Abstract
Introduction:
We evaluate the diagnostic value of bladder tumour antigen (BTA stat) tests compared with urine cytology test in detecting bladder cancer.
Methods:
We searched public databases including PubMed, MEDLINE Springer, Elsevier Science Direct, Cochrane Library and Google Scholar before December 2012. To collect relevant data of BTA stat tests and urine cytology tests in patients with bladder cancer, we studied meta-analyses of sensitivity, specificity, positive likelihood ratio (LR), negative LR and diagnostic odds ratios (DOR) of BTA stat tests and cytology tests from published studies. We applied the software of Rev. Man 5.1 and Stata 11.0 to the meta-analysis.
Results:
A total of 13 separate studies consisting of 3462 patients with bladder cancer were considered in the meta-analysis. We found that the BTA stat test had a higher sensitivity than the urine cytology test (0.67, 95% confidence interval [CI] 0.64 to 0.69 vs. 0.43, 95% CI 0.40 to 0.46), but the specificity, positive LR, negative LR, DOR, the area under the curve (AUC) and Q index of the BTA stat test were lower compared with the urine cytology test. The results of the Egger’s linear regression test showed no publication bias (p > 0.05).
Conclusions:
Specificity, positive LR, negative LR, DOR, the AUC and the Q index of the urine cytology test may be superior to the BTA stat test, but the BTA stat test has greater sensitivity than the urine cytology test.
Introduction
Bladder cancer is one of the most common urologic malignancies.1 More than 25% of bladder cancer cases are still muscle-invasive at first diagnosis.2 Early diagnosis of bladder cancer remains a challenge,3 because it has low sensitivity and specificity. In recent years, the use of diagnostic categories for extragenital cytology has increasingly been discussed as an approach to improve the quality of reports.4 However, in corresponding reported urine cytology, the accuracy and sensitivity are highly variable.5 The bladder tumour antigen (BTA stat) test is a rapid, non-invasive, qualitative urine test that detects bladder tumour associated antigen (human complement factor H related protein) in urine.6,7 It is an immunochromatographic reaction to detect the bladder tumour antigen in patients with bladder cancer.8 The limitation of current urinary tumour markers is their low specificity and positive predictive value, which clinically manifests as a high false-positive rate.9 Despite significant advances in our understanding of the molecular pathology of bladder cancer, it remains a significant health problem. Muscle-invasive bladder cancer is still associated with high morbidity and mortality.10
It is debatable whether the diagnostic value of the BTA stat test in detecting bladder cancer is superior to urine cytology.11–17 To understand the nuances of the BTA test and the urine cytology test in diagnosis, we conducted a systematic review of published findings and used meta-analysis techniques to quantitatively combine results. We made a meta-analysis to assess the sensitivity, specificity, positive likelihood ratio (LR), negative LR, diagnostic odds ratio (DOR) in patients tested by BTA stat or urine cytology.
Methods
Source of material
We retrieved several public databases, mainly PubMed, MEDLINE Springer, Elsevier Science Direct, Cochrane Library and Google Scholar before December 2012. These databases cover all the available English literature. “Bladder tumor antigen test,” “BTA stat,” “urine cytology,” “cytology,” “diagnosis,” “bladder cancer,” “study” and “trial” were the key words. Moreover, references from retrieved papers were checked for additional studies. We only collected data from published papers, excluding meeting or conference abstracts.
Search methods
Four independent investigators retrieved the electronic databases. An independent PubMed, MEDLINE and Springer retrieve was done by A and B with the same method. An independent Elsevier Science Direct, Cochrane Library and Google Scholar retrieve was done by C and D with the same method. If an investigator’s assessment was not consistent with the others, then a discussion ensued regarding the final decision to include the data.
The included papers contained investigations of the patients with bladder cancer (i.e., prospective studies, retrospective studies or cross-sectional studies) and the diagnosis of bladder cancer using the BTA stat test and cytology test. The effect size as odds ratio (OR), sample size, gender or range of age were not limited. We excluded reviews, duplicated studies or reports which only described the BTA stat test data or cytology test data.
Our evaluation included study methods, sample size and recruitment. We selected papers by reading the document title and abstract. We also read the full text of papers for secondary screening to determine whether they were going to be included in the analysis. Two investigators independently completed this task. If there were differences (i.e., extracted data or information was inconsistent with another investigator), we reached an agreement through a discussion.
