Abstract
Objectives
Early stages of peri-implant bone formation play an essential role in the osseointegration and long-term success of dental implants. Biological implant surface coatings are an emerging technology to enhance the attachment of the implant to the surrounding bone and stimulate bone regeneration. The purpose of this study was to determine the effect of coating the implant surface with fibronectin on osseointegration.
Material and methods
The experiment was conducted on a total of twelve New Zealand white mature male rabbits, weight between 2.5–4 kg. Twenty four pure titanium implants were used in this study. Each rabbits received two implants, one implant in each tibia; the implant in the right limb was coated with fibronectin (experimental group), whilst on the contralateral side the implants were placed without coating (control group). Six rabbits were sacrificed for Scanning Electron Microscopic evaluation after 4 and 8 week healing periods.
Results
The results of the present study demonstrating the mean gap distance between the bone and implant was greater in the control group compared to fibronection group at both observation periods however, the difference between these two groups was not statistically significant.
Conclusion
Thus, it could be suggested that the biological functionalization of dental implants with fibronectin, may influence the integration or biocompatibility and bonding of the implant to the surrounding bone.
Keywords: Dental implant, Osseointegration, Biofunctionalization, Extracellular matrix, Fibronectin
Introduction
Dental implants are an excellent treatment option for restoring areas that are missing a tooth. Dental implants were originally made with pure titanium, which yields a strictly bio-inert titanium-oxide surface. However, it takes a long time (3–6 months) before this implant becomes biologically attached to the bone. Various surface modifications have been introduced to improve the speed with which bone attaches to the implant surface and a new generation of dental implant have emerge.(1–3)
The new generation dental implants exhibit a large variation in surface properties including structural and chemical compositions. The surfaces have mainly underwent topographical modification aiming to achieve an enhanced biological response. (4)
The main methods that are reported in the literature to create topographical modification are acid etching, sandblasting, titanium plasma spraying and hydroxyapatite coating. A current tendency is the manufacturing of implants with micro and submicro (nano) topography. Furthermore, the biological functionalization of implant’s surfaces, by adding biomimetic bioactive substances to improve its biological characteristics, has also been recently investigated. (5–7)
Generally, the bone-to-implant interaction is complex and does not depend on surface topography only. Chemical or biochemical composition of implant surface also plays a key role in the early stages of bone formation. (8, 9) Cell recruitment onto biomaterial surface is a fundamental step within the multifaceted process responsible for implant osseointegration. This process involves several proteins from the extracellular matrix (ECM), cytoskeleton and cell membrane. (10)
ECM proteins have been studied as potential adhesive scaffolds for bone defect healing and implant integration. (11) These ECM polymers include collagen, (12–18) fibronectin (19–22), decellularized matrix, (23–24) bone sialoprotein, (25) as well as hyaluronic acid. (26, 27)
Fibronectin belongs to a group of high molecular weight glycoproteins that exist on cell surfaces. It is found in connective tissues, basement membranes, and extracellular fluids, and is known to play a role in cell-to-cell and cell-to-substrate adhesion, as well as an important role in osseointegration due to its capacity to make osteoblasts attach to ECM components. (28)
Fibronectin is also known to enhance gingival fibroblast attachment, which has beneficial effects on healing after implant surgery and during the maintenance phase by forming attachments between connective tissue and the epithelium, which can prevent inflammatory breakdown around the implant.(29, 30)
The purpose of this study was to determine the effect of coating the implant surface with fibronectin on osseointegration.
Materials and Methods
Experimental model
This experiment was conducted on a total of twelve New Zealand white mature male rabbits weight between 2.5–4 kg. The animals were housed in separate cages in temperature - controlled rooms and were fed on standard food and had free access to tap water. The animals were cared for according to the guidelines of the local Ethics Committee of the Animal Research at the Faculty of Medicine, Cairo University, which approved the project before the beginning of the experiments.
