Abstract
Introduction:
We assessed differences in results of stone analyses on subsequent sampling.
Methods:
A retrospective review of patients with stone analyses at a tertiary stone centre between March 2006 and July 2012 was performed. All stones were analyzed at a centralized laboratory using infrared spectroscopy. Patients were grouped according to the first predominant stone type on record, as defined by the predominant stone component of at least 60%. Stone groups included calcium oxalate (CaOx), calcium phosphate (CaP), uric acid (UA), cystine, struvite, mixed CaOx-CaP and mixed CaOx-UA. All patients had a full metabolic stone workup.
Results:
Of the 303 patients with stone analyses, 118 (38.9%) patients had multiple stone analyses. The mean age was 53.4 ± 15.1 years, and 87 (73.7%) were males. Of the 118, the initial stone analysis showed 43 CaOx, 38 CaP, 21 UA, 4 CaOx-CaP, 2 CaOx-UA, 6 cystine, and 4 struvite. There was a different stone composition in 25 (21.2%) patients with a median time delay of 64.5 days. Different compositions were found in 7 CaOx (to 3 CaP, 2 CaOx-CaP, and 2 UA), 5 CaP (to 3 CaOx and 2 CaOx-CaP), 3 UA (to 3 CaOx), 4 CaOx-CaP (to 2CaOx, 1 UA and 1 CaP), 2 CaOx-UA (to 2 CaOx) and 4 struvite (to 3 CaP and 1 UA).
Conclusions:
Stone composition was different in 21.2% of patients on subsequent analyses.
Introduction
The prevalence of urolithiasis in North America has been increasing, with an estimated lifetime diagnosis of 13% of men and 7% of women in the United States, and incurring an approximate cost of $2 billion annually.1 This disease tends to relapse, with recurrence rates after the first kidney stone of 14%, 35% and 52% after 1, 5 and 10 years, respectively.2 The Canadian Urological Association Guidelines recommend stone analysis as part of the initial workup for a patient presenting with a kidney stone.3 However, little is known regarding the variability in stone composition of different samples of stones obtained from the same patient. The aim of the present study was to assess differences in results of stone analyses on subsequent sampling.
Methods
A retrospective review was performed for 303 patients with confirmed stone analysis treated at a single tertiary stone centre between March 2006 and July 2012. Urinary stones were collected either through spontaneous passage (intact stones), or post-endourologic procedures, such as shock wave lithotripsy (SWL), ureteroscopy (URS) or percutaneous nephrolithotomy (PCNL) (fragmented stones). All retrieved stone fragments were sent for analysis. During URS, holmium laser lithotripsy was used to fragment stones rather than pulverize them. Swiss LithoClast Ultra (Boston Scientific, Natick, MA) and holmium laser were used to fragment stones during PCNL and URS, respectively. All stone fragments were analyzed by a single commercial laboratory (Gamma Dynacare Laboratories, St-Laurent, QC) using infrared spectroscopy. All patients had a full metabolic stone workup.
Patients were classified according to their first predominant stone type on record, where stone type was defined by the predominant stone component, represented by at least 60% of all components. Otherwise, stones were considered to be mixed, such as CaOx-CaP with a composition of 50% CaOx and 50% CaP. Calcium oxalate monohydrate and dihydrate were grouped as CaOx. Uric acid (UA) stones included uric acid dihydrate, while carbonate apatite and brushite were grouped as CaP stones.
Data were analyzed using the commercially available IBM Statistical Package of Social Science (IPSS, Chicago, IL) version 20. Continuous variables were presented as means ± standard deviations and were compared using the analysis of variance (ANOVA) test. Dichotomous variables were presented as numbers and percentage and were compared using the Fisher exact test. Statistical significance was defined as two-sided alpha error level of less than 0.05.
Results
Of the 303 patients with stone analyses, 118 (38.9%) patients had multiple stone analyses. The mean age was 53.4 ± 15.1 years, and 87 (73.7%) were males. The median time delay between stone analyses was 64.5 days. Of the 118 patients, 25 (21.2%) had a different stone composition on subsequent analysis (Fig. 1). From the original numbers on initial sampling, 7 patients of the 43 CaOx, 5 of the 38 CaP, 3 of the 21 UA, none of the 6 cystine, all of the 4 mixed CaOx-CaP, all of the 2 mixed CaOx-UA and all of the 4 struvite stone types were different.