Extracted data included study details (e.g., the first author’s name, research year of study, year of study publication, location of participants, design of studies, follow-up time) and patient characteristics (e.g., age, gender of patients with the BTA test and the cytology test, sample size). Two investigators (A and D) extracted the data independently using the standard protocol, and a third investigator reviewed the results. We contacted authors of incorporated studies to obtain further information for data items that needed clarification. Discrepancies were resolved by discussing with our research team or by contacting the original investigators.
The meta-analysis combined the ORs in the patients with bladder cancer. The point estimates of the ORs and its 95% confidence interval (95% CI) were pooled estimated for each study. We assessed the within- and between-study variation or heterogeneity by testing Cochran’s Q-statistic.18 We also quantified the effect of heterogeneity using I2 = 100% × (Q−df)/Q formula.19 A significant Q-statistic (p < 0.10) or I2-statistic (I2 > 50) indicated heterogeneity across studies, and then the random effect model was used for meta-analysis and to account for the possibility of heterogeneity between studies; otherwise, the fixed effect model was used.20 The overall or pooled estimate of ORs was obtained using the Mantel–Haenszel method in the fixed effect model21 and the DerSimonian and Laid method in the random effect model.22
The summary receiver operating characteristic (SROC) curve was used to represent the performance of a diagnostic test,23 based on data from a meta-analysis. The SROC curve included multiple points, and the cut-off points were determined by the maximal value points, which are the value summations of the sensitivity and specificity.24 The area under the curve (AUC) and an index Q were discussed as potentially useful summaries of the curve. An upper bound was derived for the AUC based on an exact analytic expression for the homogeneous situation, and a lower bound based on the limit case Q, defined by the point where sensitivity equals specific ity: Q is invariant to heterogeneity.23
Analyses were performed using the Meta-DiSc software v.1.4 and the STATA software package v.11.0 (Stata Corporation, College Station, TX). All p values were two-sided. P values less than 0.05 were considered statistically significant.
Results
Characteristics of eligible studies
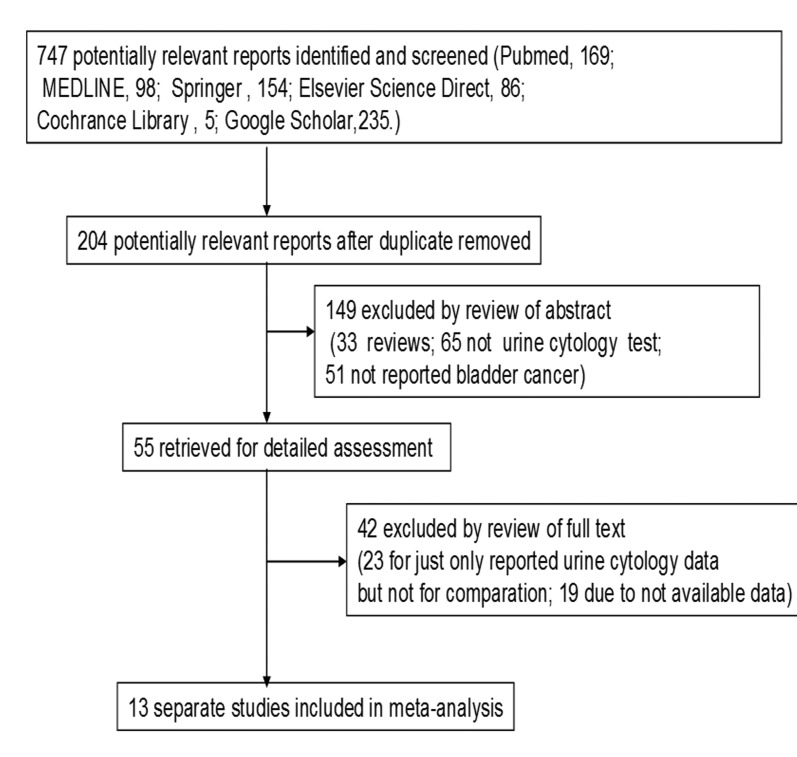
In total, we had 747 potentially relevant papers (PubMed: 169; MEDLINE: 98; Springer: 154; Elsevier Science Direct: 86; Cochrane Library: 5; Google Scholar: 235) (Fig. 1). After duplicates were removed, we had a total of 204 potentially relevant studies. During the abstract screening, 149 articles were excluded (33 were review articles, 65 did not include urine cytology tests; 51 did not report on bladder cancer). This left us with 55 studies for full publication review. Of these, 42 of these were excluded (23 because they only included urine cytology data and no comparison and 19 because there were no data available).