All rabbits received two implants, one implant in each tibia; the implant in the right limb was coated with fibronectin (experimental group), whilston the contralateral side the implants were placed without coating (control group).
Coating of implants
Twenty four titanium implants of 4.2 mm in diameter and 8.0 mm in length (Implantium, Dentium, Seoul, Korea) were used in this study. Twelve implants were implanted without coating in left limb, while the experimental side was implanted by twelve implants in right limb after coating with fibronectinas follows: 10 μg of fibronectin (Biochrom, Germany) were dissolved in 1 ml of 0.9M phosphate buffered saline, pH 7.2. Then the twelve implants were incubated for two hours in 300 μl of fibronectin solution. The treated implants were removed from the coating solutions and allowed to dry under sterile conditions for 12 hours at room temperature. (31)
Surgical Procedure
Rabbits were anaesthetized intramuscularly with a mixture of Xylazine (Chanazine, Chanelle Pharmacuetical, Ireland) 5mg/kg body weight and ketamine hydrochloride (Ketamine, Pharmazeutische Pröparate, Germany) 30mg/kg body weight. Once general anaesthesia was established, the medial aspects in the region of the proximal tibia were shaved; the skin was carefully swabbed with mixture of iodine and 70% ethanol. A 30 mm incision was made along the medial aspect of the proximal tibia and the wound advanced down to and through the periosteum. A subperiosteal dissection was then advanced up to the inferior attachment of the knee joint capsule and laterally to the full extent of the flat medial bone surface.
Under continuous irrigation with sterile saline, the implants installation procedure in tibiae bone was carried out according to the manufacturer’s instructions. Closure of the wound should be performed in layers using catgut for the subcutaneous layer and silk for the skin layer. The prophylactic administration of procaine penicillin (Wyeth Pharmaceuticals, Parramatta, New South Wales.) 60,000 units/kg intramuscularly was commenced during the surgery and continued for three postoperative days to reduce the potential for wound infection.
Animal sacrifice
Six rabbits were sacrificed at 4 and 8 weeks using an intramuscular injection of overdose of 60mg/ml/kg body weight sodium phenobarbitone (Phenobarbitone, Fawns & McAllan Pty Ltd, Melbourne, Victoria).
Electron microscopic analysis
Block section of the tibial bone, containing the implants were obtained using a stryker bone saw (Stryker; Kalamazoo, Mich, United States of America). The samples were immersed into 10% buffered formicacid for 48 hours for decalcification. These specimens were dehydrated in ascending ethyl alcohol concentration 70%, 80% and 90% for 6 hours each and 100% for 10 hours. Then, to displace the alcohol the specimen were immersed in acetone for 12 hours. These specimen were embedded in polymethylmethacrylate resin under vacuum and after polymerization for 24 hours, sections were cut at 150μm by a diamond wafering blade. The specimens were coated with layer of gold with the aid of magnetron-spattering device. Analysis was performed using scanning electron microscopy (SEM, JXA-840A, JEOL, Japan). The mean gap distance (μm) between the bone and implant in areas among the five threads was calculated.
