Abstract
Epinephrine, released into blood from the adrenal medulla in response to arousing experiences, is a potent enhancer of learning and memory processing. This review examines mechanisms by which epinephrine exerts its effects on these cognitive functions. Because epinephrine is largely blocked from moving from blood to brain, it is likely that the hormone's effects on memory are mediated by peripheral actions. A classic effect of epinephrine is to act at the liver to break down glycogen stores, resulting in increased blood glucose levels. The increase in blood glucose provides additional energy substrates to the brain to buttress the processes needed for an experience to be learned and remembered. In part, it appears that the increased glucose may act in the brain in a manner akin to that evident in the liver, engaging glycogenolysis in astrocytes to provide an energy substrate, in this case lactate, to augment neuronal functions. Together, the findings reveal a mechanism underlying modulation of memory that integrates the physiological functions of multiple organ systems to support brain processes.
Keywords: epinephrine, glucose, astrocytes, brain metabolism and memory, vagus, memory consolidation and modulation
MEMORY CONSOLIDATION AND MEMORY MODULATION
Many treatments enhance memory when administered soon after an experience and do so in retrograde time-dependent manner (McGaugh and Petrinovich, 1965; McGaugh, 1966, 2000; McGaugh and Roozendaal, 2009; Gold, 2008; Gold and Korol, 2012). These findings complement the extensive evidence that amnestic treatments can also act in time-dependent retrograde fashion.
Retrograde enhancement of memory studies, together with retrograde amnesia studies, provided much of the bases for ideas about memory consolidation, i.e. that the temporal properties of post-training treatments revealed the time needed to form new memories. Similar ideas come from examination of the time courses of cell molecular responses, and manipulations of those responses, during the time after a training experience. In these studies too, the temporal functions for different responses differ widely. Some of these findings are described and discussed below.
While the findings are clear, the interpretation of these findings as a basis to define temporal properties of memory formation is not clear (Gold and McGaugh, 1975; Gold, 2006, 2008). Beginning with a consideration of retrograde amnesia and memory enhancement gradients, if there were a time-constant for memory formation, one would expect a rather narrow range of times after training when treatments were effective. However, there are very different temporal gradients across tasks, species and treatments. Differences by tasks and species might of course reflect real differences in the time needed to form memory under different conditions. Less readily incorporated into memory consolidation frameworks are the many findings that a single treatment can affect memory across widely different times after training depending on the dose or intensity of the treatment (cf. Gold and McGaugh, 1975; Gold, 2008), suggesting that such differences in the temporal characteristics of retrograde amnesia represent properties of the treatments rather than those of an underlying memory process (Gold and McGaugh, 1975). Similarly, anterograde amnesia gradients are often taken as evidence of decay of a short-term memory process that can be seen in the absence of impaired long-term memory formation. (A few of many examples: Bourtchouladze et al., 1998; Wang et al. 2006; Taubenfeld et al., 2001). However, in these instances too the time-course varies with the specific treatment and often with the dose or intensity of that treatment (cf. Gold, 2008), sometimes even revealing non-monotonic decay functions (Schafe and LeDoux, 2000).
The vastly different functions for the temporal gradients of anterograde and retrograde impairments and enhancements of memory suggest that, while the underlying construct of memory consolidation may be valid, the literature does not provide direct support for a unitary function that represents the time necessary for memory formation.
Instead, it appears on the basis of the results above that there may be multiple biological factors responsible for the formation of new memories. Some of these factors may occur soon after an experience while others have rather long times to onset (Izquierdo et al., 2006) and still others appear in waves after training (Bekinschtein et al., 2007; Bourtchouladze et al.,1998; Nader et al., 2000; Igaz et al., 2002; Abel and Lattal, 2001). For example, as described by Izquierdo et al. (2006), cGMP levels peak within minutes of inhibitory avoidance training and PKC levels peak about 2 hr after training. PKA levels peak within minutes of training, return toward baseline at 1 hr, and then peak again at 3 hrs, returning to baseline at 8–9 hrs after training. Moreover, some molecular responses, e.g pCREB (Taubenfeld et al., 2001) and pERK (Trifilieff et al., 2007) have durations that peak early, decrease, and then increase and remain increased beyond 24 hr. In addition, the duration of these responses to experience can themselves be short or long. These multiple temporal properties for amnesia contribute important information about the sensitivity of memory to pharmacological and other manipulations but also complicate the interpretation of the effects of post-training treatments on memory processes. It seems likely that molecular mechanisms of memory involve a network of serial and parallel molecular responses with multiple time courses to regulate memory processing (Izquierdo et al., 2006).
Given the diverse temporal characteristics of anterograde and retrograde treatment effects on memory and of molecular players in the formation of memory, questions about the neurobiology may gain focus by studying how the multiple changes are initiated and regulated by physiological responses to experiences. This view captures the distinction between modulation and consolidation of memory. Consolidation of memory refers to the formation of a neural product of memory while modulation of memory refers to up- and down-regulation of processes that participate in the storage of new memories. While modulation of memory is often used to describe enhancement of memory formation, the term applies as readily to down- as well as up-regulation of memory processing.
EPINEPHRINE AND MEMORY
The multiple time courses seen for retrograde and anterograde enhancement and impairment of memory led directly to the development of early ideas about modulation of memory. The question became: What was the purpose of changes in memory and in susceptibility to treatments that enhanced and impaired memory? One answer is that there are endogenous responses to an experience - arousal, neuroendocrine changes, etc. - that regulate memory processes (for reviews: Gold and McGaugh, 1975; Korol and Gold, 2007; Gold, 2008; McGaugh and Roozendaal, 2009).
One of the earliest identified and most potent hormonal regulators of memory is epinephrine (Gold and van Buskirk, 1975, 1978a). Epinephrine is released from the adrenal medulla into blood in response to training experiences in a graded manner related to the arousal and emotion of the triggering experience (McCarty and Gold, 1981). Many of the demonstrations of enhancement of memory with epinephrine have used inhibitory avoidance tasks. In these tasks, epinephrine is particularly effective at enhancing memory for training with low footshock intensities, apparently by mimicking a physiological response seen with an experience of higher arousal (McCarty and Gold, 1981). In addition to inhibitory avoidance tasks, the efficacy of epinephrine as a treatment with which to enhance memory has also been demonstrated using active avoidance, conditioned emotional response, one-trial water-motivated appetitive, visual discriminated avoidance, spatial working memory, and object recognition memory tasks (Sternberg et al., 1985; Introini and McGaugh, 1986; Stone et al., 1992; Talley et al., 2000; Dornelles et al., 2007). Epinephrine also enhances memory in humans (Cahill and Akire, 2003; Cahill et al., 2003). This broad base of findings likely represents the involvement of multiple memory systems, providing evidence that epinephrine enhancement of learning and memory is itself probably mediated by effects at multiple memory systems.
The relationship between circulating levels of epinephrine and memory is not a monotonic function. Low doses of epinephrine have little effect on memory, moderate doses enhance memory, and high levels impair memory. The dose-dependent profile for epinephrine effects on memory may be a biological instantiation of the inverted-U function relating arousal to learning and memory characterized long ago by Yerkes and Dodson (1908). The inverted-U dose-response curve, with enhancement and impairment of memory at different doses, applies to most treatments that modulate memory, apparently including even protein synthesis inhibitors (Gold and Wrenn, 2011), suggesting that a wide range of treatments my act on memory through shared cellular mechanisms.
Although the inverted-U is most directly shown with dose-response curves, interactions of exogenous treatments with endogenous arousal are also evident. A single dose of epinephrine enhances 24-hr memory for inhibitory avoidance training with a single footshock of low intensity but impairs 24-hr memory for training with a single footshock of high intensity (Gold and van Buskirk, 1978a). Results like these suggest that endogenously released epinephrine may be additive with exogenously administered epinephrine to produce the inverted-U relationship with memory. The results suggest further that epinephrine, as well as other endogenous modulators of memory including glucose described below, may provide elements of the biological underpinnings of for both memory enhancement and impairment.
The ways by which high doses of modulators of memory produce amnesia are unclear and at present quite speculative (cf. Gold, 2006; Calabrese, 2008; Mattson, 2008; Gold and Korol, 2012). One suggestion is that the memory is erased by overly active mechanisms of plasticity, making and breaking connections too rapidly to retain memory for a new experience. Another is that there is too much memory formation at the high end of the inverted-U, resulting in memories that are embedded within a cognitive framework that includes high-density details that interfere with memory retrieval. Yet another possibility is that the inverted-U is the additive function of an ascending memory enhancement process and a descending memory impairment process, a view supported by noting that opiates may initiate forgetting and that opiate antagonists attenuate memory impairments at the high end of inverted-U dose-response curves (Izquierdo, 1982; Introini-Collison and McGaugh, 1987).
