Abstract
Background
Treatments are being developed for metachromatic leukodystrophy (MLD), suggesting the need for eventual newborn screening. Previous studies have shown that sulfatide molecular species are increased in the urine of MLD patients compared to samples from non-MLD individuals, but there is no data using dried blood spots (DBS), the most common sample available for newborn screening laboratories.
Methods
We used ultra-high performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS) to quantify sulfatides in DBS and dried urine spots from 14 MLD patients and 50 non-MLD individuals.
Results
Several sulfatide molecular species were increased in dried urine samples from all MLD samples compared to non-MLD samples. Sulfatides, especially low molecular species, were increased in DBS from MLD patients, but the sulfatide levels were relatively low. There was good separation in sulfatide levels between MLD and non-MLD samples when dried urine spots were used, but not with DBS, because DBS from non-MLD individuals have measurable levels of sulfatides.
Conclusion
Sulfatide accumulation studies in urine, but not in DBS, emerges as the method of choice if newborn screening is to be proposed for MLD.
Keywords: metachromatic leukodystrophy, tandem mass spectrometry, newborn screening, dried blood spots, lysosomal storage diseases, urine, sulfatides
1. Introduction
Metachromatic Leukodystrophy (MLD) is a recessively inherited lysosomal storage disorder caused by the deficiency of the enzyme arylsulfatase A (ASA) resulting in the accumulation of 3-O-sulfogalactosyl ceramides (sulfatides) in tissues. The prevalence is 1:40,000 to 1:100,000 births [1,2]. Interest in newborn screening for MLD is increasing because of the recent development of potential therapies [3]. Although direct assay of lysosomal enzymes in dried blood spots is a powerful method for newborn screening of lysosomal storage diseases [4,5], application of this approach to MLD is problematic because trace amounts of ASA activity are sufficient to minimize disease severity and ASA pseudodeficiency is common in the population [6]. Healthy individuals with ~5% residual ASA activity are prevalent in the population [6], and even ~1-2% ASA activity is sufficient to lead to a non-MLD phenotype (unpublished data from A. Fluharty). Thus, it will be be extremely difficult to develop a newborn screening of ASA enzymatic activity or protein abundance [7] that will not suffer from a high rate of false positives. It has been reported based on mass spectrometry results that the sulfatide substrates for ASA accumulate in urine of MLD patients [8-12]. However, almost all newborn screening laboratories only collect dried blood spots (DBS).
2. Materials and Methods
2.1. Materials
C17:0-Sulfatide (sulfatide with a heptadecanoyl acyl chain on the amino group of the sphingosine backbone) and C24:0-sulfatide were obtained from Avanti Polar Lipids. These were also synthesized by treating psychosine with the appropriate fatty acyl N-hydroxysuccinimidyl ester followed by sulfation of the galactosyl unit using dibutyltin oxide and trimethylamine-SO3 complex [13] using published procedures. Sulfatide species are abbreviated with the name of the fatty acid attached to the amino group of the sphingosine group, i.e. 24:1 is a 24-carbon fatty acid with 1 double bond, and 24:1-OH has a hydroxy group in the fatty acid chain.
Dried urine and blood samples were obtained with the help of the MLD Foundation. Institutional Review Board approval was obtained from the Univ. of Washington IRB panel. DBS were collected by puncturing the fingertip with lancet and letting the blood drip onto filter paper, which was air dried for ~2 hrs and then mailed over a few days to the Univ. of Washington. DBS from random, anonymous newborns were obtained from the Washington State Newborn Screening Lab after being stored at 18 °C for 8-10 months. Urine was collected on Whatmann 5 paper disks (70 mm), which were allowed to air dry for ~2 h at room temperature, then placed in a zip-lock plastic bag for shipment at ambient temperature. After arrival, all urine and DBS samples were stored at −10 to −20 °C in a closed jar with dessicant.