Fig. 1.
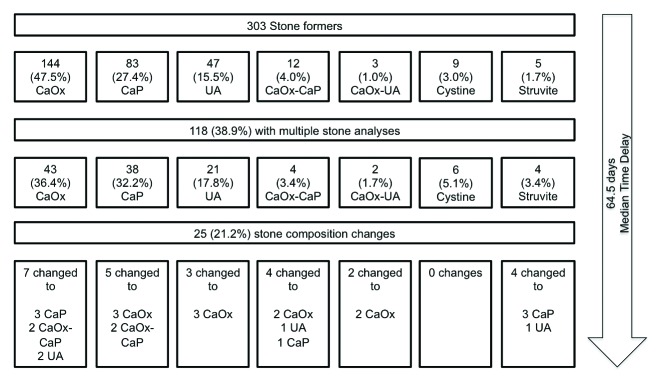
Changes in stone analyses in patients with multiple stone analyses.
In total, 25 patients had different compositions on subsequent stone analysis. They were grouped into 1 of the following 3 categories: (1) patients who had multiple procedures that yielded different fragments from the same stone (Table 1); (2) patients who had stones from different kidneys (Table 2); and (3) patients who had multiple stones from the same kidney (Table 3).
Table 1.
Characteristics of patients with multiple procedures for the same stone
| Subject | Sex | Age | First stone analysis | Second stone analysis |
|---|---|---|---|---|
| A1 | M | 62 | 60% Struvite, 30% CaP, 10% UA (Right URS) | 90% UA, 10% Struvite (Right URS) |
| A2 | M | 60 | 70% CaOx, 30% CaP (Spontaneous) | 70% CaP, 30% CaOx (Right PCNL) |
| A3 | M | 43 | 70% CaOx, 30% CaP (Left PCNL) | 50% CaOx, 50% CaP (Left PCNL) |
| A4 | M | 49 | 60% CaOx, 40% CaP (Right SWL) | 60% CaP, 40% CaOx (Right URS) |
| A5 | F | 45 | 60% CaP, 40% CaOx (Right URS) | 50% CaOx, 50% CaP (Right URS) |
| A6 | F | 45 | 60% CaP, 40% CaOx (Right PCNL) | 50% CaOx, 50% CaP (Right URS) |
| A7 | M | 59 | 60% CaP, 40% CaOx (Left PCNL) | 100% CaOx (Left URS) |
| A8 | M | 67 | 50% CaOx, 50% UA (Left SWL) | 80% CaOx, 20% UA (Left URS) |
| A9 | M | 61 | 100% UA (Right SWL) | 100% CaOx (Right URS) |
M: male; F: female; CaP: calcium phosphate; UA: uric acid; URS: ureteroscopy; CaOx: calcium oxalate; PCNL: percutaneous nephrolithotomy; SWL: shock wave lithotripsy.
Table 2.
Characteristics of patients with stone analyses from bilateral kidneys
| Subject | Sex | Age | 1st Stone Analysis | 2nd Stone Analysis |
|---|---|---|---|---|
| B1 | M | 72 | 80% CaP, 20% CaOx (Left PCNL) | 90% CaOx,10% CaP (Right SWL) |
| B2 | M | 76 | 50% CaP, 50% CaOx (Right PCNL) | 60% CaOx, 40% CaP (Left PCNL) |
| B3 | M | 36 | 90% CaOx, 10% CaP (Right SWL) | 50% CaP, 50% CaOx (Left SWL) |
| B4 | F | 63 | 80% CaP, 20% CaOx (Left PCNL) | 100% CaOx (Right URS) |
| B5 | M | 59 | 70% CaOx, 30% UA (Left URS) | 80% UA, 20% CaOx (Right URS) |
M: male; F: female; CaP: calcium phosphate; UA: uric acid; URS: ureteroscopy; CaOx: calcium oxalate; PCNL: percutaneous nephrolithotomy; SWL: shock wave lithotripsy.
Table 3.