BASES FOR EPINEPHRINE EFFECTS ON MEMORY
Although epinephrine can both enhance and impair memory depending on circulating levels, it is enhancement of memory that has generated the greatest research interest. Circulating epinephrine has very limited access to the brain (Weil-Malherbe et al., 1961; Axelrod et al., 1959; Hardebo and Owman, 1980). Considered together with findings that direct infusions of epinephrine into the brain fail to enhance memory (de Almeida et al., 1983), most ideas about how epinephrine enhances memory have focused on peripheral actions that can transduce epinephrine actions into effects on brain mechanisms.
There are two major views regarding the mechanisms by which epinephrine, released from the adrenal medulla, can influence brain functions. One is based on the idea that the vagus nerve has β-adrenergic receptors on afferent neuronal axons and that activation of these receptors by epinephrine might transmit to the brain the information identifying the circulating levels of epinephrine. A second view is that glucose, released into the blood mainly after activation of hepatic adrenoreceptors, mediates the effects of epinephrine on memory. Consideration of these alternative perspectives will be described next. It is important to note at the outset of this discussion that both views share an effort to find convergent mechanisms that explain a wide swath of modulators of learning and memory. Particularly in the context of the multiple temporal profiles described above in studies of memory, complete answers about the mechanisms of memory modulation of memory, like those for memory processing itself, will most likely involve a set of serial and parallel physiological responses.
The vagus nerve as a mediator of epinephrine effects on memory
The vagus nerve provides a conduit for information to and from the brain related to the functions of peripheral organ systems. As described here and elsewhere, there is good evidence that electrical stimulation of the vagus nerve can modulate many brain functions, including learning and memory. The important role of the vagus in monitoring multiple organ functions supports a possible role for the vagus in modulating of memory processes. Less compelling, however, is the idea that the vagus has a primary and direct response to epinephrine levels that regulates memory processes or, more generally, that vagal afferent activity is a key modulator of learning and memory under physiological conditions.
Several papers suggest that afferent vagal fibers provide the foremost transduction mechanism responsible for enhancement of memory by epinephrine, linking circulating epinephrine levels to brain functions responsible for modulation of memory (e.g., Miyashita and Williams, 2006; Williams and Miyashita, 2006; Roozendaal et al., 2006, 2009; Chen and Williams, 2012). Indeed, that view is accepted as settled evidence by some in reviews of related literatures (e.g., Wong et al., 2012; Roozendaal et al., 2009). The evidence supporting vagal participation in epinephrine effects on memory takes several forms. An often-stated feature of vagus biology included in papers with this perspective is that the vagus nerve is densely embedded with β-adrenergic receptors that bind epinephrine (e.g. Miyashita and Williams, 2006; Chen and Williams, 2012). This statement is based largely on the results contained in a short communication by Schreurs et al. (1986). That report demonstrated that dissected and desheathed vagus (and sciatic) nerves responded in vitro to bath-applied epinephrine, norepinephrine, and the β-adrenergic agonist, isoproterenol. The response measured was an increase in cAMP production in the nerves. This report appears to be the primary basis for the view that β-adrenergic receptors on afferent axons of the vagus nerve monitor epinephrine levels and convey that information to the brain, particularly at the vagal terminals in the nucleus of the solitary tract.
However, Schreurs et al. (1986) note two important caveats regarding the presence of β-adrenergic receptors in the vagus nerve that deserve more attention. First, they note that it is not evident that circulating epinephrine has access to the receptors in intact preparations, i.e. with the sheath in place as a barrier to the hormone. Second, the role of the vagal β-adrenergic receptors in nerve preparations is not clearly associated with functional activity of the vagus. Even in the desheathed preparation, the low concentration of epinephrine effective at increasing cAMP levels was approximately 1 micromolar, compared to plasma concentrations of 1–20 nanomolar in unstressed and stressed conditions, respectively (e.g., Popper et al., 1977; Mabry et al., 1995). The comparison of effective concentrations with typical plasma concentrations, together with the presence of the vagus sheath in situ, suggests that the effects of epinephrine on cAMP levels in the vagus nerve may not be evident under physiological conditions.
Another paper used to support the importance of these vagal receptors (Lawrence et al., 1995) showed that ligation of the vagus nerve results in accumulation of β2-adrenergic receptors adjacent to and on both sides of the block. These findings show transport of the receptors both toward and away from the brain but do not necessarily reveal receptors on the nerve axonal membranes per se vs. transport of the receptors to nerve endings, an interpretation offered by the authors (Lawrence et al., 1995). Overall, these reports provide at best weak bases for the view that receptors along the vagal nerves participate in monitoring epinephrine levels to control memory functions. Interestingly, there are parallel findings regarding the localization and transport of β-adrenergic receptors for the sciatic (Schreurs et al. 1986; Horn and McAfee, 1977) and other nerves (Zarbin et al. 1983).
Additionally, evidence of vagal β-adrenergic receptors in nerve preparations is not clearly associated with functional activity of the vagus in situ. Signals from the periphery to the brain via vagal afferents may monitor changes in the functions of peripheral organs, in part regulated by circulating hormones such as epinephrine (cf. Berthoud and Neuhuber, 2000; Mravec, 2011). These signals may well underlie the increase in afferent action potentials recorded from the vagus nerve after injection of epinephrine (Miyashita and Williams, 2006) seen at a dose (0.3 mg/kg) that may result in supraphysiological epinephrine levels (McCarty and Gold, 1981) sufficient to impair memory (Talley et al. 2000). Less clear is the notion that the β-adrenergic receptors associated with the vagus nerve themselves increase vagal afferent neural activity. Of note, the measure in the Schruers et al. (1986) paper is an increase in cAMP levels with adrenergic agonists bath-applied to the desheathed vagus. As Schruers et al. note, increases in cAMP may decrease rather than increase excitability in peripheral nerves (Seelig et al., 1983). Therefore, it is unlikely that epinephrine reaches these receptors under intact physiological conditions and, even if it does, such receptor binding may not result in vagus nerve activation. It seems more likely that if there is increased vagus activity in response to epinephrine injections, the increase is a consequence of the hormone's actions on peripheral organs with subsequent actions at vagal sensory nerve endings (Mravec, 2006). With this information, vagal activity might still have a role in modulating memory but by indirect rather than direct actions of epinephrine on the nerve. However, this possible role in modulating memory is also brought into question by results in food-restricted rats.
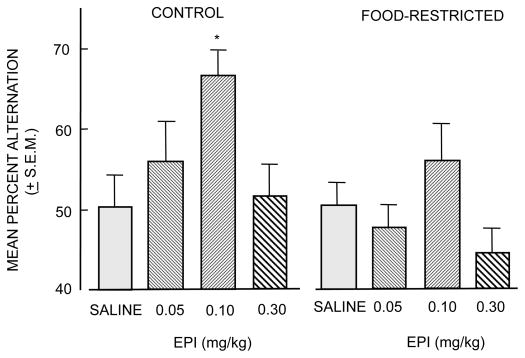
Studies of diminished epinephrine effects on memory in food-restricted rats do not support the view that epinephrine actions at the vagus are responsible for the hormone's enhancement of memory. These studies also provide evidence consistent with an alternative view that increases in blood glucose levels in response to increases in epinephrine release represent a mechanism for epinephrine enhancement of memory. One set of findings comes from a study of epinephrine effects on memory in rats 24 hrs after removal of access to food (Talley et al., 2000). A 24-hr fast is sufficient to reduce liver glycogen stores. In the absence of glycogen reserves, epinephrine binding to hepatic adrenoreceptors results in attenuated increases in blood glucose levels compared to those seen in fed rats, uncoupling epinephrine from increases in blood glucose levels. If epinephrine acts directly at vagal β-adrenergic receptors to enhance memory, the promnemonic actions of epinephrine should be retained in fasted rats. As shown in Figure 2, epinephrine did not enhance memory in rats fasted for 24 hrs; under the fasting condition, epinephrine also elicited depressed blood glucose responses (not shown).
Figure 2.
Effects of food-restriction on the ability of epinephrine to enhance memory. In free-fed control rats, epinephrine (EPI) injections 30 min prior to behavioral testing enhanced memory on a spontaneous alternation task in an inverted-U dose-response manner. In contrast, epinephrine was less effective in rats whose food had been removed for the 24 hr before testing. (From Talley et al., 2000.)
Since the vagus is intact in food-restricted rats, these findings suggest that activation of vagal β-adrenergic receptors is not sufficient to explain epinephrine enhancement of memory. Food-restriction results in decreased efficacy of epinephrine as a memory-enhancing treatment when a rise in circulating glucose is blunted. These results again point to the likelihood that peripheral increases in blood glucose levels are likely to be important mediators of epinephrine enhancement of memory, rather than a mediating mechanism involving peripheral activation of vagal afferents by direct epinephrine actions on the nerve or by indirect information conveyed from peripheral organs. Additional evidence showing diminished enhancement of memory by epinephrine but not by glucose in food-restricted rats will be presented below.