Fig. 1.
Flow diagram for selection of studies for the meta-analysis.
In the end, we had 13 studies to analyze (Table 1).7,9,11–17,25–28 These studies were published between 1998 and 2006 and included a total of 3462 patients with bladder cancer. Their sample size ranged from 71 to 739, and mean age from 60 to 70 years.
Table 1.
Characteristics of studies included in the meta-analysis (n=13)
| Study (publication year) | Country | Ethnicity | Sample size | Male (%) | Age, year | BTA stat test | Cytology test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| TP | FP | FN | TN | TP | FP | FN | TN | ||||||
| Wiener HG, et al.12 (1998) | Austria | European | 291 | 199 (68) | 65 | 52 | 64 | 39 | 136 | 54 | 0 | 37 | 200 |
| Leyh H, et al.11 (1999) | Austria, France, Germany, Italy | European | 240 | 172 (72) | 64 | 70 | 45 | 37 | 79 | 35 | 1 | 72 | 123 |
| Pode D, et al.13 (1999) | Israel | Asian | 250 | 207 (83) | NA | 106 | 38 | 22 | 84 | 51 | 5 | 77 | 107 |
| Ramakumar S, et al.25 (1999) | United States | American | 196 | 152 (78) | 66 | 42 | 38 | 15 | 101 | 24 | 3 | 30 | 55 |
| Sharma S, et al.9 (1999) | United States | American | 278 | NA | NA | 23 | 43 | 11 | 201 | 10 | 1 | 24 | 243 |
| Giannopoulos A, et al.26 (2000) | Greece | European | 168 | 145 (86) | 66 | 71 | 30 | 28 | 39 | 38 | 4 | 61 | 65 |
| Poulakis V, et al.15 (2001) | United States | American | 739 | 485 (66) | 67 | 200 | 74 | 85 | 149 | 157 | 13 | 96 | 306 |
| Raitanen MP, et al.7 (2001) | Finland | European | 445 | NA | NA | 63 | 47 | 55 | 280 | 21 | 6 | 97 | 321 |
| van Rhijn BW, et al.27 (2001) | Netherlands | European | 109 | 84 (77) | 67 | 13 | 9 | 10 | 35 | 5 | 2 | 18 | 42 |
| Fukui Y, et al.14 (2001) | Japan | Asian | 71 | NA | NA | 18 | 1 | 13 | 39 | 5 | 0 | 26 | 40 |
| Saad A, et al. (2002) | United Kingdom | European | 120 | 100 (83) | 70 | 25 | 9 | 27 | 59 | 33 | 12 | 19 | 56 |
| Boman H, et al.28 (2002) | Sweden | European | 304 | NA | NA | 89 | 54 | 65 | 91 | 60 | 12 | 73 | 131 |
| Sun Y, et al.16 (2006) | China | Asian | 251 | 177 (71) | 60 | 116 | 13 | 35 | 87 | 55 | 0 | 96 | 100 |
BTA: bladder tumour antigen; TP: true positive; FP: false positive; FN: false negative; TN: true negative.
