Abstract
Hyaluronan, a macromolecular glycosaminoglycan, is normally synthesized by hyaluronan synthases at the plasma membrane using cytosolic UDP-GlcUA and UDP-GlcNAc substrates and extruding the elongating chain into the extracellular space. The cellular metabolism (synthesis and catabolism) of hyaluronan is dynamic. UDP-GlcNAc is also the substrate for O-GlcNAc transferase, which is central to control of many cytosolic pathways. This Perspective outlines recent data for regulation of hyaluronan synthesis and catabolism that support a model that hyaluronan metabolism can be a rheostat for controlling an acceptable normal range of cytosolic UDP-GlcNAc concentrations in order to maintain normal cell functions.
Introduction
The review article in this issue by Vigetti et al. (2014) provides an insightful description of the mechanisms for regulating the stability and activity of hyaluronan synthase 2 (HAS2), the HAS that is required for development in vertebrates (McDonald and Hascall, 2002). Cytosolic UDP-GlcNAc is one of the substrates for hyaluronan synthesis, and is also the substrate for the cytosolic enzyme, O-GlcNAc transferase (OGT). OGT transfers GlcNAc from UDP-GlcNAc (O-GlcNAcylation) to serines/threonines in many intracellular proteins, which is often essential for regulating their functions. For example, O-GlcNAc is often the alternate structure for sites of phosphorylation on kinases. The activity of OGT depends on cytosolic UDP-GlcNAc levels. For example, hyperglycemic glucose stress, with consequent increases in UDP-GlcNAc concentration, leads to aberrant O-GlcNAcylation on many more cytosolic proteins, which can compromise cellular functions (Ma and Hart, 2013). The following perspective outlines how regulation of HAS2 activity could be an important regulator for maintaining cytosolic UDP-GlcNAc concentrations within an acceptable level for normal cell function.
Hyaluronan Biosynthesis
Hyaluronan is a glycosaminoglycan composed of a disaccharide, (glucuronate-β1,3-N-acetylglucosamine-β1,4-), that can be repeated more than 10,000 times to form a macromolecule >10 MDa in size. Unlike the other glycosaminoglycans (chondroitin sulfate, heparin/heparan sulfate, keratan sulfate), biosynthesis of hyaluronan does not require a core protein and is not synthesized in the Golgi. Instead, it is normally produced at the plasma membrane by one or more of the 3 hyaluronan synthases (HAS1, 2, 3) in the mammalian genomes (Weigel et al., 1997; Tammi et al., 2011).
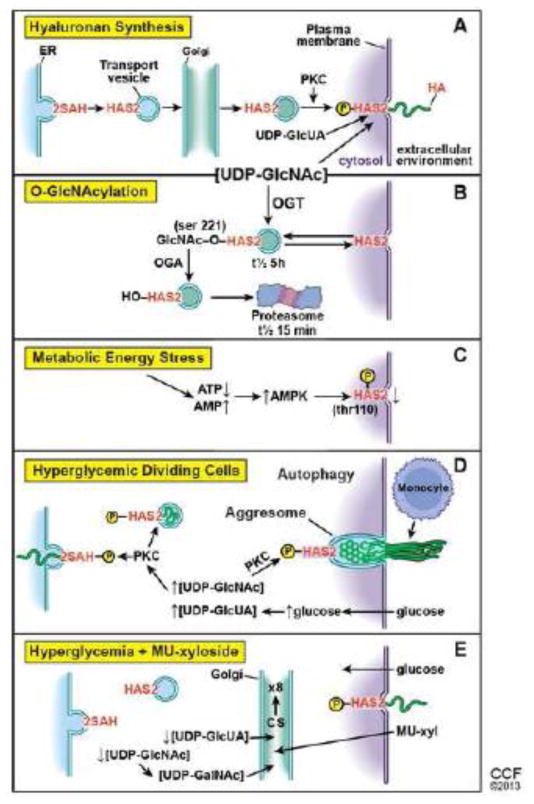
HASes are synthesized in the endoplasmic reticulum (ER) and carried by transport vesicles, likely through the Golgi, to the plasma membrane in an inactive form. Once embedded in the plasma membrane they can be activated, most likely through a phosphokinase C (PKC) phosphorylation pathway (Fig. 1A) (Wang and Hascall, 2004). The cytosolic domains of HASes then utilize cytosolic substrates (UDP-GlcUA and UDP-GlcNAc) and add them alternately onto the reducing end of the chain at the expense of removing the anchoring UDP, and the elongating chain is extruded through the plasma membrane into the extracellular space. During transport to the plasma membrane, the cytosolic domain is exposed to the cytosol. Therefore, it is important not to activate HASs before they are properly embedded in the plasma membrane.