Relating glucose to vagal function is evidence that the nerve may monitor circulating glucose levels (Niijima, 1989; Mei, 1978). These suggestions preceded the elegant recent demonstrations that glucose can support axonal functions in peripheral nerves by providing glycogen stores in Schwann cells, probably permitting the production of lactate to support axonal energy needs (cf. Brown et al., 2012). While important to nerve function, the necessity of glucose actions on the vagus nerve to modulate memory is unlikely given findings described below showing that direct brain injections of glucose also enhance memory, thereby indicating that vagal activation is not an essential component of the mechanisms by which glucose enhances memory.
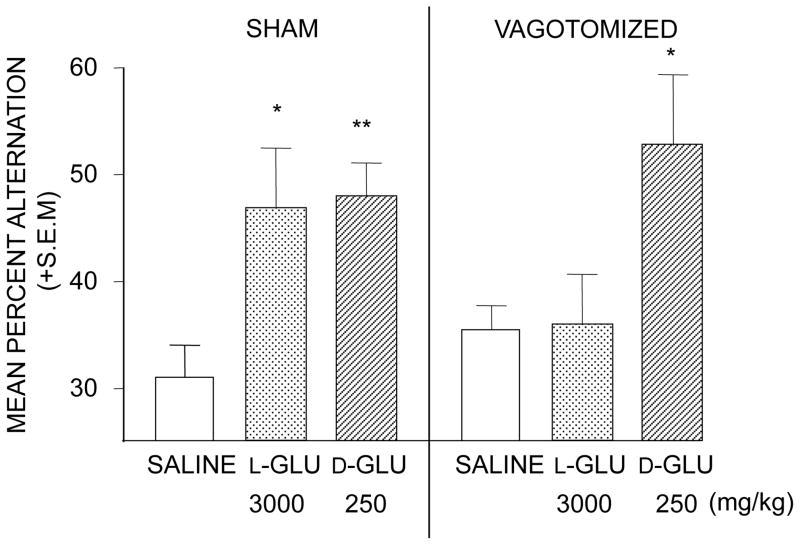
The specific question about the role of vagal afferents in epinephrine enhancement of memory is whether afferent activity initiated by epinephrine receptors on the vagus contributes to the epinephrine enhancement of memory. Vagotomy blocks the effects of some memory-enhancing treatments on memory, including bombesin and gastrin releasing hormone (Flood and Morley, 1988), leu-enkephalin (Williams and Jensen, 1993), substance P (Nogueira et al., 1994), and cholecystokinin (Itoh and Lal, 1990). Talley et al. (2002) examined the effects of vagotomy on enhancement of memory by glucose. Vagotomy does not block memory enhancement by a physiological dose of glucose. However, D- glucose has two sets of enhancing doses, the higher of which appears to be supraphysiological and is matched in efficacy at that dose by the artificial isomer, L-glucose. Enhancement of memory with either isomer of glucose at the higher dose range is attenuated by vagotomy (Talley et al., 2002) and by removal of the coeliac ganglion (White, 1991), suggesting that non-physiological doses alter the function of peripheral organs and that sympathetic afferents, including those in the vagus nerve, convey that the information to the brain but that memory enhancement by physiological doses of glucose is mediated by other mechanisms. Nonetheless, there are examples of vagotomy attenuation of memory enhancement by several treatments. In contrast, there appear to be no published tests similarly assessing vagotomy effects on enhancement of memory by epinephrine at either physiological or non-physiological doses.
In addition to effects on memory, epinephrine also has other effects on brain function, including slowing of epileptogenesis in a kindling model (Welsh and Gold, 1984, 1986). Perhaps related to the discussion of the vagus mediation of epinephrine effects on memory, vagotomy blocks seizure-protective effects of epinephrine. This effect too is seen only at a very high epinephrine dose (1 mg/kg) (Krahl et al., 2000) and may, as above, be a result of epinephrine actions on multiple organs with subsequent changes in afferent, and efferent, vagal activity. Together, these findings suggest that sympathetic nerve endings may convey to the brain information about the peripheral actions of epinephrine. The findings do not, however, offer strong support for the view that epinephrine acts on the vagus nerve directly to mediate the hormone's effects on memory, particularly under normal physiological conditions.
Although the evidence that vagal activation underlies enhancement of memory by epinephrine is not convincing, there is good evidence that vagal stimulation robustly enhances memory in rats and humans (Clark et al., 1995, 1998, 1999). The mechanism underlying these effects on memory is unclear, as is the relationship of these findings to analogous results seen with epinephrine. Activation of the vagus nerve transmits information to the brain regarding many peripheral organ systems (Berthoud and Neuhuber, 2000; Mravek, 2006, 2011; Messier, 2004) and has effects on multiple brain neurotransmitters, including increasing the release of norepinephrine in the amygdala and hippocampus (Williams et al., 1998; Miyashita and Williams, 2004). While norepinephrine may provide an intermediary step for modulation of memory, vagal stimulation also increases release of dopamine (Szczerbowska-Boruchowska et al., 2012) and serotonin (Manta et al., 2012) across multiple brain areas. In addition, vagal stimulation activates the hypothalamus-pituitary-adrenal axis via afferent projections and peripheral cholinergic systems via efferent projections (cf. Dantzer et al., 2000; Bonaz et al., 2013; Tracy, 2007). Stimulation of the vagus also results in increases in glucagon and insulin secretion, together with increases in blood glucose levels (e.g., Kaneto et al., 1974). Thus, there are many central and peripheral physiological responses to vagal stimulation that might mediate enhancement of memory by vagal stimulation (Berthoud and Neuhuber, 2000; Mravec, 2006, 2011; Messier, 2004).
Another form of evidence in support of the view that vagal afferents mediate epinephrine effects on memory is that lesions or inactivation of the nucleus tractus solitarius, the terminal region for vagal afferents, block the effects of epinephrine on memory (e.g., Clayton and Williams, 2000). These lesions would, of course, by virtue of producing dysfunctions in rostral and caudal brain systems as well as dysfunctions of the peripheral autonomic nervous system (cf.: Daulatzai, 2012; Iwasaki and Yada, 2012; Mandel and Schreihofer, 2008), have multiple consequences beyond the integration of vagal afferent activity that might influence memory and its modulation. In this respect, the findings that lesions of the nucleus tractus solitarius block effects of epinephrine on memory provide only rather indirect evidence for involvement in the underlying mechanisms of actions of epinephrine on memory.
Thus, the necessity of vagal participation in the mechanisms for epinephrine effects on memory is brought into question by the findings of food restriction experiments, the uncertain access of vagal β-adrenergic receptors to circulating epinephrine, the multiple and unresolved non-adrenergic mechanisms that might mediate effects of vagus stimulation on brain functions, and the indirect nature of the results obtained with lesions of the nucleus of the tractus solitarius. The conclusion here is that the evidence does not support the view that the vagus nerve is embedded with adrenoreceptors that monitor circulating epinephrine levels and convey that information as neural input to the brain. Moreover, the evidence does not support the idea that vagal monitoring of the functions of peripheral organs, except perhaps for non-physiological conditions, contributes to modulation of memory.
Theories of vagal mediation of epinephrine enhancement of memory often include vagal indirect control of norepinephrine release in the amygdala as a downstream mediator of epinephrine actions on memory. There is substantial evidence that the amygdala is a focal point for controlling modulation of memory by multiple hormones and neurotransmitters, including epinephrine, and that the amygdala may collate the modulatory substances to control multiple memory systems (McIntyre et al., 2012; McGaugh, 2000, 2013). To be clear, while theories of the role of the vagus often meld the amygdala into their models (Packard et al., 1995; McIntyre et al., 2012), the issue of whether vagal afferents provide a key mechanism underlying epinephrine effects on memory has no direct bearing on the role of the amygdala as a downstream target of epinephrine or other factors that modulate memory. The amygdala may well collect the information provided by diverse up- and down-modulators of memory to identify those memories that will be stored strongly and those that will be quickly forgotten.
Blood glucose as a mediator of epinephrine enhancement of memory
A classic physiological response to increases in circulating epinephrine is an increase in blood glucose levels, largely mediated by activation of hepatic adrenoreceptors and subsequent breakdown of glycogen stores (Sutherland and Rall, 1960). The mechanism underlying this action of epinephrine is the basis for the first identification of a second messenger system, cAMP, which increases upon activation of adenylate cyclase when epinephrine binds to membrane receptors on liver cells. The foundational experiments identifying second-messenger mechanisms provide the basis for current studies of signaling cascades initiated by activation of membrane receptors on neurons and other cell types. This fundamental biological process provides an important step between epinephrine release and the availability of glucose for brain functions, including regulation of learning and memory processing.