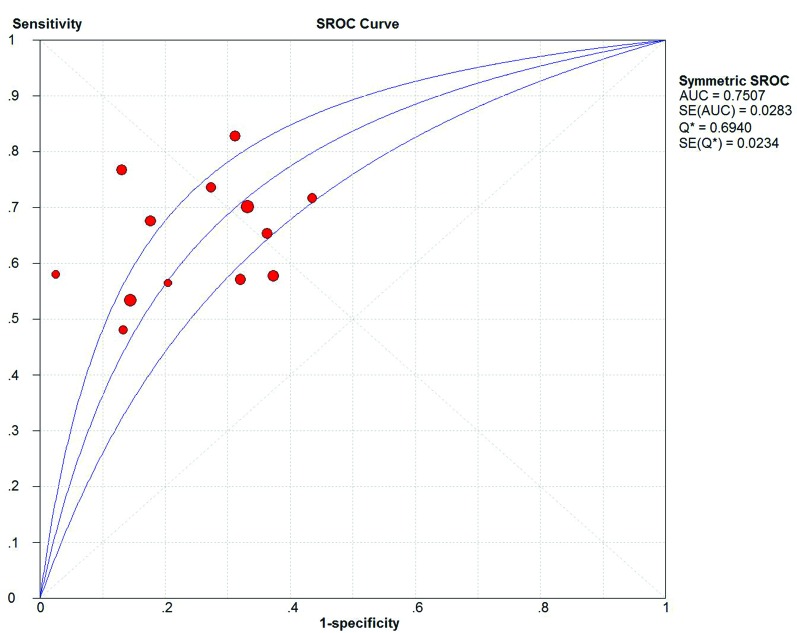
We summarized the overall meta-analysis of bladder cancer patients with the BTA stat test (Table 2). Of the 13 studies, 3175 patients with bladder cancer were considered in the meta-analysis. We used the random effect model (Q2 = 56.23, I2 = 96.4%, p < 0.01) to combine the data of true positive (TP), false positive (FP), false negative (FN) and true negative (TN) numbers. The overall meta-analysis showed that sensitivity, specificity, positive LR, negative LR and DOR of BTA stat test were 0.67 (95% CI=0.64 to 0.69), 0.75 (95% CI=0.73 to 0.77), 2.58 (95% CI=2.07 to 3.20), 0.47 (95% CI=0.39 to 0.55), 5.88 (95% CI=4.06 to 8.63), respectively. The AUC and Q index were 0.75 and 0.69, respectively (Fig. 2, Fig. 3).
Table 2.
The indexes of bladder cancer diagnose with by BTA stat test and urine cytology test
| Diagnostic methods | Parameter | Test of association | Test of heterogeneity | Model | Egger’s test for publication bias | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Estimates | 95% CI | Q | p value | I2 (%) | t | p value | |||
| BTA stat test | Overall | — | — | 56.23 | <0.01 | 96.4 | Random | 1.16 | 0.27 |
| Sensitivity | 0.67 | 0.64 to 0.69 | 55.80 | <0.01 | 78.5 | — | — | — | |
| Specificity | 0.75 | 0.73 to 0.77 | 105.39 | <0.01 | 88.6 | — | — | — | |
| Positive LR | 2.58 | 2.07 to 3.20 | 62.08 | <0.01 | 80.7 | Random | — | — | |
| Negative LR | 0.47 | 0.39 to 0.55 | 46.73 | <0.01 | 74.3 | Random | — | — | |
| DOR | 5.88 | 4.06 to 8.63 | 53.82 | <0.01 | 77.7 | Random | — | — | |
| Cytology test | Overall | — | — | 68.47 | <0.01 | 97.1 | Random | −0.63 | 0.54 |
| Sensitivity | 0.43 | 0.40 to 0.46 | 116.29 | <0.01 | 89.7 | — | — | — | |
| Specificity | 0.97 | 0.96 to 0.98 | 72.26 | <0.01 | 83.4 | — | — | — | |
| Positive LR | 10.56 | 6.21 to 17.96 | 37.69 | <0.01 | 68.2 | Random | — | — | |
| Negative LR | 0.62 | 0.54 to 0.72 | 131.77 | <0.01 | 90.9 | Random | — | — | |
| DOR | 18.24 | 10.54 to 31.57 | 30.72 | <0.01 | 60.9 | Random | — | — | |
BTA: Bladder tumour antigen; CI: confidence interval; LR: likelihood ratio; DOR: diagnostic odds ratio.
Fig. 2.
The summary receiver operating characteristic (SROC) curve of the bladder tumour antigen stat test.
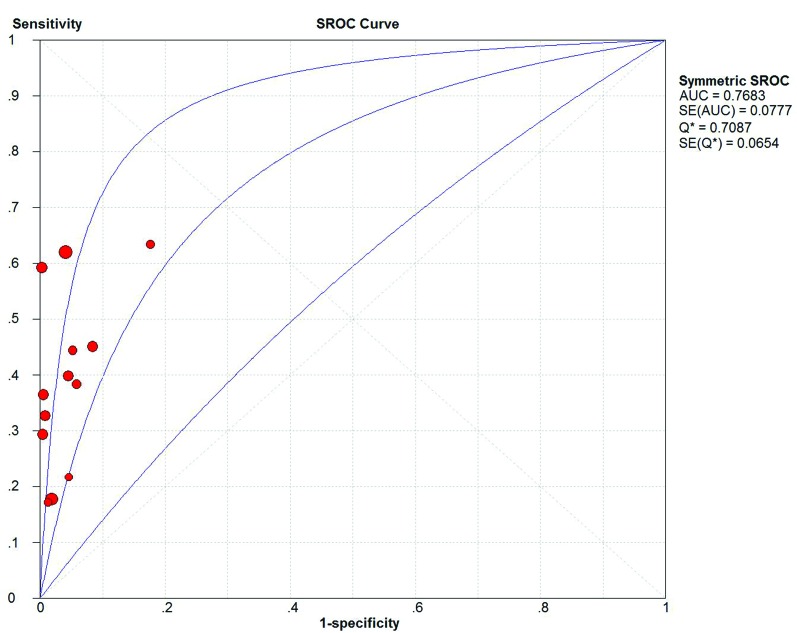
Fig. 3.