Figure 1.
Further, regulation of hyaluronan synthesis is relatively simple as it depends only on single enzymes. It is also relatively energy efficient as synthesis of one of the substrates, UDP-glucuronic acid (UDP-GlcUA), recovers the equivalent of two ATPs, 5 from double oxidation of NADPH, minus 3 from the oxidation of UDP-glucose. It is also quite likely that the rate of hyaluronan synthesis depends on the cytosolic concentrations of the UDP-sugar substrates (Jokela et al., 2008; Rilla et al., 2013).
Hyaluronan Synthase 2 (HAS2) (see the Vigetti et al. review 2014)
Hyaluronan synthase 2 is expressed by most, if not all, cells and is essential for life. While the HAS1 and 3 null mice are developmentally normal, the HAS2 null mouse dies at an early embryonic stage when the heart is formed. Normal heart development requires endothelial cells to undergo epithelial-mesenchymal transition (EMT), which occurs when HAS2 is upregulated. Organ cultures of the wild type heart tube endothelial cells undergo EMT at the initiation of hyaluronan synthesis by HAS2. In contrast, organ cultures of the HAS2 null heart endothelium do not undergo any EMT, but do so if hyaluronan is added to the culture medium (McDonald and Hascall, 2002; Camenish et al., 2002).
HAS2 is dynamically regulated by cytosolic pathways that are involved in regulation of O-GlcNAcylation. The stability of cytosolic HAS2 is significantly increased when serine 221 is O-GlcNAcylated (t1/2 of ~5 h). In the absence of O-GlcNAc on this serine, HAS2 is rapidly degraded in proteasomes (t1/2 ~15 min) (Fig. 1B) (Vigetti et al., 2012). It is possible that O-GlcNAcylation of this serine is key for regulating whether or not HAS2 remains inactivated, which would allow the enzyme to migrate to the surface after its synthesis in the ER. Further, it would allow HAS2 to be internalized in an inactive form into cytosolic vesicles for storage or for eventual lysosomal or proteasomal degradation as outlined in figure 1B. It is also possible that removal of the GlcNAc and phosphorylation of this site or of another serine residue is critical for its normal activation in the plasma membrane. In any event, it is necessary to maintain HASs in non-active configurations when they are in any membrane exposed to cytosol except the plasma membrane, as shown by the pathological autophagic response of dividing hyperglycemic cells discussed below.
During energy stress, the ratio of AMP/ATP increases with consequent activation of AMP kinase. AMP kinase phosphorylates threonine 110 in HAS2, which inactivates its ability to synthesize hyaluronan (Fig. 1C) (Vigetti et al., 2011). This is a rapid way to help restore ATP levels back to normal, which would be needed to synthesize the UDP-GlcNAc substrate.
Regulation of Hyaluronan Synthesis by HAS2
These results indicate several ways for post-transcriptional regulation of hyaluronan synthesis by HAS2. 1) The concentrations of the UDP-sugar substrates in the cytosol can vary greatly depending on carbohydrate metabolism, which could increase or decrease hyaluronan synthesis accordingly. 2) The number of active HAS2 enzymes in the plasma membrane could effectively be altered by vesicular transport to and from the plasma membrane. 3) The active enzyme can be rapidly inactivated by AMP kinase under metabolic stress. Central to these mechanisms are the cytosolic concentrations of the UDP-sugar substrates, critically UDP-GlcNAc, because it is also the substrate for OGT. In normal conditions, the concentration of UDP-GlcNAc can vary from metabolism of carbohydrates. When UDP-GlcNAc is increased, hyaluronan synthesis is rapidly increased, which can effectively reduce the UDP-GlcNAc pool size to an acceptable cytosolic concentration. Further, when hyaluronan synthesis is significantly increased by overexpression of HAS, UDP-GlcNAc pools decrease significantly (Rilla et al., 2013). It is also possible that transfer of HAS to and from the plasma membrane can modulate hyaluronan synthesis. During ATP energy stress, synthesis of the UDP-sugar substrates can be inhibited, and the rapid inactivation of active HAS2 can help to maintain effective cytosolic UDP-GlcNAc concentrations. In these circumstances, hyaluronan synthesis by HAS2 could function as a rheostat for effective maintenance of UDP-GlcNAc within an acceptable cytosolic concentration.
