Abstract
Objective
To evaluate hypoglycemic and hypolipidemic effects of Salvadora persica aqueous extracts on streptozotocin-induced diabetic rats by measuring fasting blood glucose levels, lipid profiles and histopathological analysis of pancreas.
Materials and Methods
Experimental Diabetes was induced by single intraperitoneal injection of streptozotocin (60 mg/kg) to albino Wistar rats. Salvadora persica extracts were administered orally at 250 and 500 mg/kg dose levels for 21 days. Glucose tolerance test (GTT) was performed on 16 h fasted rats and changes in blood glucose levels, total cholesterol, triglycerides, low-density lipoprotein (LDL), very low density lipoprotein (VLDL), high density lipoprotein (HDL) and histopathology of pancreas were performed.
Results
At a dose level of 500 mg/kg, blood glucose 85.25 ± 13.20 mg/dl, total cholesterol (TC) 114.57 ± 15.81(mg/dl), triglycerides (TG) 75.40 ± 16.47(mg/dl), LDL 42.63 ± 13.17(mg/dl), VLDL 22.78 ± 1.88(mg/dl), and elevation of HDL 44.88 ± 11.61(mg/dl) were found in comparison with diabetic control on 28th day by Arabic origin Salvadora persica. It also accelerated the regeneration of β-cells in experimental animal’s pancreas to 32.6 ± 2.4 compared to diabetic control animal’s pancreas of 8.1 ± 0.5 at the end of 28th day.
Conclusion
This study confirmed that Arabic Salvadora persica aqueous extracts at 500 mg/kg dose level, in comparison to other extracts (Indian Salvadora persica, 250 and 500 mg/kg, Arabic Salvadora persica 250 mg/kg) possessed significant hypoglycemic and hypolipidemic activities and regenerated pancreatic β-cells in streptozotocin treated diabetic rats.
Keywords: Salvadora persica, geo-comparison, antidiabetic activity, hypoglycemic activity, serum lipid profile, glucose tolerance test, histopathology, pancreatic β-cells,
Introduction
Traditionally, many herbal preparations have been used for the treatment of diabetes as alternative medicines, Miswak being one of the oldest. (1) Miswak is a product of Salvadora persica L. (Salvadoraceae) which grows in different areas of the world and occurs as a shrub from northwestern India to Africa, especially used in Saudi Arabia as a sunnah of the prophet. (2) Miswak, also known as tooth brush tree, is 4–6 m tall with a short trunk, white bark and smooth green leaves, with a life span of 25 years and has been used as a brushing stick for more than 1,300 years. (3) (4) The history and use of miswak (tooth stick) as an oral and dental cleaning tool as well as its biological effects have been reviewed. (5) (6)
The roots have been mainly used as a weak anti-inflammatory agent. (7) Leaves are prescribed to treat cough, asthma, scurvy, rheumatism like symptoms, and other common disorders. (8) The bark is scratched and the latex obtained is applied to heal sores. (9) Pharmacological studies showed that Salvadora persica plant possessed antimicrobial, antiplaque, aphrodisiac, alexiteric, antipyretic, astringent, diuretic, stomachic activities. It has great medicinal uses by herbalists and common man in treatment of nose troubles, piles, scabies, leucoderma, gonorrhea, boils, toothache, hook worm, venereal diseases, to lower cholesterol plasma levels, re-establishment of the components of gastric mucosa, and as a laxative. (10) (11) Salvadora persica extract contains several organo-sulphur compounds and it is well documented that certain sulphur derivatives show hypoglycemic effect. Many plants containing organo-sulphur compounds are used traditionally as potent hypoglycemic for symptoms related to the condition. (12)
Trovato et al. observed hypoglycemic effect, an increase in plasma immunoreactive insulin (IRI) and an incremented oral-glucose tolerance in normal rats with significant decrease in mean body weights, when treated with a stem decoction of Salvadora persica. (13) According to Trovato et al. the ability of stem decoction to improve the utilization of glucose following a glucose load, may indicate the possible mechanism of hypoglycemic activity of this plant decoction, which further may facilitate peripheral utilization of the glucose, either by direct stimulation of glucose uptake or by enhanced insulin secretion. Trovato et al. used alloxan to induce experimental diabetes to the albino Wistar rats and no comparison with a standard anti-diabetic drug was carried out.
The present study confirmed the hypoglycemic and hypolipidemic activities of Salvadora persica obtained from two distinct geographical sources, i.e., Saudi Arabia and India, in streptozotocin (STZ) induced diabetic Wistar rats observed over 4 weeks. This study also includes the histopathological findings on control and treated experimental rats. Glibenclimide was used as a standard drug.
Materials and methods
Animals
Male albino Wistar rats (150–200 g) were used. The animals were housed in macrolon cages under standard laboratory conditions (12h light/12h darkness cycle, 21 ± 2°C room temperature). The animals were given standard pellets diet (Lipton Rat Feed, Ltd., Pune, India) and unrestricted water ad libitum throughout the experimental period. The animal study was approved by the Institutional Animal Ethical Committee (IAEC) 173/CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) of Jamia Hamdard, New Delhi, on 25th of March 2010.