2.2. Extraction of sulfatides from dried urine and blood spots
To determine the extraction yield of sulfatides from the filter paper, a 3 mm paper punch (urine or blood) was spiked with 100 pmole of C17:0- and C24:0-sulfatide (from a stock solution in methanol (Fisher Scientific)), and the paper was allowed to dry for ~2 h. The punch was placed in a 1.5 ml polypropylene microfuge tube, and 1 ml of ethyl acetate (Macron Fine Chemicals) was added. The sample was mixed on a vortex mixer for 5 min and centrifuged at 3,000 rpm for 3 minutes. Supernatant (0.8 ml) was transferred to another tube, and solvent was removed with a stream of nitrogen at room temperature. The residue was dissolved in 0.1 ml of methanol for injection into the mass spectrometer.
2.3 Processing of DBS and urine spots for sulfatide analysis
Samples for sulfatide analysis were processed as follows. A 10 mm punch of the DBS or urine spot was placed in a glass tube, and 1 ml of ethyl acetate was used for extraction as described above. The ethyl acetate added to each sample contained 2 pmole of C17:0-sulfatide as internal standard.
2.4 UHPLC/MS/MS analysis of sulfatides in DBS and urine spots
UHPLC was carried out with a Waters AQUITY system with a HSS T3 C18 analytical column (50 × 2.1 mm, 1.8 μm) with a HSS T3 (5 × 2.1 mm, 1.8 μm) VanGuard guard column (Waters Corp.). The elution solvent was water with 0.1% formic acid (solvent A) and 2-propanol/methanol (80/20) with 0.1% formic acid (solvent B) (all solvents are LC-MS Optima grade from Fisher Scientific). The solvent program was 82% solvent B to 92% solvent B over 1.3 min, hold at 92% solvent B for 1.7 min, all at a flow rate of 0.4 ml/min. The total run time was 3 min. Tandem mass spectrometry was carried out on a Waters Xevo TQ instrument used in negative ion mode. The injection volume was 10 μl. Mass spectrometer settings are given in Supplemental Material Tables 1 and 2.
3. Results
3.1. Extraction of sulfatides from dried blood and urine spots
We explored a number of organic solvents for extraction of sulfatides from filter paper (CHCl3/methanol (2/1), hexane/2-propanol (3/2) and ethyl acetate) and found extraction yields for sulfatide of 69, 37 and 73%, respectively, thus ethyl acetate was used. When a methanol solution of C17:0- and C24:0-sulfatides was spotted onto filter paper followed by solvent removal, 74% and 76% of the sulfatide, respectively, were recovered after ethyl acetate extraction and subsequent quantification by UHPLC/MS/MS. Next we spotted known amounts of C17:0-sulfatide onto 1 cm diameter DBS or dried urine spots, and measured the recovery by UHPLC/MS/MS following extraction with ethyl acetate (100% was taken as the MS/MS signal obtained when the same amount of C17:0-sulfatide was submitted to UHPLC/MS/MS analysis without extraction, i.e. directly added to the autosampler well from the stock solution). Supplemental Fig. 1. shows a highly linear curve when the MS/MS response of extracted samples is plotted versus the MS/MS response of sulfatides (0 – 6 pmole) directly injected into the instrument without extraction. The slopes for urine and DBS were 0.49 and 0.56, respectively, indicating that about 50% of the sulfatide was extracted from filter paper containing the biological fluid. C17:0-sulfatide was used for these studies since this molecular species is not present in urine and blood. Subsequent studies of sulfatide levels in urine spots and DBS made use of C17:0-sulfatide as an internal standard.
3.2. Sulfatide levels in dried urine spots
Sulfatides were detected using negative mode tandem mass spectrometry. All sulfatide [M-H]− precursor ions produce a common fragment ion at m/z 96.9 corresponding to [HSO4]−, and this was used for tandem mass spectrometry quantification. The precursor ion and fragment masses for all sulfatide species analyzed are given in Supplementary Material Table 2. Additional mass spectrometry instrument settings are given in Supplemental Material Table 1. C17:0-sulfatide was used as an internal standard for sulfatide quantification in all samples.