Results
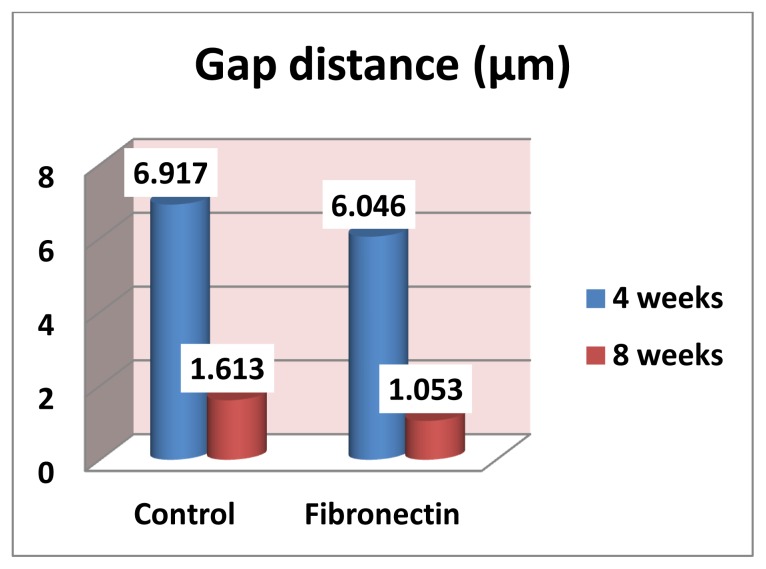
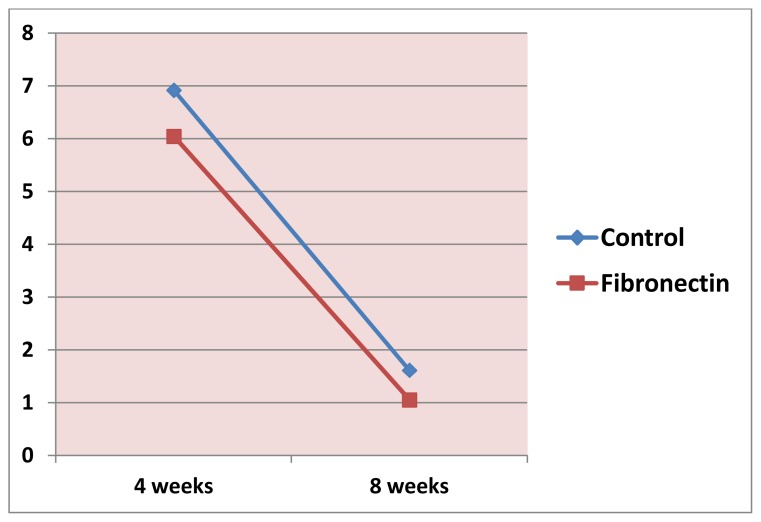
Measurements by the aid of the SEM revealed that the gap distance was greater in the control group compared to fibronection group at both observation periods (Fig 1–4). However, Student’s t test revealed that the difference between these two groups was not statistically significant at 4 and 8 weeks (p= 0.4364, 0.2021 respectively), (Table 1, Fig. 5), also there was no statistically significant change in mean gap distance (μm) through all period by time within each group (Fig. 6).
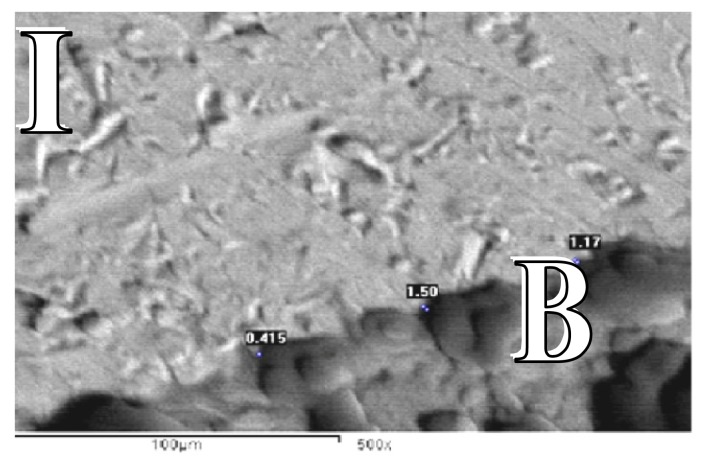
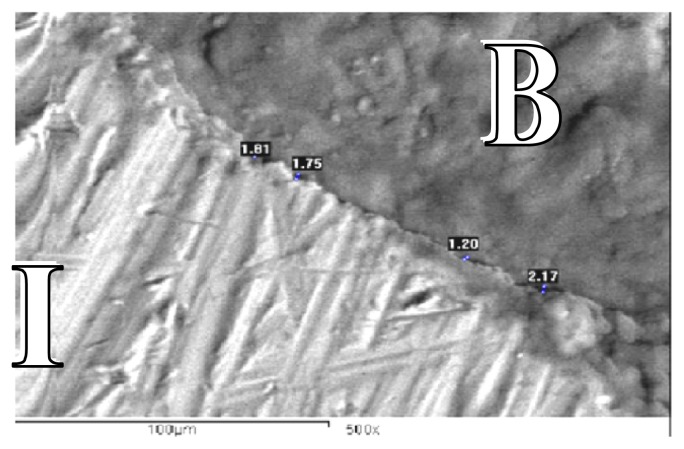
Figure 1.