Preparation of plant extract
Salvadora persica roots for Indian sample were procured one week before the study from local herbal drugs market at Khari Baoli, Delhi, India and the roots of Salvadora persica for Arabic origin sample were obtained from sources in, Medina Munawwarah, K.S.A. The identity of the plant material was confirmed by bibliographic data and authenticated by a plant Taxonomist (8) (14) Department of Botany, Faculty of Science, Jamia Hamdard, New Delhi (voucher number PRD/JH/08/56). The finely cut air-dried roots (500 g) were extracted with hot water (100° C) in a Soxhlet apparatus for 6 h each. The extracts were evaporated to dryness under reduced pressure and temperature below 45°C. The dried extracts were stored at 0–4°C for further use.
Chemicals
Various biochemical estimations in blood viz. fasting blood glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), very low density lipoprotein (VLDL) and high density lipoprotein (HDL) were carried out by using commercially available kits (Sigma-Aldrich, USA) as per manufacturer’s instruction. Streptozotocin was obtained from (Sigma-Aldrich, USA). All the other chemicals used were of analytical grade.
Induction of diabetes
Streptozotocin (STZ) was dissolved in freshly prepared 0.1 M cold citrate buffer (pH 4.5) and administered through injection intraperitoneal (i.p.) route (60 mg/kg) to the overnight fasted animals. (15) After 6 h of administering STZ injection, rats received 5% dextrose solution for the next 24 h to prevent STZ induced fatal hypoglycemia as a result of massive pancreatic insulin release after its administration. (16) Diabetes was confirmed 72 h after STZ administration by measuring blood glucose level using glucose meter (Ames One Touch Glucometer, Accu Check Roche, Germany). Further development of polydipsia and polyuria confirmed diabetes. The blood glucose level was again determined on day 7th and animals with a blood glucose level higher than 250 mg/dl were selected for further study.
Experimental design
The rats were randomized into seven groups comprising of six animals in each group. The aqueous extracts of Indian origin Salvadora persica (ISpAq) and Arabic origin Salvadora persica (ASpAq) (250 and 500 mg/kg dose levels) were administered orally (p.o.) as aqueous suspensions (3% v/v with Tween 80 in water) once per day.
Group 1: Normal control rats received vehicle (3% v/v Tween 80 in water), (1 ml/kg, i.p.).
Group 2: Diabetic control rats, received STZ in single dose (60 mg/kg, i.p.).
Group 3: ASpAq treated diabetic rats (250 mg/kg/day, p.o.), 7 days after STZ (60 mg/kg) treatment and continued for 21 days.
Group 4: ASpAq treated diabetic rats (500 mg/kg/day, p.o.), 7 days after STZ (60 mg/kg) treatment and continued for 21 days.
Group 5: ISpAq treated diabetic rats (250 mg/kg/day, p.o.), 7 days after STZ treatment (60 mg/kg) and continued for 21 days.
Group 6: ISpAq treated diabetic rats (500 mg/kg/day, p.o.), 7 days after STZ treatment (60 mg/kg) and continued for 21 days.
Group7: Diabetic rats were treated with Glibenclamide (5 mg/kg/day, p.o.), 7 days after STZ treatment (60 mg/kg) and continued for 21 days.
ISpAq and ASpAq, standard drug Glibenclamide (5 mg/kg) and vehicle were administered orally with the help of feeding cannula. On the last day of experiment, blood samples were collected for biochemical estimations and, the animals were sacrificed, pancreas removed, cleaned and washed in ice-cold normal saline for histological studies.(17)
Glucose tolerance test
Intraperitoneal glucose tolerance test (GTT) was performed on 16 h fasted rats using 2 g glucose/kg body weight. For all groups, the blood samples were collected from animals at 0, 30, 90, and 120 min after glucose load at the end of 21 days (GTT TESTS ONLY AFTER 21 DAYS FOR EACH GROUP?) of drug treatment.
Histological examination preparation
After sacrificing the animals receiving 500 mg/kg of both ASpAq and ISpAq extract samples (as pronounced results were obtained at 500 mg/kg than 250 mg/kg), the pancreas were removed, washed with normal saline and part of, cleared tissue was fixed in 10% natural buffered solution (pH 7.0–7.2). After proper fixation, the tissue was processed for dehydration in ascending grade of ethanol, clearing with toluene, followed by impregnation in paraffin wax, and then sections of 5 μm in thickness were cut with the help of semiautomatic rotary microtome. The histopathological examination and grading were carried out on chromealum hematoxiline-phloxine stained sections at 5 μm thickness of tissue with 30 μm distances used for morphometric analysis. (18)
The number of islets and the number of β-cells of each islet were counted by Olympus BX-51T-32E01 research microscope connected to DP 12 Camera with 3.34 million pixel resolution and Olysia Bio software (Olympus Optical Co. LTD, Tokyo, Japan).