Like epinephrine, glucose enhances memory in an inverted-U dose-response manner in rats (Messier and White, 1984; Gold, 1986). When injected systemically, the doses of glucose and epinephrine that enhance memory elicit similar increases in blood glucose levels (Hall and Gold, 1986). Also like epinephrine, the inverted-U function interacts with the footshock used during training: the same glucose dose that enhances memory after training with low footshock impairs memory after training with a high footshock, providing another example of an interaction between the endogenous release of a modulator and its exogenous administration. Another similarity between glucose and epinephrine affects on learning and memory is that glucose enhances learning and memory across many tasks, including inhibitory avoidance, conditioned emotional response, appetitive mazes, and extinction tasks in laboratory rodents (e.g., Wenk, 1989; Kopf et al., 1996; White and Messier, 1988; Messier et al., 1997; Messier, 2004; Gold, 2001, 2004; Canal et al., 2005; Winocur and Gagnon, 1988). Glucose also enhances memory for many tasks in young and elderly individuals with normal memory functions as well as in several populations with impaired memory functions (Scholey et al., 2013; Benton et al., 2003; Kaplan et al., 2001; Watson and Craft, 2004; Manning et al., 1990, 1993; Parsons and Gold, 1992; Stone et al., 2003, 2005; Smith et al., 2011; Gold, 2001).
While these similarities are important in supporting the role of glucose as a mediator of epinephrine effects on memory, it is actually the differences between the pharmacology of epinephrine and glucose effects on brain functions that are particularly important in terms of identifying increases in blood glucose levels as a downstream mediator of epinephrine actions on learning and memory. One set of findings is that adrenergic antagonists block the effects of epinephrine on memory (Introini-Collison et al., 1992; Sternberg et al., 1985, 1986; Gold and van Buskirk, 1978b) but do not do so for glucose (Gold et al., 1986). In addition, blood glucose levels attained after injections of glucose to enhance memory are similar to those achieved after memory enhancing doses of epinephrine, and are associated with the effects of adrenergic antagonists on blood glucose levels (Hall and Gold, 1992). The findings that epinephrine but not glucose effects on memory are blocked by peripherally administered adrenergic receptor antagonists are consistent with the idea that increases in blood glucose after epinephrine injection or release mediate the hormone's affects on memory. Of course, the findings are also consistent with epinephrine actions via other adrenergic receptors, including those mediated by autonomic afferents to the brain as discussed above.
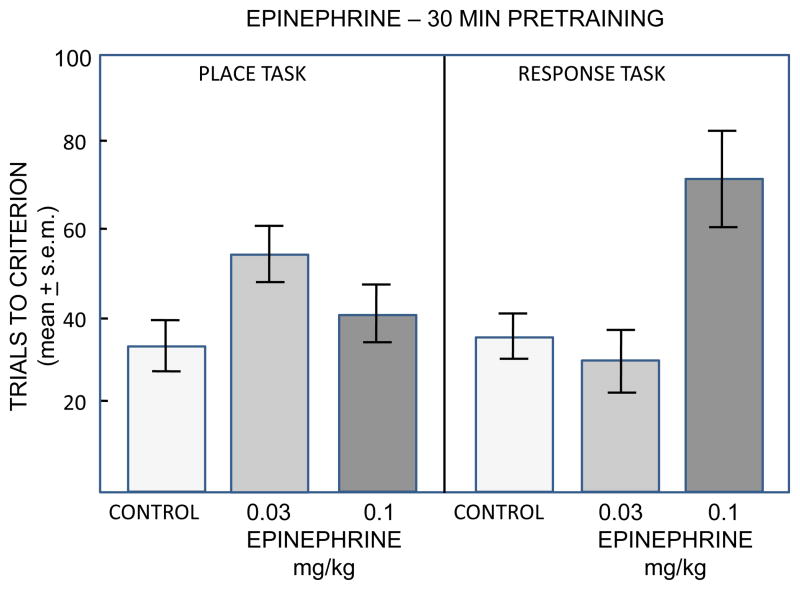
As noted earlier (Figure 2), another key piece of evidence suggesting that glucose is a substrate downstream from epinephrine comes from studies showing that epinephrine loses its efficacy in enhancing memory in food-restricted rats, which do not have the hepatic glycogen reserves needed to increase blood glucose levels (Talley et al., 2000). Most studies of epinephrine effects on memory have used tasks that do not involve food restriction. These include several avoidance tasks and also include tasks with relatively low arousal, such as spontaneous alternation working memory (e.g., Ragozzino et al., 1996, 1998; Stone et al., 1992), object recognition (Messier, 1997), one-trial appetitive learning in water-restricted rats (Sternberg et al., 1985), and drug extinction (Schroeder and Packard, 2003). In a direct test of epinephrine effects on learning in food-motivated radial arm mazes, epinephrine did not enhance but impaired memory for both place and response versions of the mazes (Sadowski et al., 2009; Figure 3). In contrast, systemic injections of glucose enhanced memory for training in food-motivated radial arm maze tasks (Winocur and Gagnon, 1998; Packard and White, 1990) and operant tasks (Messier and Destrade, 1988), and intrahippocampal injections of glucose enhanced memory for training in a food-motivated T-maze (Canal et al., 2005). There are, however, two examples of epinephrine enhancement of memory in food-restricted rats, one on an avoidance task (Williams and McGaugh, 1993) and the other a visual discrimination maze (Clayton and Williams, 2000). Additional attention is needed for more complete evaluation of the relative efficacy of epinephrine and glucose in enhancing memory in food-restricted rats.
Figure 3.
Epinephrine effects on learning in place and response versions of food-motivated 4-arm radial mazes. In the place version of the maze, rats were trained to find food at the end of an arm located in a particular position relative to the room cues. In the response version of the maze, rats were trained to find food by using a specific body turn on each trial. A deceases in trials to criterion beyond control values indicates enhanced acquisition while an increase indicates impaired acquisition. Note that epinephrine did not enhance but instead impaired learning on these tasks. (From Sadowski et al., 2009.)
A final example of the importance of glucose as a putative mediator of epinephrine effects on memory comes from studies of aging. Footshock, as used in inhibitory avoidance training, results in increases in both epinephrine and glucose in young adult rats, including young adult Fischer-344 rats (Mabry et al., 1995). In addition, post-training injections of both epinephrine and glucose enhance memory in young adult rats, including 3-month-old Fischer-344 rats (Morris et al., 2010; Morris and Gold, 2013). Compared to young adult rats, two-year-old Fischer-344 rats exhibit rapid forgetting after one-trial inhibitory avoidance training, with substantial and significant impairments in memory tested 1–7 days after training (Gold et al., 1982; Morris et al., 2010; Morris and Gold, 2012, 2013). The senescent rats also have changes in the profiles of circulating epinephrine and glucose after training (Mabry et al., 1995; Morris et al., 2010). In young rats, both substances increase after training. In aged rats, epinephrine is released at higher levels than those seen in young rats, but the epinephrine release is uncoupled from blood glucose levels, which do not increase in old rats as they do in young rats. Because of the difference in responsiveness of epinephrine vs. glucose, aged Fischer-344 rats provide a 'natural experiment' with which to study the roles of epinephrine and glucose in regulating memory. Enhancement of memory with post-training injections of epinephrine is attenuated in aged rats, together with a diminished rise in blood glucose levels after epinephrine injections. However, enhancement of memory with post-training injections of glucose is comparable to that seen in young rats, fully reversing the age-related memory impairments (Morris et al., 2010). These findings add support to the view that glucose is an important downstream mediator of epinephrine effects on memory.
GLUCOSE ACTIONS IN THE BRAIN CONTRIBUTING TO MEMORY ENHANCEMENT
Early views on glucose availability to the brain suggested that blood levels were adequate to saturate brain levels until times of near-starvation. However, later findings indicated that neuronal glucose uptake transporters are saturated at levels above 1.3 mM extracellular glucose (Braun et al., 1985; Fellows et al., 1992), a value higher than basal values in brain. For example, extracellular glucose concentrations in the hippocampus of rats appear to be ~1.0 mM (Fellows et al., 1993; McNay and Gold, 1999), levels similar to those seen in NMR studies in humans (Gruetter et al., 1998). Glucose levels differ by brain region in rats, with levels ~ 0.2 – 0.5 mM for the striatum (Fellows and Bouttelle, 1992; Fellows et al., 1992) and between 0.3 – 1.4 mM for the hypothalamus, varying with feeding status (de Vries et al., 2003; Dunn-Meynell et al., 2009).