SEM for control group at the end of 4 weeks showing gap distance of bone along the implant surface (SEM X 500).
B: Bone
I: Implant
Figure 4.
SEM forfibronectin group at the end of 8 weeks showing gapdistance of bone along the implant surface (SEM X 500).
B: Bone
I: Implant
Table 1.
Mean and standard deviation of gap distance (μm) between the bone and implant in control and fibronectin group and statistical significance of the difference (Student’s t test)
| Control | Fibronectin | t value | p value | |
|---|---|---|---|---|
| 4 weeks | 6.917±3.268 | 6.046±1.138 | 0.7959 | 0.4364 |
| 8 weeks | 1.613±1.195 | 1.053±0.601 | 1.3239 | 0.2021 |
Figure 5.
Mean of gap distance (μm) between the bone and implant in control and fibronectin group
Figure 6.
Change by time in gap distance (μm) between the bone and implantin control and fibronectin group
Discussions
Surface modifications of titanium implants using various modalities aim to improve the initial healing and promote faster healing times those are of increasing importance in modern dentistry, with this surface modification, immediate or early loading has become a predictable treatment protocol. (32,33)
The selective adsorption of beneficial molecules has been attempted by modifying the implant surfaces. It has been demonstrated that coating of the surface with cell-adhesive proteins, such as fibronectin, collagen, and/orlaminin, improved initial cell attachment, cell spreading, and cell activity. (34–37)
Biomimetic coating with fibronectin has been tested in previous studies, (20–23) its adsorption onto solid substrates has already been evaluated in previous studies, though the interactions between this protein and biomaterial surfaces are not fully understood.(30, 38)
Although many studies reported about the applications of fibronectin to the implant surface can enhance the osseointegration of dental implants to the bone, (39,40) the results of the present study demonstrating the difference between two groups was not statistically significant at both observation periods and this could be due to:
Firstly exfoliation of fibronectin coating from implant surface during surgery.
Secondly, it is possible that fibronectin-coated implants exhibit enhanced osseointegration around dental implant in rabbits because fibronectin has the ability to promote osteoblast attachment more via distance osteogenesis than contact osteogenesis which was found to be enhanced more by a rough implant surface than by a smooth implant surface due to the increased surface area available for fibrin attachment and surface features to which fibrin could become attached while, distance osteogenesis takes place on the surface of the bone around the implant through appositional growth, while contact osteogenesis occurs directly on the surface of the implant, two osteogenetic phenomena (distance and contact osteogenesis) have been proposed to occur around dental implants.(41)
Thirdly, the observation periods of healing might be not enough to reveal any differences in healing due to coating the surfaces. Fibronectin affects healing in the early healing period. (28) It is suggested that a difference would have been revealed if the healing had been evaluated after only 2 weeks and long-term prognosis.
Although no statistically significant difference between the two groups at both observation periods, the mean gap distance between the bone and implant was greater in the control group compared to fibronection group at both observation periods and this could be due to prevention of inflammatory process around the implant by the action of fibronetin in enhancing the gingival fibroblast attachment, which has beneficial effects on healing after implant surgery and during the maintenance phase by forming attachments between connective tissue and the epithelium. (42)
Apical migration of the long junctional epithelium, which is the result of poor attachment between the subepithelial connective tissues and the epithelium around the implant, leads to the formation of peri-implant pockets. Inflammatory breakdown is caused by bacterial invasion through the peri-implant pocket (42, 43) which can extend directly to the underlying bone in the absence of a transgingival seal between the gingival tissue and the implant leading to peri-implantitis, and implant failure. (42)
Thus, it could be suggested that the biological functionalization of dental implants with fibronectin, may influence the integration or biocompatibility and bonding of the implant to the surrounding bone.