Biochemical estimations
Blood glucose level was estimated by Ames One Touch Glucometer (Accu Check Roche, Germany). Serum TC, LDL, VLDL and HDL were estimated at the end of 4th week by using standard enzymatic colorimetric kits (Span diagnostics Ltd. Surat, India). (19) (20) (21)
Statistical analysis
Data were expressed as mean ± SEM and statistical analysis was carried out using Graph Pad Prism, V 5.02, San Diego, CA, USA software. The means of data were compared with an analysis of variance (ANOVA) followed by Dunnett’s t-test. All the values were considered statistically significant when p<0.05.
Results
Glucose tolerance test
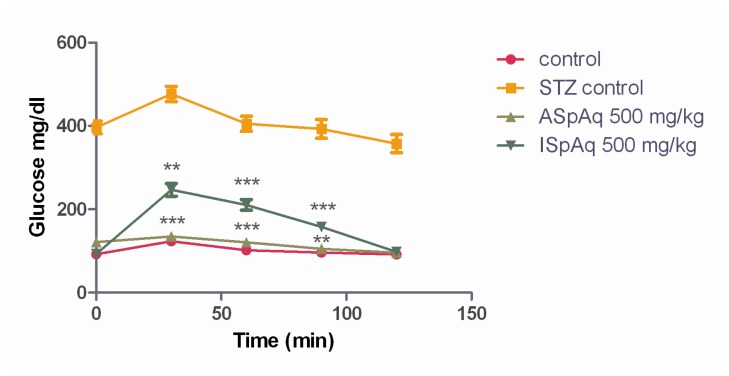
At the end of 120 min GTT results has shown the glucose concentration in normal control, STZ control, with ISpAq and AspAq extracts at a dose of 500 mg/kg viz 91.9 ± 2.9, 357.4 ± 21.6 mg/dl and 97.7 ± 4.3, 95.6 ± 3.5 mg/dl that were found to be significant with STZ control (Figure 1).
Figure (1).
Glucose tolerance test for control, STZ, ASpAq and ISpAq at 500 mg/kg dose. Values are given in mean ± SD, and n=6.
Effect of Arabic and Indian origin Salvadora persica aqueous extracts on hyperglycemia
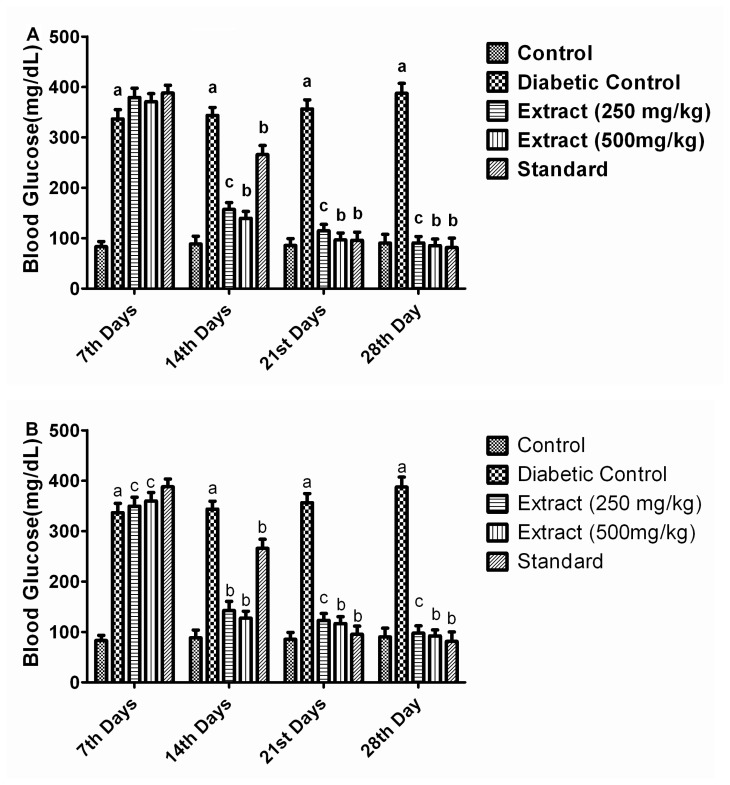
In all the groups, prior to STZ administration, basal level of blood glucose was nearly same for any statistical implication. However, there was a significant elevation in glucose levels 72 h after the administration of STZ. Although a significant hypoglycemic effect was evident from the first week onwards, decrease in blood glucose levels was more prominent in the third week in groups receiving ISpAq and ASpAq (250 and 500 mg/kg). After STZ administration, increase in blood glucose was observed in diabetic control rats (Group 2) when compared with normal control rats (Group 1). The administration of ISpAq and ASpAq at 500 mg/kg (Groups 4 and 6) decreased the glucose levels in diabetic rats as compared with diabetic control rats (Group 2). The administration of ISpAq and ASpAq at 250 mg/kg (Groups 3 and 5) decreased the glucose levels in diabetic rats as compared to diabetic control rats. Thus, the levels of blood glucose returned to near normal range in diabetic rats treated with ISpAq and ASpAq (250 and 500 mg/kg) by the end of the 4th week.