Characteristics of patients with stone analyses from multiple stones from a single kidney
| Subject | Sex | Age | 1st Stone Analysis | 2nd Stone Analysis |
|---|---|---|---|---|
| C1 | M | 41 | 70% Struvite, 30% CaP (Left URS) | 80% CaP, 20% Struvite (Left PCNL) |
| C2 | M | 44 | 90% Struvite, 10% CaP (Left PCNL) | 70% CaP, 30% Struvite (Left URS) |
| C3 | F | 20 | 60% Struvite, 40% CaP (Right PCNL) | 80% CaP, 20% Struvite (Right PCNL) |
| C4 | M | 67 | 50% CaOx, 50% CaP (Spontaneous) | 70% CaP, 30% CaOx (Left PCNL) |
| C5 | M | 58 | 60% CaOx, 40% CaP (Right SWL) | 60% CaP, 40% CaOx (Spontaneous) |
| C6 | M | 35 | 50% CaOx, 50% CaP (Right SWL) | 60% CaOx, 40% CaP (Right URS) |
| C7 | M | 64 | 50% CaOx, 50% UA (Left SWL) | 100% CaOx (Left URS) |
| C8 | M | 39 | 100% CaOx (Left URS) | 100% UA (Left PCNL) |
| C9 | M | 52 | 50% CaP, 50% CaOx (Left SWL) | 100% UA (Left URS) |
| C10 | M | 44 | 90% UA, 10% CaOx (Spontaneous) | 70% CaOx, 30% UA (Spontaneous) |
| C11 | M | 73 | 80% UA, 20% CaOx (Right SWL) | 90% CaOx, 10% UA (Right URS) |
M: male; F: female; CaP: calcium phosphate; UA: uric acid; URS: ureteroscopy; CaOx: calcium oxalate; PCNL: percutaneous nephrolithotomy; SWL: shock wave lithotripsy.
Of the 9 patients with multiple procedures for the same stone (Table 1), 8 yielded subsequent analyses that had the same components as the initial stone, but with different proportions: struvite and UA components (A1), CaOx and CaP components (A2–A7), and CaOx and UA components (A8). The stone composition in patient A9 changed completely from 100% UA (from SWL) to 100% CaOx (from URS extraction). Computed tomography (CT) scan at initial presentation showed a single stone with a dense core (995 Hounsfield unit [HU]) surrounded by a softer shell (480 HU).
Of the 5 patients with bilateral renal stone analyses (Table 2), 4 (B1–B4) had different proportions of CaOx and CaP on subsequent analyses. One patient (B5) had bilaterally obstructing ureteral stones, and had different proportions of CaOx and UA components on subsequent stone analyses. A corresponding CT scan showed a stone density at 450 HU in the right ureter, and 2 different stone densities (697 and 251 HU) in the left ureter. The stone analysis from the left URS and stone extraction showed 70% CaOx and 30% UA. The second analysis came 23 days later from a right URS and stone extraction of the right ureteral stone, showing 80% UA and 20% CaOx.
Of the 11 patients with multiple stones from the same kidney (Table 3), 3 had different proportions of struvite and CaP components (C1–C3), 3 had different proportions of CaOx and CaP components (C4–C6), and 5 had different proportions of UA and CaOx components (C7–C11), 2 of whom had subsequent analyses containing no components of the initial analysis (C8–C9). Patient C11 had an initial CT scan that showed a single stone with a dense core (740 HU), surrounded by a softer shell (338 HU) (Fig. 2, part A). Two patients had subsequent analyses containing no components of the initial analysis (C8–C9). Patient C8 had fragments from the initial stone extracted from the left ureter via URS which showed 100% CaOx, while fragments from the subsequent stone extraction from the left kidney via PCNL showed 100% UA (with no remnants of CaOx). Patient C9 had an initial CT scan that showed 2 stones in the left kidney with different densities (438 HU and 838 HU, Fig. 2, part B). In addition, a third stone in the left ureter had a density of 1216 HU. The patient underwent SWL for the obstructing ureteral stone. The stone analysis post-SWL yielded 50% CaP and 50% CaOx. Subsequently, patient underwent left URS for the left renal stones (Fig. 2, part B) and stone analysis showed 100% UA.
Fig. 2.