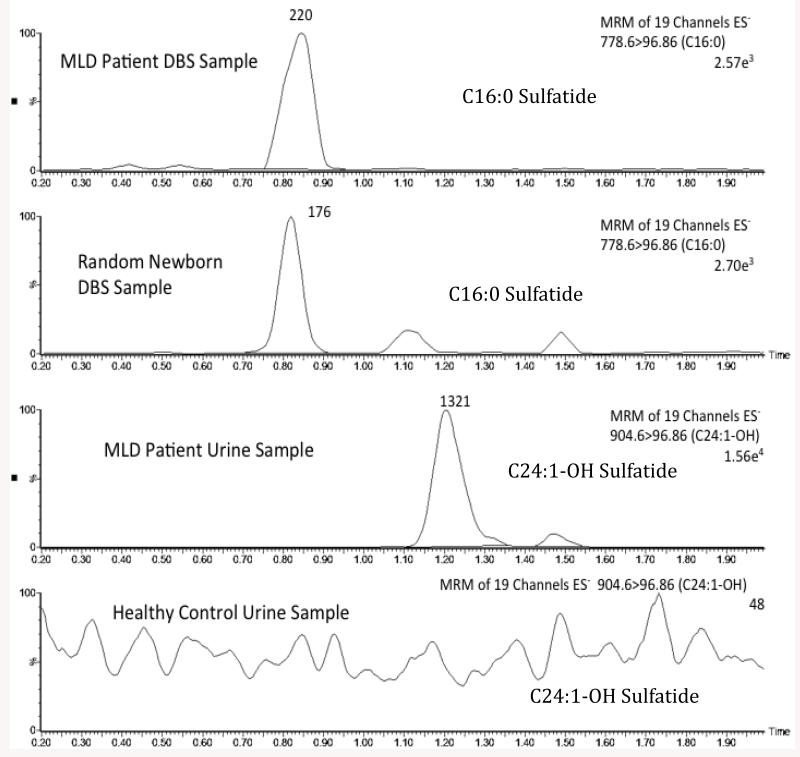
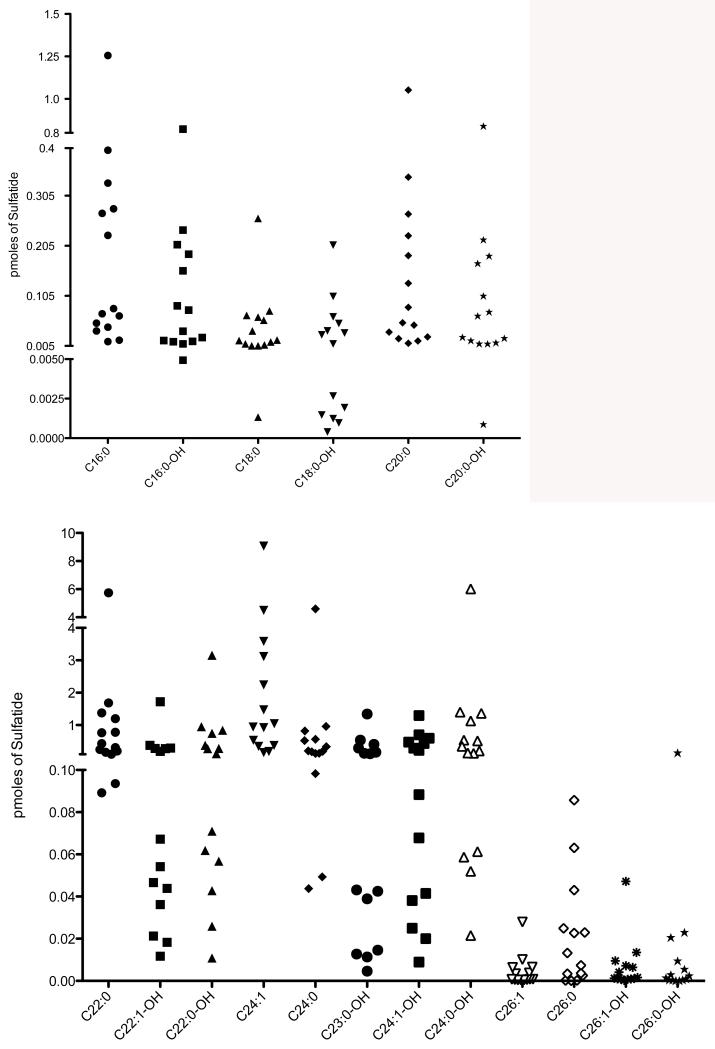
Fig. 1 (third panel) shows the well-defined peak in the ion chromatogram for the sulfatide species C24:1-OH, as an example, present in urine from an MLD patient. By contrast, no discernable peak was seen for this species when urine from a non-MLD individual was analyzed (Fig. 1, bottom panel). Sulfatide levels for all molecular species detected in all 14 MLD patients are shown in Fig. 2. No data is shown for non-MLD individuals as no discernable ion trace peak was seen for all sulfatide species among the 8 non-MLD individuals analyzed (not shown, analogous to Fig. 1, bottom panel). A lack of detectable urinary sulfatide species in non-MLD individuals was also reported previously [8]. The most abundant sulfatides in the MLD patients were C22:0, C22:0-OH, C24:0-OH, and C24:1. These were well increased in all 14 MLD patients. The next most abundant group was C16:0, C16:0-OH, C20:0, C20:0-OH, C22:1-OH, C23:0-OH, C24:0, C24:1-OH. These were also well increased in all 14 MLD patients. The least abundant sulfatide species were 18:0, 18:0-OH, 26:0, 26:0-H, 26:1, and 26:1-OH. These were increased in most but not all of the 14 MLD patients. Some of the MLD patients showed no detectable MS/MS signal for a subset of these low abundant sulfatides. Numerical data for all sulfatide species in all urine samples are provided as Supplemental