The animals treated with the Arabic extract, ASpAq, have shown the glucose level (mg/dl) in the groups as: Control, Diabetic control, ASpAq 250 mg/kg, ASpAq 500 mg/kg and standard Glibenclamide at 83.16 ± 10.16, 336.65 ± 18.41, 379±18.71, 370.83 ± 15.95, 388 ± 15.31 on the 7th day; whereas on 14th day it was 88.32 ± 15.60,343.67 ± 15.71, 157.17 ± 13.29, 139.50 ± 13.73, 265.83 ± 18.21; on 21st day 85.45 ± 13.78, 356.57 ± 18.11, 114.67 ± 12.71, 96.50 ± 13.78, 95.83 ± 16.34, and on the 28th day the level was found to be 90.23 ± 17.45, 387.50 ±19.76, 90.53 ± 12.84 (P < 0.05), 85.25 ± 13.20 (P < 0.01), 81.35 ± 18.67 (P < 0.01). Whereas the animals treated with the Indian extract, ISpAq, has shown the glucose level (mg/dl) in the groups as: Control, Diabetic control, ISpAq 250 mg/kg, ISpAq 500 mg/kg and standard Glibenclamide at 83.16 ± 10.16, 336.65 ± 18.41, 349.45 ± 17.71, 359.73 ± 16.75, 388 ± 15.31 on the seventh day; whereas on 14th day 88.32 ± 15.60, 343.67 ± 15.71, 142.73 ± 17.88, 127.50 ± 13.73, 265.83 ± 18.21;on 21st day 85.45 ± 13.78, 356.57 ± 18.11, 123.21 ± 13.71, 116.65 ± 13.78, 95.83 ± 16.34, and on 28th day the level was found to be 90.23 ± 17.45, 387.50 ± 19.76, 97.83 ± 14.64 (P < 0.05), 92.33 ± 11.70(P < 0.01), 81.35 ± 18.67 (P < 0.05) (All other significant comparisons have been illustrated in Figure 2).
Figure (2).
A. Effect of Arabic origin aqueous extract (ASpAq) of Salvadora persica on hyperglycemia. B. Effect of Indian origin aqueous extract (ISpAq) of Salvadora persica on hyperglycemia. aP<0.001 diabetic control compared with control group; bP<0.001 compared with diabetic control group; cP<0.05 compared with diabetic control group; Values are given in mean ± SEM, and n=6.
Effect of ISpAq and ASpAq on hyperlipidemia
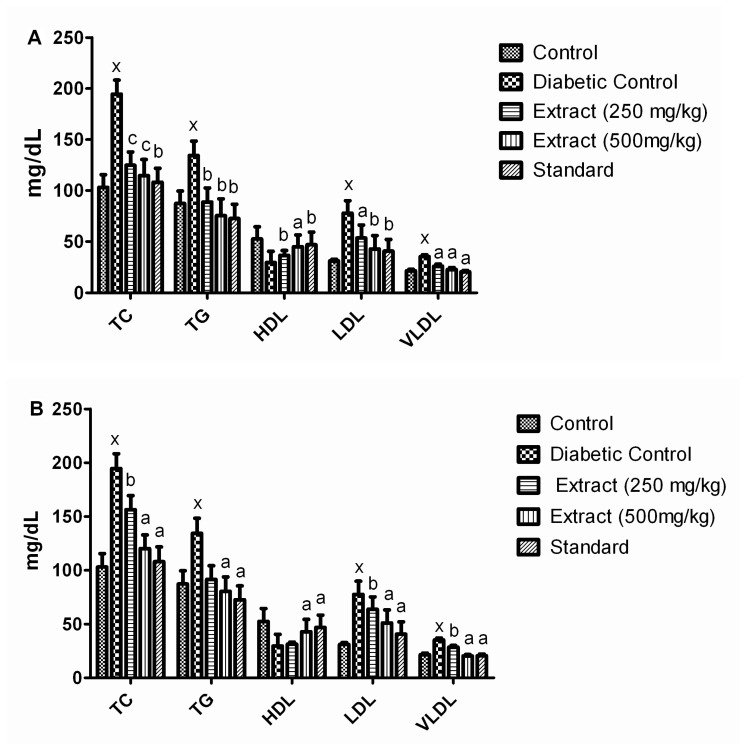
In STZ-induced diabetic rats, TC, TG, LDL and VLDL levels were increased and HDL level was decreased as compared to normal control rats. In diabetic rats, administration of ISpAq 250 mg/kg (P<0.01) and ASpAq 500 mg/kg (P<0.001) dose showed reduction in TC, TG, LDL and VLDL levels as compared to diabetic control rats. Increased level of HDL was observed in diabetic rats treated with 250 and 500 mg/kg doses of ISpAq and ASpAq and diabetic rats treated with Glibenclamide as compared to diabetic control rats.