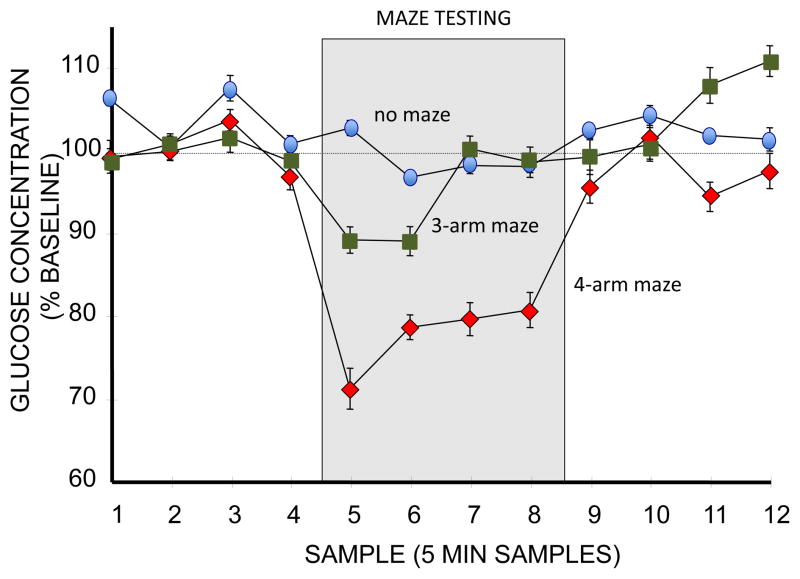
Brain levels of glucose also vary during learning and memory processing. Extracellular glucose levels in the hippocampus decrease while rats are tested on a 4-arm plus-shaped spontaneous alternation task (McNay et al., 2000, 2001; McNay and Gold, 2001; McNay and Sherwin, 2004; de Bundel et al., 2009; Pearson-Leary and McNay, 2012). The decreases in brain glucose levels are not simply the effects of motor or sensory functions associated with the maze, a conclusion supported by the absence of depletion of extracellular glucose levels in the hippocampus when rats are tested on a simpler task in which alternation is assessed in a 3-arm Y-shaped maze (McNay et al. 2000) (Figure 4). The number of arm entries and distance traveled are approximately the same in the two mazes, suggesting that the main difference between behavioral conditions responsible for glucose depletion is cognitive load. Extracellular glucose levels are also sensitive to avoidance training, with amygdala extracellular glucose levels decreasing after training and during and after a retrieval trial the next day (Sandusky et al., 2013).
Figure 4.
Effects of spontaneous alternation testing (during gray section of graph) on levels of glucose in the extracellular fluid (ECF) of the hippocampus, as measured with microdialysis procedures applied before, during, and after testing. Glucose levels remained generally constant in rats not tested on the maze (top line, blue ovals). Testing on a 4-arm alternation maze resulted in substantial and significant decreases in ECF glucose levels (bottom line, red diamonds). Testing a a 3-arm maze resulted in smaller decreases in glucose (middle line, green boxes); these values were significantly different than either controls or rats tested on the 4-arm maze. Because rats tested on the 3-arm maze had similar locomotor activity and exhibited alternation scores above baseline, the smaller decrease in glucose appears to be based on the different cognitive loads between the more-demanding 4-arm and less-demanding 3-arm mazes. (From McNay et al., 2000).
The depletion of glucose during spontaneous alternation tests of memory is largely attenuated by peripheral injections of glucose that enhance memory. For example, injections of a memory-enhancing dose of glucose prior to spontaneous alternation testing blocks the depletion of glucose otherwise seen during memory testing in both young adult and senescent rats, while also enhancing memory scores (McNay and Gold, 1999, 2001; McNay et al., 2000). Thus, the efficacy of glucose in enhancing memory seems to be accomplished through replenishment. In this model, glucose depletion leads to suboptimal processing that can be restored by exogenous glucose. In aged rats, the depletion of hippocampal glucose is more extreme, coincident with an absence of a rise in blood glucose levels in aged rats as compared to that seen in young rats and coincident also with memory impairments in the alternation task (McNay and Gold, 2001). In aged rats, peripheral injections of glucose restore memory scores to those seen in young rats and block the depletion of glucose during behavioral testing (McNay and Gold, 2001; Morris et al., 2010; Morris and Gold, 2013).
Although blood glucose levels can enhance memory while replenishing and maintaining brain glucose levels near baseline levels even during memory testing, it is not necessary for blood glucose per se to increase to see the effects on memory. Direct injections of glucose into the hippocampus, striatum and amygdala have all been shown to enhance memory as well, generally for those tasks canonically associated with these brain regions (Schroeder and Packard, 2003; Ragozzino et al., 1996, 1998; Stefani and Gold, 2001; Krebs and Parent, 2005; Pych et al., 2006; Morris and Gold, 2013).
Neurochemical bases of glucose enhancement of memory
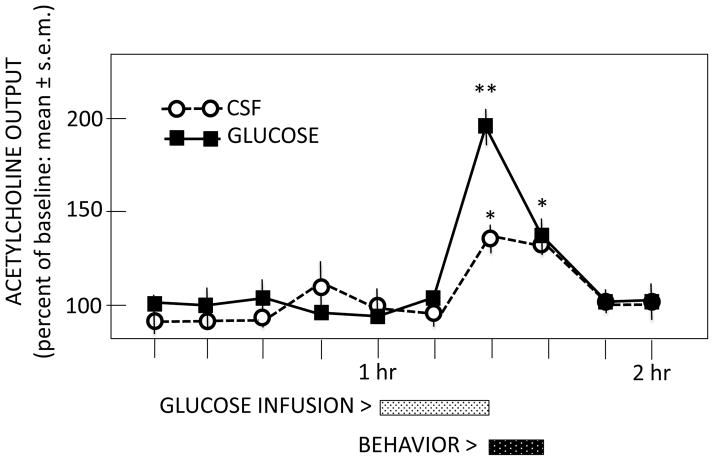
There is considerable evidence that acetylcholine release contributes to a wide range of learning and memory categories, regulating the participation of multiple neural systems during cognitive processing (Gold et al., 2013). The examination of glucose effects on acetylcholine release has been assessed in the hippocampus in relation to both spontaneous alternation and inhibitory avoidance training. During spontaneous alternation tests, the magnitude of training-related release of acetylcholine is augmented when glucose is administered peripherally or directly into the hippocampus to enhance memory (Ragozzino et al., 1998) (Figure 5), apparently supplementing available glucose within the hippocampus. Systemic injections of glucose also enhance acetylcholine release in the hippocampus when rats are trained on a one-trial inhibitory avoidance task (Morris et al., 2010). Of interest, glucose augments training-initiated increases in acetylcholine release in the hippocampus in both young and old rats but epinephrine, which does not increase blood glucose levels in old rats, augments acetylcholine release in young rats but is less effective in aged rats. These findings once again provide evidence that glucose may be an important mediator of epinephrine effects on memory, with the possible participation of acetylcholine release in those effects. The possibility that these effects extend to other neurotransmitters implicated in memory processing is understudied.
Figure 5.
Effects of intrahippocampal glucose infusions on release of acetylcholine in the hippocampus before, during, and after spontaneous alternation testing. Note that spontaneous alternation testing resulted in increases in acetylcholine release (open circles). Intrahippocampal infusions of glucose augmented the magnitude of that release during memory testing. (From Ragozzino et al., 1998.)
Recent findings open the intriguing possibility that glucose effects on learning and memory processing may be mediated by control of metabolic substrates by astrocytes (cf. Bélanger et al., 2011; Magistretti, 2006; Pellerin et al., 2007; Gibbs et al., 2008; Pirttimaki and Parri, 2013; Bezzi and Volterra, 2011; Gold and Korol, 2012; Gold et al., 2013). These findings support a sequence by which glucose is taken into astrocytes for production of glycogen stores, with glycogenolysis and production of lactate initiated during learning by activation of neurotransmitter membrane receptors on astrocytes. This function of astrocytes may thereby link glucose effects on memory with astrocytic provision of lactate to neurons as an additional energy substrate.