Recommendations
Shortening or extending the period of observation and the use of other modifying implant surfaces with cell-adhesive proteins such as collagen, hyaluronic acid and laminin, may be recommended in further studies.
Figure 2.
SEM for fibronectin group at the end of 4 weeks showing gap distance of bone along the implant surface (SEM X 500).
B: Bone
I : Implant
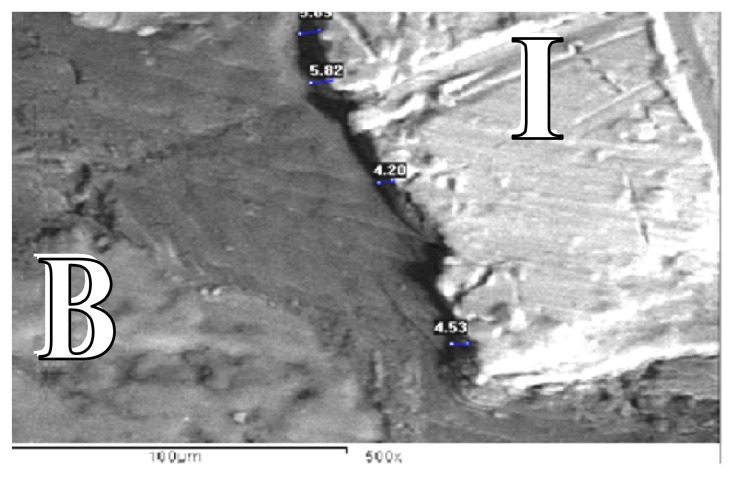
Figure 3.
SEM forcontrol group at the end of 8 weeks showing gap distance of bone along the implant surface (SEM X 500).
B: Bone
I : Implant
References
- 1.Ellingsen J. Surface configurations of dental implants. Periodontology 2000. 1998;17:36–46. doi: 10.1111/j.1600-0757.1998.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000;84:522–534. doi: 10.1067/mpr.2000.111966. [DOI] [PubMed] [Google Scholar]
- 3.Klokkevold Perry R, Nishimura Russell D, Adachi Moriyasu, Caputo Angelo. Osseointegration enhanced by chemical etching of the titanium surface. A torque removal study in the rabbit. Clin Oral Impl Res. 1997;8:442–447. doi: 10.1034/j.1600-0501.1997.080601.x. [DOI] [PubMed] [Google Scholar]
- 4.Xia Wei, Lindahl Carl, Lausmaa Jukka, Engqvist Håkan. Biomimetic Hydroxyapatite Deposition onTitanium Oxide Surfaces for Biomedical Application. Advances in Biomimetics. 2011. [Acessed August, 2011]. Available from: http://www.intechopen.com/books/advances-inbiomimetics/biomimetic-hydroxyapatite-deposition-on-titanium-oxide-surfaces-for-biomedical-application.