The animals treated with the Arabic extract of Salvadora persica have shown serum lipid profile in normal control, diabetic control, ASpAq 250 mg/kg, ASpAq 500 mg/kg and standard Glibenclamide as: for TC 103.13 ± 12.39, 194.50 ± 13.82, 124.77 ± 13.05, 114.57 ± 15.81, 108.10 ± 13.62; for TG 87.34 ± 12.17, 134.30 ± 14.13, 88.73 ± 13.81, 75.40 ± 16.47, 72.63 ± 3.86; for HDL 52.60 ± 11.86, 29.53 ± 11.02, 36.38 ± 4.92, 44.88 ± 11.61, 46.88 ± 12.40; for LDL 31.02 ± 1.81, 77.72 ± 12.25, 53.57 ± 12.62, 42.63 ± 13.17, 40.69 ± 11.41; for VLDL 21.55 ± 1.43, 35.34 ± 1.64, 25.94 ± 1.96, 22.78 ± 1.88, 20.52 ± 1.40. Whereas animals treated with the Indian origin extract have shown serum lipid profile in normal control, diabetic control, ISpAq 250 mg/kg, ISpAq 500 mg/kg and standard Glibenclamide as: for TC 103.13 ± 12.39, 194.50 ± 13.82, 156.37 ± 13.05, 120.15 ± 12.81, 108.10 ± 13.62; for TG 87.34 ± 12.17, 134.30 ± 14.13, 91.54 ± 12.71, 80.40 ± 13.41,72.63 ± 3.86; for HDL 52.60 ± 11.86, 29.53 ± 11.02, 31.33 ± 1.92, 42.85 ± 11.61, 46.88 ± 12.40; for LDL 31.02 ± 1.81, 77.72 ± 12.25, 63.77 ± 11.61, 51.03 ± 12.14, 40.69 ± 11.41; for VLDL 21.55± 1.43, 35.34 ± 1.64, 28.62 ± 1.54, 20.31 ± 1.40, 20.52 ± 1.40.
Effect of ISpAq and ASpAq on β cell of pancreas (histopathology)
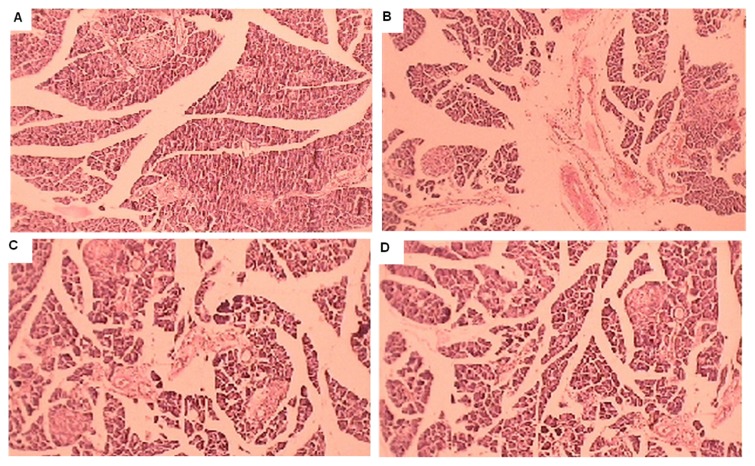
Histopathological studies showed normal acini and cellular populations in the pancreatic islets of control rats (Group 1). Extensive damage to the islets of Langerhans and reduced dimensions of islets to diabetic control (Group 2), restoration of normal cellular population size of islets with hyperplasia by Glibenclamide (Group 7) were observed thus resulting in lowering of blood glucose caused by increase secretion of insulin in Group 7. The partial restoration of normal cellular population and enlarged size of β-cells with hyperplasia were shown by aqueous Salvadora persica extracts (Group 3, 4, 5 and 6) (Table-1), Figure 4A, 4B, 4C, 4D.
Table (1).
Effect of Indian origin aqueous extract (ISpAq) and Arabic origin aqueous extract (ASpAq) of Salvadora persica on hyperglycemia (mg/dl).
| Arabic Origin, ASpAq | Indian Origin, ISpAq | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Control | Diabetic Control | Extract (250 mg/kg) | Extract (500mg/kg) | Extract (250 mg/kg) | Extract (500mg/kg) | Standard | |
| 7th Day | 83.16 ± 10.16 | 336.65 ± 18.41a | 379.00 ± 18.71 | 370.83 ± 15.95 | 349.45 ± 17.71c | 359.73 ± 16.75c | 388.00 ± 15.31 |
| 14th Day | 88.32 ± 15.60 | 343.67 ± 15.71a | 157.17 ± 13.29c | 139.50 ± 13.73b | 142.73 ± 17.88b | 127.50 ± 13.73b | 265.83 ± 18.21b |
| 21st Day | 85.45 ± 13.78 | 356.57 ± 18.11a | 114.67 ± 12.71c | 96.50 ± 13.78b | 123.21 ± 13.71c | 116.65 ± 13.78b | 95.83 ± 16.34b |
| 28th Day | 90.23 ± 17.45 | 387.50 ± 19.76a | 90.53 ± 12.84c | 85.25 ± 13.20b | 97.83 ± 14.64c | 92.33 ± 11.70b | 81.35 ± 18.67b |
P<0.001 diabetic control compared with control group;
P<0.001 compared with diabetic control group;
P<0.05 compared with diabetic control group;
Values are given in mean ± SEM, and n = 6.
Figure (4).
Effect of Arabic origin Aqueous extract (ASpAq) and Indian origin Aqueous extract (ISpAq) of Salvadora persica on β cell of pancreas (histopathology). A Normal control. B. β-cell destruction of Diabetic control. C. β-cell regeneration of ISpAq (500mg/kg) group. D. β-cell regeneration of ASpAq (500mg/kg) group.