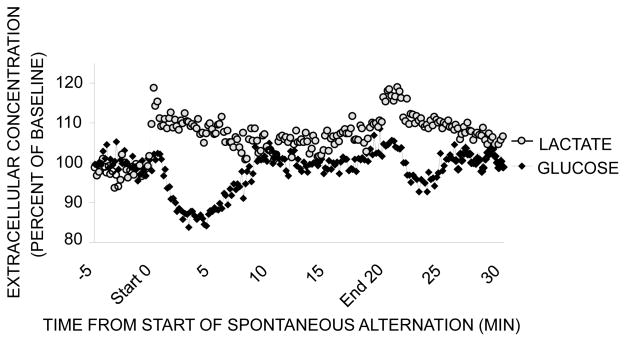
Brain astrocytes store a modest level of glycogen, which can be metabolized to lactate and provided to neurons to supplement glucose in meeting the energy demands of neurons when activated (Magistretti et al., 1999). Perhaps important toward understanding the mechanisms of glucose effects on memory, brain activation is accompanied by an increase in glucose uptake largely into astrocytes rather than into neurons (Chuquet et al., 2010). While glycogen-derived lactate cannot sustain brain activity for long in the absence of glucose provisions, glycogenolysis and production of lactate appear to be very important regulators of learning and memory processing by providing a rapid and short-term supplement of lactate as an energy supplement to neurons (Suzuki et al., 2011; Newman et al., 2011; Gibbs et al., 2006, 2008). Newman et al. (2011) assessed glucose and lactate levels in the hippocampus using bioprobes with sampling periods of 1 sec, much faster than the 5 min or so needed for microdialysis in past experiments of this type (Figure 6). During spontaneous alternation testing of working memory in rats, extracellular lactate levels in the hippocampus increased in response to decreasing glucose levels. The importance of the lactate to memory processing has been demonstrated by pharmacological and genetic interventions with glycogenolysis to lactate, lactate administration, and blockade of lactate transporters (Suzuki et al., 2011; Newman et al., 2011; Gibbs et al., 2006, 2008).
Figure 6.
Effects of spontaneous alternation testing on extracellular glucose (black diamonds) and lactate (gray circles) levels in the hippocampus. These levels were measured using bioprobes with 1-sec sampling periods, summarized here as 10-sec sampling epochs. Lactate in particular is largely provided to extracellular fluid and then to neurons from astrocytes after metabolism of glucose via glycolysis and/or breakdown of glycogen stores. Note first that glucose levels decreased during alternation testing, as shown before with microdialysis methods. Note also that lactate levels increased during testing, mirroring the changes in glucose. Not shown here, pharmacological manipulations that block and restore lactate availability impair and enhance memory, respectively. (From Newman et al., 2011.)
The mechanisms responsible for triggering glycogen breakdown and lactate production in the context of learning and memory studies are not yet clear in rats. However, in the chick model, it appears that adrenergic receptors on astrocytes may be especially important (Gibbs, 2008). Astrocytes have membrane receptors for many neurotransmitters, raising the possibility that norepinephrine modulation of memory, as well as modulation with other neurotransmitters including acetylcholine, may be mediated by receptors on the astrocytes rather than, or in addition to, receptors on neurons. The incorporation of astrocytes and energy provisions into the mechanisms regulating learning and memory processing may include several points of action for glucose: establishment of glycogen stores, lactate production by glycolysis, and augmentation of the release of neurotransmitters that activate astrocytic glycogenolysis to provide energy on demand (Magistretti et al., 1999).
CONCLUSIONS
The findings across many experiments show that physiological regulation of memory processing in the brain requires participation of not only central but also peripheral functions. In particular, epinephrine released from the adrenal medulla is a very important hormonal modulator of memory, with actions that can enhance or impair memory in an inverted-U dose-response manner. The evidence reviewed here suggest that epinephrine effects on memory may be mediated in large part by epinephrine actions at the level of the liver to initiate the metabolism of hepatic glycogen stores and an increase in blood glucose levels. Increases in blood glucose, in turn, can act directly on the brain to provide additional metabolic substrates to neurons. The recent findings involving astrocytes in this mechanism suggest that activation involves glucose transport first into astrocytes, generating lactate from glycogen, which can then shuttle to neurons to meet energy demands of cognitive functions (cf. Pellerin et al., 2007; Magistretti, 2006; Bélanger et al., 2011). The involvement of astrocytes in these mechanisms suggests that some modulators of memory may act on astrocyte receptors rather than on neuronal receptors (Bekar et al., 2008), a possibility that needs attention in the future. Furthermore, in addition to regulating neuronal plasticity, the astrocytes themselves may exhibit plasticity (Pirttimaki and Parri, 2013), which may well impact the contributions of astrocytes to learning and memory processing. The involvement of the sympathetic nervous system and the adrenomedullary hormone, epinephrine, the liver, the circulatory system, the blood-brain barrier uptake of glucose into brain, and astrocytes as well as neurons in the modulation of learning and memory processing, make it clear that these cognitive functions are regulated by the integrative physiology of multiple organ systems.
Figure 1.
Enhancement of working memory in a spontaneous alternation task by L- and D-glucose, an artificial and natural sugar, respectively. In rats, a full dose-response curve for both sugars revealed optimal enhancement of memory at 3000 and 250 mg/kg for L- and D-glucose. Left graph: Enhancement of alternation scores with both sugars in rats that had undergone sham surgery for vagotomy. Right graph: Vagotomy blocked the enhancement of memory produced by L-glucose but did not block enhancement produced by the natural D-glucose. (GLU = glucose) (From Talley et al., 2002.)
Highlights.
Learning and memory are regulated in part by epinephrine (EPI) responses to experience
EPI does not readily enter the brain but controls energy substrates available for brain function
EPI initiates increases in blood glucose, which acts centrally to enhance memory
Glucose acts centrally to generate lactate in support of memory processing
Regulating memory involves integrative physiology of many organ systems besides brain
Acknowledgments
Research described here was supported by NIA R01 AG07648, NIDA DA024129, NSF IOS 08-43175 and 13-18490, the Syracuse University Center for Aging and Policy Studies (NIA P30 AG034464), and by a grant from the Alzheimer’s Association.
Footnotes
Research described here was supported by NIA R01 AG07648, NIDA DA024129, NSF IOS 08-43175 and 13-18490, the Syracuse University Center for Aging and Policy Studies (NIA P30 AG034464), and by a grant from the Alzheimer’s Association.
Conflict of Interest Statement
The author declares that there are no conflicts of interest regarding financial, personal, or organizational relationships that could inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Weil-Malherbe H, Tomchick R. The physiological disposition of H3-epinephrine and its metabolite metanephrine. J Pharm Exp Ther. 1959;127:251–256. [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic–mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis-and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Benton D, Ruffin MP, Lassel T, Nabb S, Messaoudi M, Vinoy S, Desor D, Lang V. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacol. 2003;166:86–90. doi: 10.1007/s00213-002-1334-5. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonom Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonom Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Volterra A. Astrocytes: powering memory. Cell. 2011;144:644–645. doi: 10.1016/j.cell.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Picq C, Sinniger V, Mayol JF, Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Braun LD, Miller LP, Pardridge WM, Oldendorf WH. Kinetics of regional blood-brain barrier glucose transport and cerebral blood flow determined with the carotid injection technique in conscious rats. J Neurochem. 1985;44:911–915. doi: 10.1111/j.1471-4159.1985.tb12903.x. [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR. Schwann cell glycogen selectively supports myelinated axon function. Ann Neurol. 2012;72:406–418. doi: 10.1002/ana.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn Mem. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev. 2008;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Canal C, Stutz SJ, Gold PE. Glucose injections into the hippocampus or striatum of rats prior to T-maze training: modulation of learning rates and strategy selection. Learn Mem. 2005;12:367–374. doi: 10.1101/lm.88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Williams CL. Interactions between epinephrine, ascending vagal fibers, and central noradrenergic systems in modulating memory for emotionally arousing events. Front Behav Neurosci. 2012;6:35. doi: 10.3389/fnbeh.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci. 2010;30:15298–15303. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Williams CL. Noradrenergic receptor blockade of the NTS attenuates the mnemonic effects of epinephrine in an appetitive light–dark discrimination learning task. Neurobiol Learn Mem. 2000;74:135–145. doi: 10.1006/nlme.1999.3946. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthé RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonom Neurosci. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Daulatzai MA. Dysfunctional nucleus tractus dolitarius: Its crucial role in promoting neuropathogentic cascade of Alzheimer’s Dementia— a novel hypothesis. Neurochem Res. 2012;37:846–868. doi: 10.1007/s11064-011-0680-2. [DOI] [PubMed] [Google Scholar]