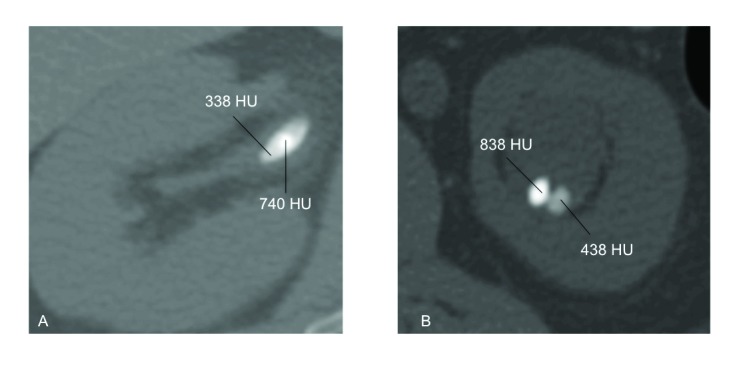
Axial computed tomography scan (bone view) of kidney stones for 2 subjects with different stone analyses on subsequent analyses. A: The right kidney of subject C11, showing a stone with a dense core of 740 Hounsfield unit (HU) and a surrounding shell of 338 HU. B: The left kidney of subject C9, showing 2 stones of different densities (838 HU and 438 HU) coexisting in the same calyx.
Discussion
The prevention of stone recurrence relies on the accurate diagnosis of stone composition and metabolic stone work-up.4 This allows us to correctly identify the composition of the stone so that we can provide targeted medical or dietary prophylaxis. In the present study, 21.2% of patients with multiple stone analyses had a different composition on subsequent analysis. We have several hypotheses for these differences.
One hypothesis is that sampling error may have contributed to different stone compositions on subsequent analysis. Most stones are heterogeneous in nature,5,6 and different parts of the same stone could have been sampled by multiple procedures and sent for analysis (Table 1). Most of the patients who underwent multiple procedures for the same stone from the same kidney (A1–A8) or multiple stones from the same kidney (C1–C6) had different proportions of the same stone components on subsequent analysis. Therefore, sampling error could have contributed to the differences in stone compositions on subsequent sampling.
Another explanation for the differences seen in stone compositions could be due to the nomenclature used to define a particular stone type. In the present study, stone type was defined based on at least 60% of a specific chemical composition. For example, a stone containing 60% CaOx and 40% CaP was categorized as CaOx stone. If its composition changed to 50% CaOx and 50% CaP, it was categorized as a mixed CaOx-CaP stone, and if it had further changed to 40% CaOx and 60% CaP, it was then categorized as a CaP stone. Thus, an incremental change as small as 10% of a single chemical composition could change the classification of the predominant stone type, as reflected in the results of the patients who switched between mixed CaOx-CaP, CaP and CaOx with as little as 10% change in CaP or CaOx components (Table 3). Therefore, the differences in the stone categories were arbitrary for these patients. Perhaps a higher cut-off of a specific component, for example 80% instead of 60%, could have been used to define predominant stone types. However, in the present study, 60% majority was used as cut-off similar to previous published studies.7–10
A third hypothesis is that stones of different compositions could coexist in the same kidney, based on observations of stones with variable densities or even stones with different densities coexisting in the same kidney (Fig. 2). For example, Figure 2, part A demonstrates the CT scan of the right kidney with a stone with variable density; there is a dense core of 740 HU with a softer shell of 338 HU (patient C11 in Table 3). Based on previously published correlations of stone densities and compositions, the dense core is most likely to contain predominantly CaOx while the softer shell is most likely to contain predominantly UA.11 Differences in stone compositions obtained through different procedures could be further explained by the different mechanisms of stone fragmentation and extraction. Since softer UA stones are more friable than the denser CaOx stones with SWL, it is plausible that the stone fragments obtained post-SWL would yield mostly UA from the softer shell of the stone. On the other hand, residual fragments of the denser core retrieved via laser lithotripsy and URS would likely contain mostly harder CaOx stone components (Patient C11, Table 3, Fig. 2, part A).12,13 Therefore, the same stone with different densities and compositions yielded different results on subsequent stone analyses of fragments obtained through different endourologic procedures. Similarly, patient C9 had simultaneously 2 left renal stones with different stone densities 838 HU and 438 HU (Fig 2, part B). This CT scan was obtained when the patient presented with an obstructing left ureteral stone. He underwent SWL of the obstructing ureteral stone that yielded a CaP-CaOx stone. Subsequently, he underwent URS for the left renal stone yielding a 100% UA stone. Although the 2 different left renal stones were not simultaneously sampled at the time of URS, it is obvious from the CT scan that stones of different densities and compositions coexisted within the same renal unit, which yielded different results on subsequent stone analyses. To further support this hypothesis, multiple stone analyses of different stones from the same renal unit obtained during ureteroscopic or percutaneous extraction of stone fragments need to be performed in future studies.