An interesting feedback inhibition of HAS2 gene expression comes from the size of the cellular UDP-GlcNAc pool. Increasing and decreasing cellular UDP-GlcNAc concentration reduces and increases, respectively, HAS2 mRNA levels in keratinocytes by O-GlcNAcylation of HAS2 gene regulating transcription factors (Jokela et al., 2011). This can dampen changes in hyaluronan synthesis from excessive fluctuations in UDP-GlcNAc levels. However, the feedback appears to fade, at least in smooth muscle cells subjected to chronic hyperglycemia (Sainio et al., 2010).
Hyaluronan Catabolism (Fig. 2) (reviewed by Stern, 2004)
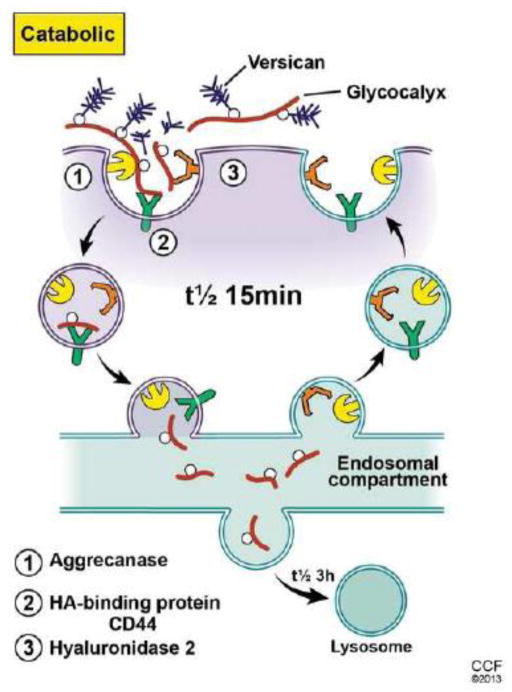
Figure 2.
Because the hyaluronan being synthesized is extruded into the extracellular space, an efficient catabolic process would be needed to remove it if its synthesis is continuously dynamic. This could be by a diffusive process as occurs in the production of synovial fluid hyaluronan, which flows into lymphatics that can recover most of it before it enters circulation. Once in circulation, hyaluronan is very effectively removed by hepatic endothelial cells. This efficient process recovers the sugars by internalization and transport to lysosomes (Pandey et al. 2008). Most cells do not have this option, but do have a metabolically active pericellular matrix (glycocalyx). For example, keratinocytes catabolize hyaluronan by a mechanism that involves the CD44 hyaluronan receptor (Tammi et al., 2001) and a hyaluronidase, most likely GPI-anchored hyaluronidase 2 (Andre et al. 2011). The presence of a protease, such as ADAMTS5 (aggrecanase) is likely also involved in order to remove associated proteoglycans (aggrecan and versican) (Hattori et al., 2011). CD44 rapidly transports (t1/2 ~15 min) the fragmented hyaluronan (20–30 kDa) with any remaining bound proteins into an endosomal compartment distinct from coated pits and pinocytotic uptake pathways. The fragments are then transported to lysosomes for complete degradation (t1/2 ~3 h) (Tammi et al., 2001). Therefore, distinct sites for biosynthesis and catabolism of hyaluronan on the surface of cells could effectively cooperate in controlling its dynamic metabolism.
Pathological Activation of HAS in Hyperglycemic Dividing Cells (Wang et al., 2011)
Glucose stress from hyperglycemia (> ~2.5 × normal glucose levels) cannot be tolerated by dividing cells. To cope with the increases in cytosolic glucose and UDP-sugars, the cells activate a PKC pathway that activates HAS enzymes in intracellular membrane compartments (Fig. 1D). Synthesis and deposition of hyaluronan in the endoplasmic reticulum initiates an ER stress mechanism that induces an autophagy that upregulates cyclin D3 synthesis, which is then involved in extrusion of hyaluronan into an extracellular monocyte-adhesive matrix. Mesangial cells that undergo this process are no longer able to sustain glomerular function in diabetic rats. The presence of 4-methylumbelliferyl-xyloside (4MU-xyl), an unnatural substrate for extension of glycosaminoglycan chains in lieu of core proteins, prevents the indirect intracellular activation of HASes and the autophagy, which allows the cells to complete normal cell division. Xylosides enter the cell and Golgi where they stimulate synthesis of chondroitin sulfate chains that are secreted into the medium (Fig. 1E). Mesangial cells increase chondroitin sulfate synthesis ~8 fold in the presence of 4MU-xyl (Nigro et al., 2009). The UDP-sugar substrates for synthesis of chondroitin sulfate are synthesized in the cytosol and transported into the Golgi by specific transporters that internalize one UDP-sugar while removing one UMP downstream product. Increasing chondroitin sulfate synthesis ~8-fold requires antiporters to remove 8 times as many UDP-sugar substrates (UDP-GalNAc and UDP-GlcUA) from the cytosol. This can effectively diminish cytosolic UDP-GlcNAc (a precursor for UDP-GalNAc) and UDP-GlcUA, the substrates for hyaluronan synthesis. Dividing mesangial cells in hyperglycemia in the presence of 4MU-xyl do not activate the HAS enzymes in intracellular compartments, which allows the cells to complete cell division without the pathological autophagy response. This result provides direct evidence that control of the cytosolic concentration of UDP-GlcNAc, and possibly of UDP-GlcUA, is critical for maintaining normal cellular function during cell division.