- 5.Beutner R, Michael J, Schwenzer B, Scharnweber D. Biological nano-functionalization of titanium-based biomaterial surfaces: a flexible toolbox. J R Soc Interface. 2010;7:S93–S105. doi: 10.1098/rsif.2009.0418.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros RR, Novaes AB, Jr, Papalexiou V, Souza SL, Taba M, Jr, Palioto DB, et al. Effect of biofunctionalized implant surface on osseointegration: a histomorphometric study in dogs. Braz Dent J. 2009;20:91–98. doi: 10.1590/s0103-64402009000200001. [DOI] [PubMed] [Google Scholar]
- 7.Lutz R, Srour S, Nonhoff J, Weisel T, Damien CJ, Schlegel KA. Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration. Clin Oral Implants Res. 2010;21:726–734. doi: 10.1111/j.1600-0501.2009.01904.x. [DOI] [PubMed] [Google Scholar]
- 8.Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Enhanced bone apposition around biofunctionalized sandblasted and acidetched titanium implant surfaces. A histomorphometric study in miniature pigs. Clin Oral Impl Res. 2006;17:251–257. doi: 10.1111/j.1600-0501.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- 9.Schuler M, Owen GR, Hamilton DW, de Wild M, Textor M, Brunette DM, et al. Biomimetic modification of titanium dental implant model surfaces using the RGDSP-peptide sequence: a cell morphology study. Biomaterials. 2006;27:4003–4015. doi: 10.1016/j.biomaterials.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, et al. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007;40:693–699. doi: 10.1016/j.bone.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Barros Raquel RM, Novaes Arthur B, Jr, Sérgio Vula Papalexiou, Souza MárioTaba LS, Jr, Palioto Daniela B, Gris Márcio FM. Effect of biofunctionalized implant Surface on osseointegration - A histomorphometric study in dogs. Braz Dent J. 2009;20(2):91–98. doi: 10.1590/s0103-64402009000200001. [DOI] [PubMed] [Google Scholar]
- 12.Morra M, Cassinelli C, Meda L, Fini M, Giavaresi G, Giardino R. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: a rabbit model. Int J Oral Maxillofac Implants. 2005;20(1):23–30. [PubMed] [Google Scholar]
- 13.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res. 2005;16(5):563–9. doi: 10.1111/j.1600-0501.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 14.Svehla M, Morberg P, Bruce W, Walsh WR. No effect of a type I collagen gel coating in uncemented implant fixation. J Biomed Mater Res B Appl Biomater. 2005;74(1):423–8. doi: 10.1002/jbm.b.30256. [DOI] [PubMed] [Google Scholar]
- 15.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27(32):5561. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Li X, Fan Y, Zhang G, Li D, Dong W, Sha Z, Yu X, Feng Q, Cui F, et al. Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2010;94(1):44–52. doi: 10.1002/jbm.b.31622. [DOI] [PubMed] [Google Scholar]
- 17.Caiazza S, Colangelo P, Bedini R, Formisano G, De Angelis G, Barrucci S. Evaluation of guided bone regeneration in rabbit femur using collagen membranes. Implant Dent. 2000;9(3):219–25. doi: 10.1097/00008505-200009030-00007. [DOI] [PubMed] [Google Scholar]
- 18.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ari A, Rivkin R, Frishman M, Gaberman E, Levdansky L, Gorodetsky R. Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng Part A. 2009;15(9):2537–46. doi: 10.1089/ten.tea.2008.0567. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Jang JD, Lee SK. Treatment of long tubular bone defect of rabbit using autologous cultured osteoblasts mixed with fibrin. Cytotechnology. 2007;54(2):115–20. doi: 10.1007/s10616-007-9084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone-tissue engineering: Anin vivo study. J Biomed Mater Res A. 2004;71(1):162–71. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 22.Perka C, Schultz O, Spitzer RS, Lindenhayn K, Burmester GR, Sittinger M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000;21(11):1145–53. doi: 10.1016/s0142-9612(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 23.Kurkalli BG, Gurevitch O, Sosnik A, Cohn D, Slavin S. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr Stem Cell Res Ther. 2010;5(1):49–56. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 24.Suckow MA, Voytik-Harbin SL, Terril LA, Badylak SF. Enhanced bone regeneration using porcine small intestinal submucosa. J Invest Surg. 1999;12(5):277–87. doi: 10.1080/089419399272395. [DOI] [PubMed] [Google Scholar]