Discussion
Currently, diabetes is controlled by a handful of available drugs such as oral hypoglycemic agents and insulin, but their use is going to be limited due to their own drawbacks like secondary failure of hypoglycemic drugs etc. To overcome the side effects or unwanted effects of synthetic drugs and hormones, there is a need to find safer and more effective antidiabetic drugs that can also take care of the associated disorders and can be used as maintenance therapy. Considerable research work is being carried out to find supplements to delay the onset and severity of the disease. An alternative cure, which could work through early to late stages of the disease, is needed as the effectiveness of any hypoglycemic drug in lowering blood glucose to a desired level decreases in many patients over a period of time. This may happen due to progression of the severity of diabetes or due to diminished responsiveness to the drug. This phenomenon is known as secondary failure.
The glucose tolerance of the ISpAq and ASpAq (500 mg/kg) showed that the serum glucose of treated animals significantly increased at 30 min, the serum glucose concentration was higher than at zero time but decreased significantly from 30 to 120 min for all the extract doses administered. Fasting plasma glucose (measured before the OGTT) for normal control and ASpAq (500mg/kg) were nearly below 6.1 mmol/L (110mg/dl) showing normal state and ISpAq (500mg/kg) had fasting plasma glucose between 6.1 and 7.0 mmol/L (110 and 125 mg/dl) thus showing borderline/impaired fasting glycaemia. The effect of ASpAq (500 mg/kg) on oral glucose tolerance is evidently better than ISpAq (500mg/kg) as shown in the graph (Figure 1).
The decrease in blood glucose is statistically significant in the first week of treatment and is more evident in the 3rd week of treatment with ISpAq and ASpAq (250 and 500 mg/kg). The decrease in blood glucose showed in a dose dependent manner, 500 mg/kg produced better results than 250 mg/kg and also showed a better response to ASpAq than ISpAq.
Administration of ISpAq and ASpAq extract for 21 days exhibited dose-dependent hypoglycemic effect in STZ-induced diabetic rats. This was found to be in contrast to the hypoglycemic activity reported by Trovato et al. (13) in which the Salvadora persica decoction was not capable of lowering blood glucose in alloxan-diabetic rats. The data of their study showed that Salvadora persica stem decoction at two doses lowered the plasma glucose and increased plasma insulin levels in normal and glucose loaded rats, in a dose-dependent fashion. These results indicated that the hypoglycemic activity of the decoction was mediated through an increase of plasma insulin levels. Since the decoction did not show a hypoglycemic effect in the alloxan-diabetic rats, they hypothesized that in the drug there were water-soluble active principles which could influence the mechanism to produce and/or liberate insulin from the β-cells of the pancreas, but no regenerative properties regarding β-cells were observed. (13)
The histopathological studies performed on STZ treated diabetic rats with ISpAq and ASpAq showed partial restoration of normal cellular population and enlarged size of β-cells with hyperplasia by the end of 4th week. This is indicative of the action of ISpAq and ASpAq extract at the cellular level with evident regenerative action on the β-cells of the pancreas.
The present study showed significant lowering of TC, TG, LDL and VLDL plasma levels in rats and an increase in HDL during the 4 weeks of treatment. In contrast to earlier studies performed on the anti-hyperlipidemic activities of Salvadora persica with STZ untreated rats in which HDL and triglycerides were found unchanged. (22)
Salvadora persica has proved to contain trimethylamine, related to urea alkaloids, sulphur monocline, organic sulphur compounds, β-sitosteroland small amounts of saponins. (7) (23) (24) Five lignan glycosides were isolated from the stems. (25) Antioxidant potential of β-sitosterol was proved using an experimental model for diabetes-induced oxidative damage. Results showed a decrease in glycated haemoglobin, serum glucose and nitric oxide, with concomitant increase in serum insulin levels as insulin secretagogue. β-Sitosterol has promising antidiabetic as well as antioxidant effects. (26) From aqueous extract of Salvadora persica many amides were reported which may be responsible for its antidiabetic action as insulin secretagogue. (27) Several amides have been reported to have α-glucosidase inhibition activity, mainly N-p-coumaroyl-N-feruloylputrescine and N-N′-diferuloylputrescine. These compounds were active in reducing the post-prandial glucose levels. (28) The presence of CONH group and its position in the molecule is critical for activity in the said compounds. The difference in the activities observed in plants from Arabic and Indian origin is probably due to genetic variability re-stressing the fact that Salvadora persica is a native of Middle Eastern region and may contain a rich source of sulphorganic and other relevant Phytoconstituents. This in turn can be the basis for a novel work to find active phytoconstituents from the plants and their identification through modern spectroscopic methods.