Material Table 4. These trends are similar to those reported previously for urinary sulfatides in MLD patients [8-12]. Supplemental Material Table 3 gives the age of onset of symptoms among the 14 MLD patients. In general, there is no good correlation between the extent of sulfatide levels in urine spots and the age of onset of symptoms. Thus, urinary sulfatide levels appear to be predictive of MLD but not of the age of onset of the disease.
Fig. 1.
Selective ion chromatograms of the indicated sulfatide molecular species in DBS from an MLD patient (top), in DBS from a non-MLD individual (second panel), in urine from an MLD patient (third panel), and in urine from a non-MLD individual (bottom panel). The Y-axis is Relative Intensity. The C17:0-sulfatide internal standard elutes at 0.85 min and gives a typical peak area of 3840 with a percent standard deviation of 17%.
Fig. 2.
Levels of sulfatide molecular species in urine samples from 14 MLD patients. The internal standard gives a typical MS/MS peak area of 3780 with a percent standard deviation of 13%.
3.3 Sulfatide levels in dried blood spots
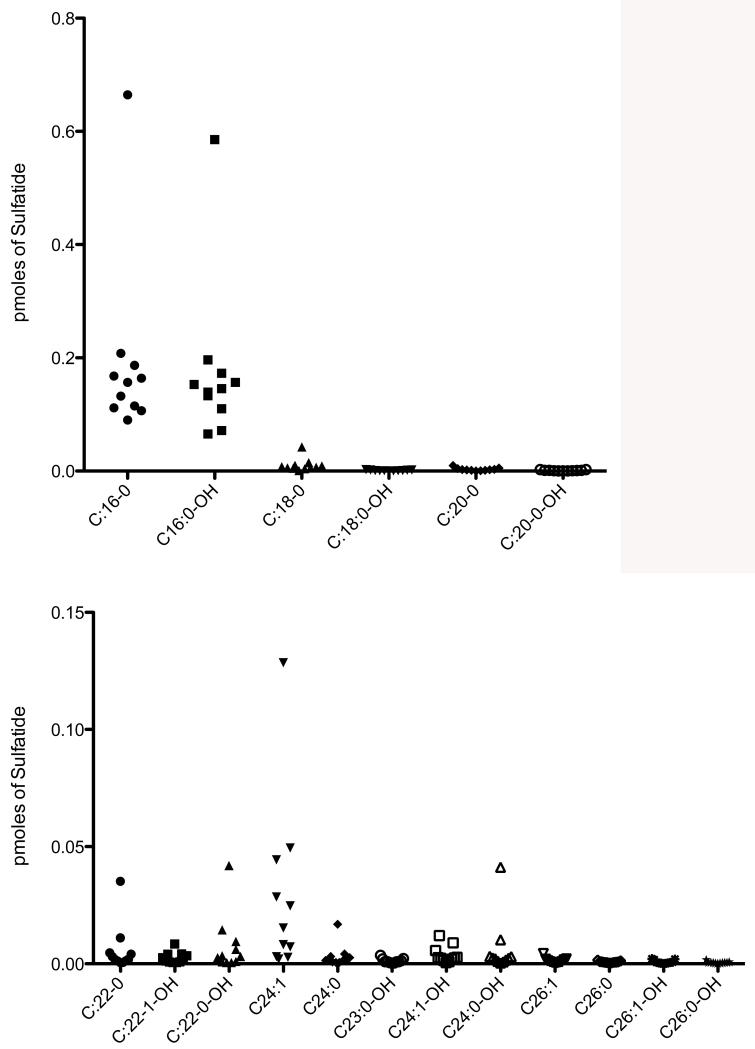
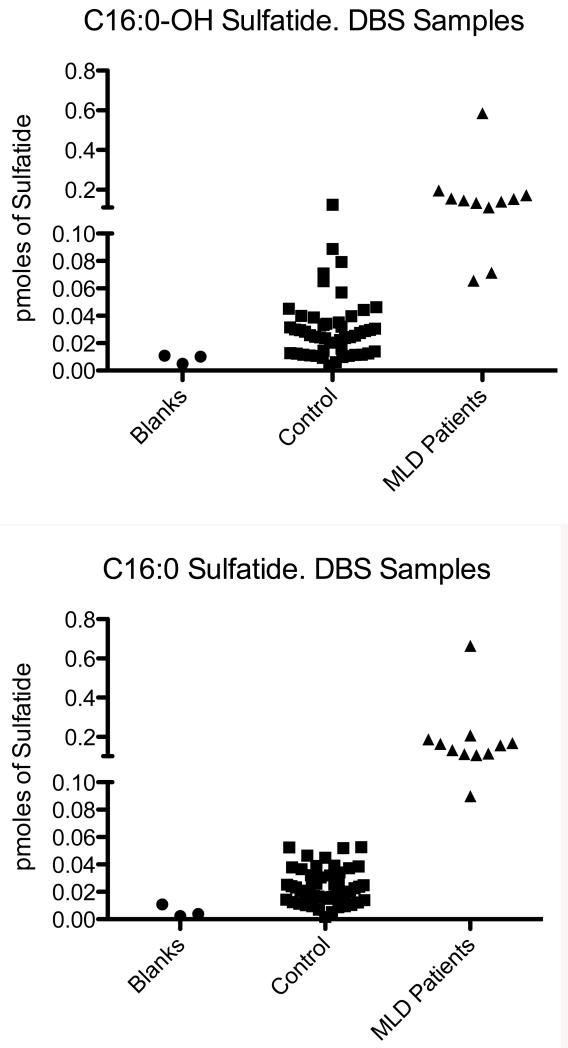
Fig. 1 (top panel) shows a well defined peak in the ion trace for C16:0-sulfatide in DBS from an MLD patient. The peak has the expected retention time for this species. Fig. 1 (second panel) shows that the same sulfatide species is detected with confidence from a DBS from a non-MLD individual. These results show that sulfatides can be detected in DBS samples. In general, we find that sulfatide species can be detected in DBS from non-MLD individuals, in contrast to urine spots where no discernable sulfatide peaks are seen in the ion chromatogram as noted above. Sulfatide levels in DBS from 50 non-MLD individuals and in 11 MLD patients are given in Supplemental Table 5. We analyzed only 11 of 14 MLD patients since we could not obtain a 1 cm punch from the filter paper cards for 3 of the MLD patients. Fig. 3 shows the levels of all sulfatide species among 11 MLD patients. Interestingly, the low molecular weight species C16:0 and C16:0-OH were increased, whereas the high molecular weight species were generally not. Some high molecular weight sulfatide species were increased in a subset of MLD patients (i.e. C24:1), but it is clear that urine and DBS give a very different distribution of sulfatides with respect to molecular species. The most abundant sulfatides in DBS were well below the levels of the most abundant species in the urine spots. Fig. 4 gives the levels of C16:0 and C16:0 sulfatides in 11 MLD patients versus 50 non-MLD individuals. Although the level of these sulfatides is generally higher in the MLD patients, there is significant overlap in the sulfatide levels for some of the MLD patients and the MLD individuals.