- de Almeida MA, Kapczinski FP, Izquierdo I. Memory modulation by post-training intraperitoneal, but not intracerebroventricular, administration of ACTH or epinephrine. Behav Neur Biol. 1983;39:277–283. doi: 10.1016/s0163-1047(83)90961-5. [DOI] [PubMed] [Google Scholar]
- De Bundel D, Smolders I, Yang R, Albiston AL, Michotte Y, Chai SY. Angiotensin IV and LVV-haemorphin 7 enhance spatial working memory in rats: effects on hippocampal glucose levels and blood flow. Neurobiol Learn Mem. 2009;92:19–26. doi: 10.1016/j.nlm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Dornelles A, de Lima MNM, Grazziotin M, Presti-Torres J, Garcia VA, Scalco FS, Roesler R, Schröder N. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88:137–142. doi: 10.1016/j.nlm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki JI, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG. Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res. 1993;604:225–231. doi: 10.1016/0006-8993(93)90373-u. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, Fillenz M. Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J Neurochem. 1992;59:2141–2147. doi: 10.1111/j.1471-4159.1992.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem. 1993;60:1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Effects of bombesin and gastrin-releasing peptide on memory processing. Brain Res. 1988;460:314–322. doi: 10.1016/0006-8993(88)90375-7. [DOI] [PubMed] [Google Scholar]
- Gibbs ME. Memory systems in the chick: regional and temporal control by noradrenaline. Brain Res Bull. 2008;76:170–182. doi: 10.1016/j.brainresbull.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32:927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose modulation of memory storage processing. Behav Neur Biol. 1986;45:342–349. doi: 10.1016/s0163-1047(86)80022-x. [DOI] [PubMed] [Google Scholar]
- Gold PE. Drug enhancement of memory in aged rodents and humans. In: Carroll ME, Overmier JB, editors. Animal research and human health: Advancing human welfare through behavioral science. Washington, DC: American Psychological Association; 2001. pp. 293–304. [Google Scholar]
- Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gold PE. The many faces of amnesia. Learning and Memory. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- Gold PE. Memory enhancing drugs. In: Eichenbaum H, Byrne J, editors. Memory Systems, vol. 3 of Learning and Memory: A Comprehensive Reference. Elsevier Science; Oxford: 2008. pp. 555–576. [Google Scholar]
- Gold PE, Korol DL. Making memories matter. Front Int Neurosci. 2012:6. doi: 10.3389/fnint.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, McGaugh JL. A single trace, two process view of memory storage processes. In: Deutsch D, Deutsch JA, editors. Short Term Memory. New York: Academic Press; 1975. pp. 355–390. [Google Scholar]
- Gold PE, McGaugh JL, Hankins LL, Rose RP, Vasquez BJ. Age dependent changes in retention in rats. Exp Aging Res. 1982;8:53–58. [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: From neurotransmitters to metabolic substrates. Hippocampus. 2013;23:1053–1065. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol. 1978a;23:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Effects of α- and β-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: Relationship to post-training brain norepinephrine concentrations. Behav, Biol. 1978b;24:168–184. doi: 10.1016/s0091-6773(78)93045-6. [DOI] [PubMed] [Google Scholar]
- Gold PE, Vogt J, Hall JL. Glucose effects on memory: behavioral and pharmacological characteristics. Behav Neur Biol. 1986;46:145–155. doi: 10.1016/s0163-1047(86)90626-6. [DOI] [PubMed] [Google Scholar]
- Gold PE, Wrenn SM. Cycloheximide impairs and enhances memory depending on dose and footshock intensity. Behav Brain Res. 2012;233:293–297. doi: 10.1016/j.bbr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Hall JL, Gold PE. The effects of training, epinephrine, and glucose injections on plasma glucose levels in rats. Behav Neur Biol. 1986;46:156–176. doi: 10.1016/s0163-1047(86)90640-0. [DOI] [PubMed] [Google Scholar]
- Hall JL, Gold PE. Plasma glucose levels predict the disrupting effects of adrenoceptor antagonists on enhancement of memory storage. Eur J Pharmacol. 1992;221:365–370. doi: 10.1016/0014-2999(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Annals Neurol. 1980;8:1–11. doi: 10.1002/ana.410080102. [DOI] [PubMed] [Google Scholar]
- Horn JP, McAfee DA. Modulation of cyclic nucleotide levels in peripheral nerve without effect on resting or compound action potentials. J Physiol. 1977;269:753–766. doi: 10.1113/jphysiol.1977.sp011927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Vianna MR, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Epinephrine modulates long-term retention of an aversively motivated discrimination. Behav Neur Biol. 1986;45:358–365. doi: 10.1016/s0163-1047(86)80024-3. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Naloxone and beta-endorphin alter the effects of post-training epinephrine on memory. Psychopharmacol. 1987;92:229–235. doi: 10.1007/BF00177921. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-h. [DOI] [PubMed] [Google Scholar]
- Itoh S, Lal H. Influences of cholecystokinin and analogues on memory processes. Drug Devel Res. 1990;21:257–276. [Google Scholar]
- Iwasaki Y, Yada T. Vagal afferents sense meal-associated gastrointestinal and pancreatic hormones: Mechanism and physiological role. Neuropeptides. 2012;46:291–297. doi: 10.1016/j.npep.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. The role of an endogenous amnesic mechanism mediated by brain beta-endorphin in memory modulation. Brazilian J Med Biol Res. 1982;15:119–134. [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kaneto A, Miki E, Kosaka K. Effects of vagal stimulation on glucagon and insulin secretion. Endocrinol. 1974;95:1005–1010. doi: 10.1210/endo-95-4-1005. [DOI] [PubMed] [Google Scholar]
- Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutrition. 2001;74:687–693. doi: 10.1093/ajcn/74.5.687. [DOI] [PubMed] [Google Scholar]
- Kopf SR, Baratti CM. Effects of posttraining administration of glucose on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem. 1996;65:253–260. doi: 10.1006/nlme.1996.0030. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Modulation of learning and memory by adrenal and ovarian hormones. In: Kesner RP, Martinez JL, editors. Neurobiology of Learning and Memory. Elsevier Science; NY: 2007. pp. 243–268. [Google Scholar]
- Krahl SE, Senanayake SS, Handforth A. Seizure suppression by systemic epinephrine is mediated by the vagus nerve. Epilepsy Res. 2000;38:171–175. doi: 10.1016/s0920-1211(99)00089-3. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Parent MB. The enhancing effects of hippocampal infusions of glucose are not restricted to spatial working memory. Neurobiol Learn Mem. 2005;83:168–172. doi: 10.1016/j.nlm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Watkins D, Jarrott B. Visualization of beta-adrenoceptor binding sites on human inferior vagal ganglia and their axonal transport along the rat vagus nerve. J Hypertension. 1995;13:631–635. doi: 10.1097/00004872-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine and glucose responses of F-344 rats to a single footshock as used in inhibitory avoidance training. Neurobiol Learn Mem. 1995;64:146–155. doi: 10.1006/nlme.1995.1054. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron–glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Glutamatergic inputs to the CVLM independent of the NTS promote tonic inhibition of sympathetic vasomotor tone in rats. Am J Physiol -Heart Circ Physiol. 2008;295:H1772–H1779. doi: 10.1152/ajpheart.216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CA, Hall JL, Gold PE. Glucose effects on memory and other neuropsychological tests in elderly humans. Psychol Sci. 1990;1:307–311. [Google Scholar]
- Manning CA, Ragozzino M, Gold PE. Glucose enhancement of memory in patients with Alzheimer's disease. Neurobiol Aging. 1993;14:523–528. doi: 10.1016/0197-4580(93)90034-9. [DOI] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Blier P. Novel attempts to optimize vagus nerve stimulation parameters on serotonin neuronal firing activity in the rat brain. Brain Stim. 2012;5:422–429. doi: 10.1016/j.brs.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis Defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty R, Gold PE. Plasma catecholamines: Effects of footshock level and hormonal modulators of memory storage. Horm Behav. 1981;15:168–182. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci. 2013;110 (Supplement 2):10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Petrinovich LF. Effects of drugs on learning and memory. Int Rev Neurobiol. 1965;8:139–196. doi: 10.1016/s0074-7742(08)60757-6. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36:1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: Effects of microdialysis flow rate, strain and age. J Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol: Biol Sci. 2001;56A:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- McNay EC, McCarty RM, Gold PE. Fluctuations in glucose concentration during behavioral testing: Dissociations both between brain areas and between brain and blood. Neurobiol Learn Mem. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C. Object recognition in mice: improvement of memory by glucose. Neurobiol Learn Mem. 1997;67:172–175. doi: 10.1006/nlme.1996.3755. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Messier C, Destrade C. Improvement of memory for an operant response by post-training glucose in mice. Behav Brain Res. 1988;31:185–191. doi: 10.1016/0166-4328(88)90022-8. [DOI] [PubMed] [Google Scholar]
- Messier C, Gagnon M, Knott V. Effect of glucose and peripheral glucose regulation on memory in the elderly. Neurobiol Aging. 1997;18:297–304. doi: 10.1016/s0197-4580(97)80311-9. [DOI] [PubMed] [Google Scholar]