Another hypothesis that could explain differences in stone compositions on subsequent analyses is the variation in the saturation conditions and microenvironments that exist in either the same or contralateral kidney. This could explain the different layers and compositions within the same stone (Patient C11, Fig. 2, part A), different stone types within the same kidney (Patient C9, Fig. 2, part B) and different stone types in the contralateral renal units (Patient B5, Table 2). Furthermore, these results are supported by another study that found 25.4% discordance in stone compositions of stones from contralateral renal units.10 Therefore, variations in the micro-environment within the same or the contralateral renal unit could contribute to the different stone compositions.6
Sampling error could also exist not only at the time of the stone extraction, but also when stones are analyzed at the laboratory. While all of our stones were sent to the same central laboratory for consistency, components that made up less than 10% of the entire stone were not reported, possibly masking the presence of a different trace component. Recently, micro-CT has been described as a tool for stone composition analysis and it may be more accurate in assessing stone compositions especially in heterogeneous stones.14 Currently, the role of micro-CT remains experimental and not standard clinical practice. It could be used in future studies for non-destructive assessment of stone compositions, including components that make up less than 10% of stones.
It should be noted that there were no changes in medical prophylaxis between the 2 different stone analyses in 23 out of the 25 patients with different stone compositions on subsequent sampling. Patients B1 and C6 were started on medical prophylaxis between the 2 samplings: patient B1 was started on hydrochlorothiazide 25 mg once daily and patient C6 was started on allopurinol 300 mg once daily and potassium citrate 10 mEq twice daily (Table 2, Table 3). Three other patients were already on medical prophylaxis: Patient A1 was on allopurinol 100 mg once daily, patient A9 was on potassium citrate 10 mEq twice daily, and patient B2 was on calcium citrate/Vitamin D 500mg/400units twice daily (Table 1, Table 2). The remaining patients were not on medical prophylaxis. There was no clear pattern of increased percentages of CaP stone composition on subsequent stone analyses, as opposed to the findings of Mandel and colleagues.15 Therefore, stone composition changes in the 25 patients on subsequent analyses are unlikely to be related to medical prophylaxis after a median time delay of 64.5 days. This time delay is much shorter than the 2.3-year difference between stone analyses in the Mandel study.15
What is the clinical impact of discordant stone compositions? In the present study, 7 out of the 25 patients (28%) had clinically significant differences in stone composition on subsequent sampling (Fig. 1). For example, 3 patients (A9, C10, C11) had predominantly UA on first analysis, and predominantly CaOx on subsequent analysis. Therefore, based on the first analysis, these patients would have been advised to follow a low purine diet, whereas after the second analysis, these patients would then have been advised to follow a low oxalate diet. Another example is patient A1 who had predominantly struvite stone on first analysis, while the subsequent analysis showed predominantly UA stone. Based on the first stone analysis, this patient would have been advised to be placed on prophylactic antibiotics, whereas based on the second stone analysis, he would be advised to follow a low purine diet. In 28% of patients, discordant stone compositions resulted in different dietary recommendations and medical management. Previous cost-effectiveness studies have shown that medical prophylaxis in recurrent stone formers is cheaper than the medical costs associated with acute stone episodes,16–18 in addition to reducing morbidity resulting from obstructing ureteral stones and subsequent urological procedures. Given that stone analysis is an important component of metabolic stone workup and medical prophylaxis and given its relatively inexpensive cost (around $20 at our centre), the present study argues in favour of ordering stone analysis at each stone episode.