Fig. 3.
Levels of sulfatide molecular species in DBS samples from 11 MLD patients.
Fig. 4.
Levels of C16:0 and C16:0-OH in DBS from 11 MLD patients and in 50 DBS from non-MLD individuals. Also shown is data from 3 blood-free filter paper punches (blanks).
4. Discussion
In this study we have confirmed earlier reports that sulfatide molecular species are significantly increased in urine from MLD patients versus non-MLD individuals. In our studies, we used adult urine samples from non-MLD individuals because we have no ready access to urine samples from newborns. We also used urine samples from MLD children rather than newborns, because these were available with the help of the MLD society. Because urine is not routinely collected in newborn screening laboratories, at least in the United States and in most countries, we analyzed the sulfatide molecular species in DBS using UHPLC/MS/MS. For these studies we used DBS from random newborns (non-MLD) and DBS from young children with MLD (obtained with the help of the MLD Foundation). It is clear from the results of this study that sulfatide levels are increased in some but not all of the MLD patient DBS samples compared to non-MLD individuals. However, there is significant overlap between the sulfatide levels in a subset of MLD patients and those in the non-MLD samples, so much so that the analysis of sulfatide levels in DBS seems problematic for newborn screening of MLD. Sulfatides were detectable by UHPLC/MS/MS analysis in persons without MLD. This is in contrast to urine, where we and others report essentially no detectable levels of sulfatide molecular species. The level of sulfatides detected in DBS from non-MLD individuals leads to a significant overlap in sulfatide levels between MLD and non-MLD samples. This overlap is expected to lead to problematic newborn screening results where one is trying toidentify a small number of individuals who are at risk to develop MLD.
We cannot be certain that the weak UHPLC/MSMS signals for sulfatides seen in DBS are completely due to sulfatides in blood. One has to consider that the m/z 97 is not specific for HSO4− but can also be due to H2PO4− from a phospholipid. In any case, the data show that DBS are probably not a reliable source of samples for newborn screening of MLD.
A second problem with the use of DBS for sulfatide analysis is that the ion counts for the UHPLC sulfatide peaks are low, typically a few hundred counts for the most abundant species detected using a mass spectrometer that is about 2- to 3-fold more sensitive than those typically available in a newborn screening laboratory. This will add a further obstacle to the use of DBS for newborn screening of MLD.
Since treatment options are being developed for MLD, it is important to explore the newborn screening options that can possibly be implemented. The current study comparing urine to DBS samples strongly suggests that reliable newborn screening for MLD will require that urine samples be obtained. It has already been mentioned that the measurement of ASA protein or enzymatic activity is expected to be problematic for newborns screening of MLD. Our results continue to support the use of dried urine samples for the diagnosis of MLD. Should acceptable treatment for MLD be developed and should early diagnosis lead to a better treatment outcome, the path forward will require that urine samples be collected at birth and submitted to newborn screening laboratories. This is currently not done in the United States and in many other countries, but it is done in Quebec. It should also be mentioned that urine collection will allow other diseases besides MLD to be analyzed in newborn screening laboratories. Whether or not urine collection for the newborn screening of a single rare disease such as MLD will occur remains questionable.
Supplementary Material
Highlights.
There is interest in methodoogies for newborn screening of metabolic leukodystrophy (MLD).