- Messier C, White NM. Contingent and non-contingent actions of sucrose and saccharin reinforcers: effects on taste preference and memory. Physiol Behav. 1984;32:195–203. doi: 10.1016/0031-9384(84)90129-x. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Peripheral arousal-related hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral β-adrenergic receptors. Neurobiol Learn Mem. 2006;85:116–124. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiol Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, Gold PE. Epinephrine and glucose modulate training-related CREB phosphorylation in old rats: Relationships to age-related memory impairments. Exp Gerontol. 2013;48:115–127. doi: 10.1016/j.exger.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B. Possible involvement of the vagus nerve in monitoring plasma catecholamine levels. Neurobiol Learn Mem. 2006;86:353–355. doi: 10.1016/j.nlm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mravec B. Role of catecholamine-induced activation of vagal afferent pathways in regulation of sympathoadrenal system activity: negative feedback loop of stress response. Endocr Regul. 2011;45:37–41. [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PloS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol. 1982;332:315–323. doi: 10.1113/jphysiol.1982.sp014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira PJ, Tomaz C, Williams CL. Contribution of the vagus nerve in mediating the memory-facilitating effects of substance P. Behav Brain Res. 1994;62:165–169. doi: 10.1016/0166-4328(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Effect of posttraining injections of glucose on acquisition of two appetitive learning tasks. Psychobiol. 1990;18:282–286. [Google Scholar]
- Packard MG, Williams CL, Cahill L, McGaugh JL. The anatomy of a memory modulatory system: from periphery to brain. In: Spear N, Spear L, Woodruff M, editors. Neurobehavioral Plasticity: Learning, Development and Response to Brain Insults. New Jersey: Lawrence Erlbaum Assoc; 1995. pp. 149–184. [Google Scholar]
- Parsons M, Gold PE. Glucose enhancement of memory in elderly humans: An inverted-U dose-response curve. Neurobiol Aging. 1992;13:401–404. doi: 10.1016/0197-4580(92)90114-d. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-β1-42 oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alz Dis. 2012;30:413–422. doi: 10.3233/JAD-2012-112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pirttimaki TM, Parri HR. Astrocyte plasticity implications for synaptic and neuronal activity. Neuroscientist. 2013 doi: 10.1177/1073858413504999. [DOI] [PubMed] [Google Scholar]
- Popper CW, Chiueh CC, Kopin IJ. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977;202:144–148. [PubMed] [Google Scholar]
- Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behav Brain Res. 2006;167:373–378. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, De Quervain DF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav Brain Res. 2009;205:19–25. doi: 10.1016/j.bbr.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandusky LA, Flint RW, McNay EC. Elevated glucose metabolism in the amygdala during an inhibitory avoidance task. Behav Brain Res. 2013;245:83–87. doi: 10.1016/j.bbr.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96–RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey A, Macpherson H, Sünram-Lea S, Elliott J, Stough C, Kennedy D. Glucose enhancement of recognition memory: Differential effects on effortful processing but not aspects of 'remember- know' responses. Neuropharmacol. 2013;64:544–549. doi: 10.1016/j.neuropharm.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Schreurs J, Seelig T, Schulman H. β2-adrenergic receptors on peripheral nerves. J Neurochem. 1986;46:294–296. doi: 10.1111/j.1471-4159.1986.tb12961.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur J Neurosci. 2003;17:1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- Seelig TL, Grossman Y, Kendig JJ. Cyclic AMP reduction of frequency following ability in peripheral axons. Brain Res. 1983;279:303–307. doi: 10.1016/0006-8993(83)90198-1. [DOI] [PubMed] [Google Scholar]
- Smith MA, Riby LM, Eekelen JAM, Foster JK. Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci Biobehav Rev. 2011;35:770–783. doi: 10.1016/j.neubiorev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intra-hippocampal infusions of K-ATP channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. J Neurosci. 2001;21:609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DB, Isaacs KR, Gold PE, McGaugh JL. Epinephrine facilitation of appetitive learning: attenuation with adrenergic receptor antagonists. Behav Neur Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- Sternberg DB, Korol D, Novack GD, McGaugh JL. Epinephrine-induced memory facilitation: attenuation by adrenoceptor antagonists. Eur J Pharmacol. 1986;129:189–193. doi: 10.1016/0014-2999(86)90353-5. [DOI] [PubMed] [Google Scholar]
- Sternberg DB, Isaacs K, Gold PE, McGaugh JL. Epinephrine facilitation of appetitive learning: Attenuation with adrenergic receptor antagonists. Behav Neur Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- Stone WS, Rudd RJ, Gold PE. Glucose attenuation of scopolamine-and age-induced deficits in spontaneous alternation behavior and regional brain [–3H] 2-deoxyglucose uptake in mice. Psychobiol. 1992;20:270–279. [Google Scholar]
- Stone WS, Seidman LJ, Wojcik JD, Green AI. Glucose effects on cognition in schizophrenia. Schizo Res. 2003;62:93–103. doi: 10.1016/s0920-9964(02)00406-1. [DOI] [PubMed] [Google Scholar]
- Stone WS, Thermenos HW, Tarbox SI, Poldrack RA, Seidman LJ. Medial temporal and prefrontal lobe activation during verbal encoding following glucose ingestion in schizophrenia: a pilot fMRI study. Neurobiol Learn Mem. 2005;83:54–64. doi: 10.1016/j.nlm.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. The relation of adenosine-3', 5'-phosphate and phosphorylase to the actions of catecholamines and other hormones. Pharmacol Rev. 1960;12:265–299. [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerbowska-Boruchowska M, Krygowska-Wajs A, Ziomber A, Thor P, Wrobel P, Bukowczan M, Zizak I. The influence of electrical stimulation of vagus nerve on elemental composition of dopamine related brain structures in rats. Neurochem Int. 2012;61:156–165. doi: 10.1016/j.neuint.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Talley CP, Clayborn H, Jewel E, McCarty R, Gold PE. Vagotomy attenuates effects of L-glucose but not of D-glucose on spontaneous alternation performance. Physiol Behav. 2002;77:243–249. doi: 10.1016/s0031-9384(02)00850-8. [DOI] [PubMed] [Google Scholar]
- Talley CE, Kahn S, Alexander LJ, Gold PE. Epinephrine fails to enhance performance of food-deprived rats on a delayed spontaneous alternation task. Neurobiol Learn Mem. 2000;73:79–86. doi: 10.1006/nlme.1999.3920. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Inv. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Calandreau L, Herry C, Mons N, Micheau J. Biphasic ERK1/2 activation in both the hippocampus and amygdala may reveal a system consolidation of contextual fear memory. Neurobiol Learn Mem. 2007;88:424–434. doi: 10.1016/j.nlm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Watson G, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer's disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Weil-Malherbe H, Whitby LG, Axelrod J. The blood-brain barrier for catecholamines in different regions of the brain. Reg Neurochem. 1961:284–292. [Google Scholar]
- Welsh KA, Gold PE. Attenuation of epileptogenesis: proactive effect of a single epinephrine injection on amygdaloid kindling. Behav Neur Biol. 1984;40:179–185. doi: 10.1016/s0163-1047(84)90279-6. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Gold PE. Epinephrine proactive retardation of amygdala-kindled epileptogenesis. Behav Neurosci. 1986;100:236–245. doi: 10.1037//0735-7044.100.2.236. [DOI] [PubMed] [Google Scholar]
- Wenk GL. An hypothesis on the role of glucose in the mechanism of action of cognitive enhancers. Psychopharmacol. 1989;99:431–438. doi: 10.1007/BF00589888. [DOI] [PubMed] [Google Scholar]
- White NM. Peripheral and central memory-enhancing actions of glucose. In: Frederickson RCA, McGaugh JL, Felten DL, editors. Peripheral signaling of the brain: Role in neural-immune interactions and learning and memory. Toronto: Hogrefe & Huber; 1991. pp. 421–441. [Google Scholar]
- White NM, Messier C. Effects of adrenal demedullation on the conditioned emotional response and on the memory improving action of glucose. Behav Neurosci. 1988;102:499–503. doi: 10.1037//0735-7044.102.4.499. [DOI] [PubMed] [Google Scholar]
- Williams CL, Jensen RA. Effects of vagotomy on Leu-enkephalin-induced changes in memory storage processes. Physiol Behav. 1993;54:659–663. doi: 10.1016/0031-9384(93)90073-o. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Williams CL, Miyashita T. The role of peripheral adrenergic receptors in mediating epinephrine-induced changes in vagal nerve firing. Neurobiol Learn Mem. 2006;86:356–357. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Winocur G, Gagnon S. Glucose treatment attenuates spatial learning and memory deficits of aged rats on tests of hippocampal function. Neurobiol Aging. 1998;19:233–241. doi: 10.1016/s0197-4580(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, Carlezon WA, Jr, Kvetnansky R. Epinephrine: A short-and long-term regulator of stress and development of illness. Cell Molec Neurobiol. 2012;32:737–748. doi: 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- Zarbin MA, Palacios JM, Wamsley JK, Kuhar MJ. Axonal transport of beta-adrenergic receptors. Antero-and retrogradely transported receptors differ in agonist affinity and nucleotide sensitivity. Molec Pharmacol. 1983;24:341–348. [PubMed] [Google Scholar]