(5):1153–1163. doi: 10.1093/cercor/bhp175. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Dien J, O’Hare AJ. Evidence for automatic sentence priming in the fusiform semantic area: convergent ERP and fMRI findings. Brain Res. 2008;1243:134–145. doi: 10.1016/j.brainres.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Ferguson MA, Anderson JS. Dynamical stability of intrinsic connectivity networks. Neuroimage. 2011;59(4):4022–4031. doi: 10.1016/j.neuroimage.2011.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS One. 2011;6(6):e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zheng L, Zhu L, Yang Z, Chen C, Zhang L, et al. Acquisition of conscious and unconscious knowledge of semantic prosody. Conscious Cogn. 20(2):417–425. doi: 10.1016/j.concog.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Hellyer PJ, Woodhead ZV, Leech R, Wise RJ. An investigation of twenty/20 vision in reading. J Neurosci. 31(41):14631–14638. doi: 10.1523/JNEUROSCI.2740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15(5):673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, et al. Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport. 1993;4(6):675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala T, Karma K, Ceponiene R, Belitz S, Turkkila P, Tervaniemi M, et al. Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proc Natl Acad Sci U S A. 2001;98(18):10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, et al. Human Connectome Project informatics: quality control, database services, and data visualization. NeuroImage. 2013;80:202–219. doi: 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240(4859):1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price SE, Kinsella GJ, Ong B, Storey E, Mullaly E, Phillips M, et al. Semantic verbal fluency strategies in amnestic mild cognitive impairment. Neuropsychology. 26(4):490–497. doi: 10.1037/a0028567. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Raij T, Uutela K, Hari R. Audiovisual integration of letters in the human brain. Neuron. 2000;28(2):617–625. doi: 10.1016/s0896-6273(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Ruff IM, Petrovich Brennan NM, Peck KK, Hou BL, Tabar V, Brennan CW, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol. 2008;29(3):528–535. doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Share DL. Phonological recoding and self-teaching: sine qua non of reading acquisition. Cognition. 1995;55(2):151–218. doi: 10.1016/0010-0277(94)00645-2. discussion 219-126. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biol Psychiatry. 2004;55(9):926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58(8):1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43(2):271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Watkins K, Paus T. Modulation of motor excitability during speech perception: the role of Broca’s area. J Cogn Neurosci. 2004;16(6):978–987. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- Wiederholt J, Bryant B. Gray oral reading tests- (GORT-4) Austin: Pro-Ed; 2001. [Google Scholar]
- Xiang HD, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb Cortex. 2010;20(3):549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]