Several molecular species of sulfatide are highly elevated in dried urine samples from MLD patients compared to non-MLD controls when detected by combined liquid chromatography/tandem mass spectrometry.
Dried blood spots show elevated sulfatides, especially for the low molecular species, in MLD versus non-MLD samples, but non-MLD samples contain detectable levels of sulfatides. This leads to a significant overlap between sulfatide levels in MLD and non-MLD samples.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (DK67859).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Von Figura K. Metachromatic Leukodystrophy. In: Scriver C, Beaudet A, Arthur L, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. Vol. 3. McGraw-Hill; New York: 2001. pp. 3696–3724. [Google Scholar]
- [2].Heim P, Claussen M, Hoffmann B, et al. Leukodystrophy incidence in Germany. Am. J. Med. Genet. 1997;71:151–156. [PubMed] [Google Scholar]
- [3].Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, Benedicenti F, Vallanti G, Biasco L, Leo S, Kabbara N, Zanetti G, Rizzo WB, Mehta NA, Cicalese MP, Casiraghi M, Boelens JJ, Del Carro U, Dow DJ, Schmidt M, Assanelli A, Neduva V, Di Serio C, Stupka E, Gardner J, von Kalle C, Bordignon C, Ciceri F, Rovelli A, Roncarolo MG, Aiuti A, Sessa M, Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- [4].Chamoles NA, Niizawa G, Blanco M, Gaggioli D, Casentini C. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin. Chim. Acta. 2004;347:97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- [5].Spacil Z, Tatipaka H-B, Marcenas M, Scott CR, Turecek F, Gelb MH. High Throughput Assay of Nine Lysosomal Enzymes for Newborn Screening. Clin. Chem. 2013;59:502–511. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fluharty AL. In: Arylsulfatase A Deficiency. Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. 1983. [PubMed] [Google Scholar]
- [7].Tan MA, Fuller M, Zabidi-Hussin ZA, Hopwood JJ, Meikle PJ. Biochemical profiling to predict disease severity in metachromatic leukodystrophy. Mol Genet Metab. 2010;99:142–148. doi: 10.1016/j.ymgme.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [8].Natowicz MR, Prence EM, Chaturvedi P, Newburg DS. Urine sulfatides and the diagnosis of metachromatic leukodystrophy. Clin. Chem. 1996;42:232–238. [PubMed] [Google Scholar]
- [9].Cui Y, Colsch B, Afonso C, Baumann N, Tabet JC, Mallet JM, Zhang Y. Synthetic sulfogalactosylceramide (sulfatide) and its use for the mass spectrometric quantitative urinary determination in metachromatic leukodystrophies. Glycoconj J. 2008;25:147–155. doi: 10.1007/s10719-007-9067-7. [DOI] [PubMed] [Google Scholar]
- [10].Kuchar L, Asfaw B, Poupetova H, Honzikova J, Turecek F, Ledvinova J. Direct tandem mass spectrometric profiling of sulfatides in dry urinary samples for screening of metachromatic leukodystrophy. Clin Chim Acta. 2013;425c:153–159. doi: 10.1016/j.cca.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nishio H, Kodama S, Matsuo T. Analysis of fatty acids and sphingosines from urinary sulfatides in a patient with metachromatic leukodystrophy by gas chromatography-mass spectrometry. Brain Dev. 1985;7:614–621. doi: 10.1016/s0387-7604(85)80010-3. [DOI] [PubMed] [Google Scholar]
- [12].Whitfield PD, Sharp PC, Johnson DW, Nelson P, Meikle PJ. Characterization of urinary sulfatides in metachromatic leukodystrophy using electrospray ionization-tandem mass spectrometry. Mol Genet Metab. 2001;73:30–37. doi: 10.1006/mgme.2001.3165. [DOI] [PubMed] [Google Scholar]
- [13].Blanchard S, Turecek F, Gelb MH. Short synthetic sequence for 2-sulfation of alpha-L-iduronate glycosides. Carbohydr. Res. 2009;344:1032–1033. doi: 10.1016/j.carres.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.