The limitations of the present study include its retrospective nature and small sample size. Nonetheless, the present study analyzed stone analysis of 303 patients, including 118 patients with multiple stone analyses. Another limitation is that a full dietary history was not obtained. All patients had detailed metabolic stone workup and based on these results were advised to follow specific dietary recommendations. However, the impact of dietary modifications on different stone compositions on subsequent stone analyses is unknown. Another limitation of the study is the inability to assess the time required for the initial stone to be formed. Therefore, it is difficult to predict the changes in microenvironments affecting stone composition over time, especially between different stone samplings. In addition, although patients were instructed to collect all stone fragments for analysis post-SWL, it is possible that not all fragments were collected or brought for analysis. This could be addressed in a prospective fashion in future studies.
Conclusion
This study demonstrates that stone compositions differ in 21.2% on subsequent stone analyses in patients being followed at a tertiary stone centre. Reasons include sampling error, as well as differences in microenvironments between kidneys and within the same kidney. Variability of stone composition from one sampling to the next can be mistaken for true stone changes and could affect medical therapy. Therefore, in addition to simply sending a stone at the initial presentation as per current Canadian Urological Association Guidelines,3 this study supports recommendations to send stones for analysis every time a stone fragment is obtained.
Footnotes
Competing interests: Dr. Lee, Dr. Elkoushy and Dr. Andonian all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: Urolithiasis. J Urol. 2005;173:848–57. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 2.Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989;111:1006–9. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 3.Paterson R, Fernandez A, Razvi H, et al. Evaluation and medical management of the kidney stone patient. Can Urol Assoc J. 2010;4:375–9. doi: 10.5489/cuaj.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–52. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 5.Jawalekar S, Surve VT, Bhutey AK. The composition and quantitative analysis of urinary calculi in patients with renal calculi. Nepal Med Coll J. 2010;12:145–8. [PubMed] [Google Scholar]
- 6.Abdel-Halim RE, Al-Sibaai A, Baghlaf AO. The structure of large lamellar urinary stones. A quantitative chemical analytic study applying a new classification scheme. Scand J Urol Nephrol. 1993;27:337–41. doi: 10.3109/00365599309180444. [DOI] [PubMed] [Google Scholar]
- 7.Gnessin E, Mandeville JA, Handa SE, et al. Changing composition of renal calculi in patients with musculoskeletal anomalies. J Endourol. 2011;25:1519–23. doi: 10.1089/end.2010.0698. [DOI] [PubMed] [Google Scholar]
- 8.Viprakasit DP, Sawyer MD, Herrell SD, et al. Changing composition of staghorn calculi. J Urol. 2011;186:2285–90. doi: 10.1016/j.juro.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 9.Cho ST, Jung SI, Myung SC, et al. Correlation of metabolic syndrome with urinary stone composition. Int J Urol. 2013;20:208–13. doi: 10.1111/j.1442-2042.2012.03131.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadlec AO, Fridirici ZC, Acosta-Miranda AM, et al. Bilateral urinary calculi with discordant stone composition. World J Urol. 2014;32:281–5. doi: 10.1007/s00345-013-1113-4. [DOI] [PubMed] [Google Scholar]
- 11.Nakada SY, Hoff DG, Attai S, et al. Determination of stone composition by noncontrast spiral computed tomography in the clinical setting. Urology. 2000;55:816–9. doi: 10.1016/S0090-4295(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 12.Dretler SP. Stone fragility--a new therapeutic distinction. J Urol. 1988;139:1124–7. doi: 10.1016/s0022-5347(17)42801-1. [DOI] [PubMed] [Google Scholar]
- 13.Turgut M, Unal I, Berber A, et al. The concentration of Zn, Mg and Mn in calcium oxalate monohydrate stones appears to interfere with their fragility in ESWL therapy. Urol Res. 2008;36:31–8. doi: 10.1007/s00240-007-0133-1. [DOI] [PubMed] [Google Scholar]
- 14.Zarse CA, McAteer JA, Sommer AJ, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urol. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandel N, Mandel I, Fryjoff K, et al. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026–9. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 16.Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68:1808–14. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 17.Hyams ES, Matlaga BR. Cost-effectiveness treatment strategies for stone disease for the practicing urologist. Urol Clin North Am. 2013;40:129–33. doi: 10.1016/j.ucl.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Parks JH, Coe FL. The financial effects of kidney stone prevention. Kidney Int. 1996;50:1706–12. doi: 10.1038/ki.1996.489. [DOI] [PubMed] [Google Scholar]