Conclusion
The potential of β-sitosterol and its various analogs found in Salvadora persica are well known as promising antidiabetics and antioxidants, this can provide a clue towards the hypoglycemic, anti-hyperlipidemic activities, improved oral glucose tolerance effect, and also regeneration of β-cells of pancreas recorded in the present study. In this study we also compared the difference in activity of Salvadora persica obtained from two different geographical sources as most of the studies performed by earlier scientists were done only on the plant material obtained from Middle Eastern countries but as Salvadora persica has also been used effectively in the Indian subcontinent for the treatment of symptoms related to diabetes i.e. hyperglycemia, hyperlipidemia, etc. The need to evaluate and compare their activities and chemical contents becomes indispensable to provide further insight on this subject. The extract obtained from Arabic Salvadora persica at dose 500 mg/kg showed slightly better results than Indian Salvadora persica especially with glucose tolerance test. The underlying reason may be that the plant is a native of Middle East and thus better quantity of constituents may be present. A thorough characterization, evaluation, and identification of the compounds found in the extracts should be done to confirm this, which can form the basis of a novel study.
Compounds related to sulphonyl urea alkaloids, already proved to be present in Salvadora persica by various scientists, shows the α-glucosidase inhibitory activity as demonstrated by β-sitosterol can be one of the cause of hypoglycemic and hypolipidemic effect of Salvadora persica extract in STZ-diabetic and normal rats and further extensive studies are needed to confirm this. The above study may provide a platform for further work on this topic with its strong potential as a natural hypoglycemic and hypolipidemic.
Figure (3).
A. Effect of Arabic origin aqueous extract (ASpAq) of Salvadora persica on hyperlipidemia. B. Effect of Indian origin aqueous extract (ISpAq) of Salvadora persica on hyperlipidemia. aP<0.001 diabetic control compared with control group; bP<0.001 compared with diabetic control group; cP<0.05 compared with diabetic control group; xP<0.001 compared with control group; Values are given in mean ± SEM, and n=6.
Table (2).
Effect of Indian origin aqueous extract (ISpAq) and Arabic origin aqueous extract (ASpAq) of Salvadora persica on hyperlipidemia (mg/dl).
| Arabic Origin, ASpAq | Indian Origin, ISpAq | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Control | Diabetic Control | Extract (250 mg/kg) | Extract (500mg/kg) | Extract (250 mg/kg) | Extract (500mg/kg) | Standard | |
| TC | 103.13 ± 12.39 | 194.50 ± 13.82x | 124.77 ± 13.05c | 114.57 ± 15.81c | 156.37 ± 13.05b | 120.15 ± 12.81a | 108.10 ± 13.62b |
| TG | 87.34 ± 12.17 | 134.30 ± 14.13x | 88.73 ± 13.81b | 75.40 ± 16.47b | 91.54 ± 12.71 | 80.40 ± 13.41a | 72.63 ± 3.86b |
| HDL | 52.60 ± 11.86 | 29.53 ± 11.02x | 36.38 ± 4.92b | 44.88 ± 11.61a | 31.33 ± 1.92 | 42.85 ± 11.61a | 46.88 ± 12.40b |
| LDL | 31.02 ± 1.81 | 77.72 ± 12.25x | 53.57 ± 12.62a | 42.63 ± 13.17b | 63.77 ± 11.6b | 51.03 ± 12.14a | 40.69 ± 11.41b |
| VLDL | 21.55 ± 1.43 | 35.34 ± 1.64x | 25.94 ± 1.96a | 22.78 ± 1.88a | 28.62 ± 1.54b | 20.31 ± 1.40a | 20.52 ± 1.40a |
P<0.001 diabetic control compared with control group;
P<0.001 compared with diabetic control group;
P<0.05 compared with diabetic control group;
P<0.001 compared with control group;
Values are given in mean ± SEM, and n=6.
Table (3).
Effect of ISpAq and ASpAq on glucose level (mg/dl) and number of β cells of Pancreas
| Groups | Initial blood glucose mg/dl | Blood glucose mg/dl (end of 4th week) | β-cells (end of 4th week) |
|---|---|---|---|
| Control | 83.1±10.1 | 90.2±17.4 | 208.3±12.5 |
| Diabetic control | 336.6±18.4a | 387.5±15.7a | 8.1±0.5a |
| ISpAq 500 mg/kg | 359.7±16.7c | 92.3±11.7b | 25.3±4.1c |
| ASpAq 500 mg/kg | 370.8±15.9b | 85.2±13.2b | 32.6±2.4b |
| Standard | 388.0±16.0b | 81.3±18.6b | 39.3±3.6b |
P<0.001 diabetic control compared with control group;
P<0.001 compared with diabetic control group;
P<0.05 compared with diabetic control group;
Values are illustrated as Mean ± SEM.
Acknowledgement
Author, Maria Khan, acknowledges the financial assistance rendered by University Grants Commission (UGC), Government of India as a Research Fellow.