- 25.Graf HL, Stoeva S, Armbruster FP, Neuhaus J, Hilbig H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER and pure titanium implant surfaces. Int J Oral Maxillofac Surg. 2008;37(7):634–40. doi: 10.1016/j.ijom.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17(2):205–13. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 27.Paderni S, Terzi S, Amendola L. Major bone defect treatment with an osteoconductive bone substitute. Chir Organi Mov. 2009;93(2):89–96. doi: 10.1007/s12306-009-0028-0. [DOI] [PubMed] [Google Scholar]
- 28.Park JM, Koak JY, Jang JH, Han CH, Kim SK, Heo SJ. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. Int J Oral Maxillofac Implants. 2006;21:859–66. [PubMed] [Google Scholar]
- 29.doSerro AP, Fernandes AC, de Jesus Vieira Saramago B. Calcium phosphate deposition on titanium surfaces in the presence of fibronectin. J Biomed Mater Res. 2000;49:345–52. doi: 10.1002/(sici)1097-4636(20000305)49:3<345::aid-jbm7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Tamada Y, Ikada Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J Colloid Interface Sci. 1993;155:334–9. [Google Scholar]
- 31.Wuttke M, Müller S, Nitsche DP, Paulsson M, Hanisch FG, Maurer P. Structural characterization of human recombinant and bone-derivede bone sialoprotein. J Biol Chem. 2001;276:276(3):36839–36848. doi: 10.1074/jbc.M105689200. [DOI] [PubMed] [Google Scholar]
- 32.Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Bio-technol. 2010;28:198–206. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Atieh MA, Payne AG, Duncan WJ, de Silva RK, Cullinan MP. Immediate placement or immediate restoration/loading of single implants for molar tooth replacement: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2010;25:401–15. [PubMed] [Google Scholar]
- 34.Cannas M, Denicolai F, Webb LX, Gristina AG. Bioimplant surfaces: binding of fibronectin and fibroblast adhesion. J Orthop Res. 1988;6:58–62. doi: 10.1002/jor.1100060108. [DOI] [PubMed] [Google Scholar]
- 35.Dean JW, 3rd, Culbertson KC, D’Angelo AM. Fibronectin and laminin enhance gingival cell attachment to dental implant surfaces in vitro. Int J Oral Maxillofac Implants. 1995;10:721–8. [PubMed] [Google Scholar]
- 36.El-Ghannam A, Starr L, Jones J. Laminin-5 coating enhances epithelial cell attachment, spreading, and hemidesmosome assembly on Ti-6A1-4V implant material in vitro. J Biomed Mater Res. 1998;41:30–40. doi: 10.1002/(sici)1097-4636(199807)41:1<30::aid-jbm4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Roehlecke C, Witt M, Kasper M, Schulze E, Wolf C, Hofer A, et al. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001;168:178–87. doi: 10.1159/000047833. [DOI] [PubMed] [Google Scholar]
- 38.Jönsson U, Ivarsson B, Lundström I, Berghem L. Adsorption behavior of fibronectin on well-characterized silica surfaces. J Colloid Interface Sci. 1982;90:148–63. [Google Scholar]
- 39.Chen C, Lee IS, Zhang SM, Yang HC. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco’s phosphate-buffered saline solution containing CaCl(2) with and without fibronectin. ActaBiomater. 2010;6:2274–81. doi: 10.1016/j.actbio.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa T, Yoshinari M, Nemoto K. Direct attachment of fibronectin to tresyl chloride-activated titanium. J Biomed Mater Res A. 2003;67:684–8. doi: 10.1002/jbm.a.10143. [DOI] [PubMed] [Google Scholar]
- 41.Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998;11:391–401. [PubMed] [Google Scholar]
- 42.Malmstrom HS, Fritz ME, Timmis DP, Van Dyke TE. Osseointegrated implant treatment of a patient with rapidly progressive periodontitis. A case report. J Periodontol. 1990;61:300–4. doi: 10.1902/jop.1990.61.5.300. [DOI] [PubMed] [Google Scholar]
- 43.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]