In sum, these recent findings demonstrating the inter-relationships between UDP-GlcNAc, hyaluronan synthesis and protein O-GlcNAc modifications reveal an interesting direct connection of glucose metabolism with HAS2 gene expression and regulation of its activity that can modify the pericellular matrix. Further, changes of hyaluronan metabolism in these cellular matrices can have extensive and deleterious consequences in major diseases, including inflammation, obesity, diabetes and cancer, which are often linked to disturbed glucose metabolism.
Highlights.
Hyaluronan (HA) is a macromolecular glycosaminoglycan synthesized and catabolized by most cells.
HA is synthesized by HA synthases at the plasma membrane using cytosolic UDP-GlcUA and UDP-GlcNAc substrates.
HA is catabolized from cell glycocalyces by an endosomal uptake pathway.
Dynamic HA metabolism can regulate cytosolic UDP-GlcNAc levels to sustain functions of O-GlcNAc transferase.
Acknowledgments
Original research in the authors’ laboratories is supported in part by P01HL107147 to V.C.H. and by P01HL107153 to G.W.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andre B, Duterme C, Van Moer K, Mertens-Strijthagen J, Jadot M, Flamion B. Hyal2 is a glycosylphosphatidylinositol-anchored, lipid raft-associated hyaluronidase. Biochem Biophys Res Commun. 2011;411:175–179. doi: 10.1016/j.bbrc.2011.06.125. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J Biol Chem. 2011;286:34298–34310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela TA, Jauhiainen M, Auriola S, Kauhanen M, Tiihonen R, Tammi MI, Tammi RH. Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines. J Biol Chem. 2008;283:7666–7673. doi: 10.1074/jbc.M706001200. [DOI] [PubMed] [Google Scholar]
- Jokela TA, Makkonen KM, Oikari S, Kärnä R, Koli E, Hart GW, Tammi RH, Carlberg C, Tammi MI. Cellular content of UDP-N-Acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-GlcNAc modification of transcription factors YY1 and SP1. J Biol Chem. 2011;286:33632–33640. doi: 10.1074/jbc.M111.265637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hart GW. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev Proteomics. 2013;10:365–380. doi: 10.1586/14789450.2013.820536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Hascall VC. Hyaluronan minireview series. J Biol Chem. 2002;277:4575–4579. doi: 10.1074/jbc.R100064200. [DOI] [PubMed] [Google Scholar]
- Nigro J, Wang A, Mukhopadhyay D, Lauer M, Midura R, Sackstein R, Hascall V. Regulation of heparan sulfate and chondroitin sulfate. Glycosaminoglycan biosynthesis by 4-fluoro-glucosamine in murine airway smooth muscle cells. J Biol Chem. 2009;284:16832–16839. doi: 10.1074/jbc.M109.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MS, Harris EN, Weigel JA, Weigel PH. The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J Biol Chem. 2008;283:21453–21461. doi: 10.1074/jbc.M800886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilla K, Oikari S, Jokela TA, Hyttinen JM, Kärnä R, Tammi RH, Tammi MI. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem. 2013;288:5973–5983. doi: 10.1074/jbc.M112.443879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio A, Jokela T, Tammi MI, Järveläinen H. Hyperglycemic conditions modulate connective tissue reorganization by human vascular smooth muscle cells through stimulation of hyaluronan synthesis. Glycobiology. 2010;20:1117–1126. doi: 10.1093/glycob/cwq076. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- Tammi R, Rilla K, Pienimäki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI. Transcriptional and posttranslational regulation of HA synthesis. FEBS J. 2011;278:1419–1428. doi: 10.1111/j.1742-4658.2011.08070.x. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, Heldin P, Hascall VC, De Luca G, Passi A. Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem. 2011;286:7917–7924. doi: 10.1074/jbc.M110.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A. Role of UDP-N-Acetylglucosamine and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem. 2012;287:35544–35555. doi: 10.1074/jbc.M112.402347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Viola M, Karousou E, De Luca G, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014 Oct 14; doi: 10.1016/j.matbio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–10285. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011;278:1412–1418. doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]