References
- 1.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Elvin-Lewis M. The therapeutic potential of plants used in dental folk medicine. Odontostomatol Trop. 1982;5:107–117. [PubMed] [Google Scholar]
- 3.Al-Bagieh NH, Idowu A, Salako NO. Effect of aqueous extract of miswak on the in vitro growth of Candida albicans. Microbios. 1994;80:107–113. [PubMed] [Google Scholar]
- 4.Al lafi T, Ababneh H. The effect of the extract of the miswak (chewing sticks) used in Jordan and the Middle East on oral bacteria. Int Dent J. 1995;45:218–222. [PubMed] [Google Scholar]
- 5.Sher H, Khan ZD, Khan AU, Hussain F. Ethnobotanical study on some plants in village Tigdari, district Swat, Pakistan. J Acta Botan Yunnan. 2004;10:42–54. [Google Scholar]
- 6.Abuzinada AH, Al-Wetaid YI, Al-Basyouni SZM. TheNational strategy for conservation of biodiversity in the Kingdom of Saudi Arabia. Riyadh, Saudi Arabia: The National Commission for Wildlife Conservation and Development. Conservation of Biological Diversity; 2005. [Google Scholar]
- 7.Ezmirly ST, Cheng JC, Wilson SR. Saudi Arabian medicinal plants: Salvadora persica. Planta Med. 1979;35:191–192. doi: 10.1055/s-0028-1097205. [DOI] [PubMed] [Google Scholar]
- 8.Farooqi MIH, Srivastava JG. The toothbrush tree (Salvadora persica) Quart J Crude Drug Res. 1968;8:1297–1299. [Google Scholar]
- 9.Kokwaro JO. Medicinal plants of East Africa. Nairobi, Kenya: University of Nairobi Press; 1976. [Google Scholar]
- 10.Galletti GC, Chiavari G, Kahie YD. Pyrolysis/gas chromatography/ion-trap mass spectrometry of the ‘tooth brush’ tree (Salvadora persica L.) Rapid Communications in Mass Spectrometry. 1993;7:651–655. [Google Scholar]
- 11.Alali F, Hudaib M, Aburjai T, Khairallah K, Al-Hadidi N. GC-MS Analysis and Antimicrobial Activity of the Essential Oil from the Stem of the Jordanian Toothbrush Tree Salvadora persica. Pharmaceutical Biology. 2005;42:577–580. [Google Scholar]
- 12.Kupiecki FP, Ogzewalla CD, Schell FM. Isolation and characterization of a hypoglycemic agent from Xanthium strumarium. Journal of Pharmaceutical Sciences. 1974;63:1166–1167. doi: 10.1002/jps.2600630736. [DOI] [PubMed] [Google Scholar]
- 13.Trovato A, Galati EM, Rossitto A, et al. Hypoglycemic effects of Salvadora persica L. in the rat. Phytomed. 1998;5:129–132. doi: 10.1016/S0944-7113(98)80009-3. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor LD. Handbook of Ayurvedic Medicinal Plants. Boca Raton, Florida: CRC Press; 1990. [Google Scholar]
- 15.Ramachandran S, Asok kumar K, Uma Maheswari M, et al. Investigation of Antidiabetic, Antihyperlipidemic, and In Vivo Antioxidant Properties of Sphaeranthus indicus Linn. in Type 1 Diabetic Rats: An Identification of Possible Biomarkers. Evid Based Complement Alternat Med. 2011:571721. doi: 10.1155/2011/571721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanko Y, Yerima M, Mahdi MA, et al. Hypoglycemic Activity of Methanolic Stem Bark of Adansonnia digitata Extract on Blood Glucose Levels of Streptozocin-Induced diabetic Wistar rats. Int J Appl Res Nat Prod. 2008;1:32–36. [Google Scholar]
- 17.Chandramohan G, Ignacimuthu S, Pugalendi KV. A novel compound from Casearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin-diabetic rats. European Journal of Pharmacology. 2008;590:437–443. doi: 10.1016/j.ejphar.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. London: Churchill Livingstone; 1990. [Google Scholar]
- 19.Demacker PN, Hijmans AG, Vos-Janssen HE, vant Laar A, Jansen AP. A study of the use of polyethylene glycol in estimating cholesterol in high-density lipoprotein. Clin Chem. 1980;26:1775–1779. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–595. [PubMed] [Google Scholar]
- 22.Galati EM, Monforte MT, Forestieri AM. Salvadora persica L.: hypolipidemic activity on experimental hypercholesterolemia in rat. Phytomedicine. 1999;6:181–185. doi: 10.1016/s0944-7113(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 23.Pachaly P. Chemotaxonomie der Pflanzen Band 11a: Leguminosa. von R. Hegnauer, Birkhäuser Verlag Basel 1994. 552 S., Hardcover, DM 598, sFr. 498,- ISBN 3–7643–2979–3. Pharmazie in unserer Zeit. 1995;24:170–170. [Google Scholar]
- 24.Robinson T. The organic constituents of higher plants: Their chemistry and interrelationships. North Amherst, MA: Cordus Press; 1983. [Google Scholar]
- 25.Kamel MS, Othani K, Assaf MH. WHERE ARE ET AL? Lignan glycosides from stems of Salvadora persica. Phytochem. 1992;31:2469–2471. [Google Scholar]
- 26.Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes. 2011;3:29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 27.Khalil AT. Benzylamides from Salvadora persica. Arch Pharm Res. 2006;29:952–956. doi: 10.1007/BF02969277. [DOI] [PubMed] [Google Scholar]
- 28.Niwa T, Doi U, Osawa T. Inhibitory activity of corn-derived bisamide compounds against alpha-glucosidase. J Agric Food Chem. 2003;51:90–94. doi: 10.1021/jf020758x. [DOI] [PubMed] [Google Scholar]