The summary receiver operating characteristic (SROC) curve of cytology.
We also collected the pooled meta-analysis of bladder cancer patients with the cytology test (Table 2). In the 13 studies, 3122 patients with bladder cancer were considered in this meta-analysis. We used the random effect model (Q2 = 68.47, I2 = 97.1%, p < 0.01) to merge the data of the TP, FP, FN and TN numbers. The overall meta-analysis showed that sensitivity, specificity, positive LR, negative LR and DOR of cytology test were 0.43 (95% CI=0.40 to 0.46), 0.97 (95% CI=0.96 to 0.98), 10.56 (95% CI=6.21 to 17.96), 0.62 (95% CI=0.54 to 0.72), 18.24 (95% CI=10.54 to 31.57), respectively. The AUC and Q index were 0.77 and 0.71, respectively (Fig. 3).
The Egger’s test assessed publication bias. For all samples, the Egger’s test provided no evidence of publication bias for this meta-analysis (Table 2).
Discussion
Many studies7,9,13,15,16,25–27 have reported on the diagnostic value of the BTA stat and cytology test in detecting bladder cancer. These studies, however, have demonstrated mixed results due to small sample sizes or low statistical power. In our meta-analysis, we combined 13 separate studies, consisting of 3462 patients to compare the diagnostic value of the BTA stat test with the urine cytology test in detecting bladder cancer. We found that the BTA stat test had higher sensitivity than the urine cytology test, but specificity, positive LR, negative LR, DOR, the AUC and Q index of the BTA stat test were lower compared with the urine cytology test.
The BTA stat test is a “point-of-care” rapid immunochromatographic assay for detecting the bladder tumour antigen (BTA) in the urine.17 The BTA stat test is one of non-invasive tumour markers in the urine and has become a useful marker in bladder cancer detection.16 The antigen detected is a human complement factor-H related protein.17 Similar to the results in other studies,6,11,13,29 the overall sensitivity of the BTA stat test was superior to urine cytology; the latter, however, had better specificity. Therefore, the study suggested that the BTA stat test would not replace the urine cytology test in detecting bladder cancer. Vriesema and colleagues identified that 89% of their patients would prefer cystoscopy as the diagnostic method if a bladder tumour maker’s sensitivity was less than 90%.30
Diagnostic categories reflect the adequacy of the materials for interpretation and the presence or absence of cancer cells.4 The potential value of urine cytology has been reduced by the relative inexperience of most pathologists in examining urinary specimens, and by the lack of cellular criteria specifically reflecting the morphology of low-grade papillary and flat lesions of the bladder epithelium.31 Yet, urine cytology is increasingly accepted as a diagnostic tool to detection and follow patients with bladder cancer.31 However, its low sensitivity may limit its use. Interestingly, its sensitivity is highly associated with tumour stage. Millan-Rodriquez and colleagues demonstrated that low-stage tumours had only a 37% recurrence and 0% progression rate, while high-stage tumors had a relative 54% recurrence and 15% progression rates.32 Hence, for high-stage tumours that are more likely to progress, the sensitivities of common tumour markers are high. The use of diagnostic tools to detect bladder cancer should be based on tumour stage and grade.
Our study has limitations. Only published studies were included; thus, there may be publication bias. In addition, significant between-study heterogeneities were detected in the current meta-analysis, and may have distorted the meta-analysis. The degree of heterogeneity is one of the major concerns in a meta-analysis,33 as non-homogeneous data are liable to result in misleading results. Different populations may contribute to the heterogeneity among the selected studies. Moreover, the population from each country was not uniform. Hence, the results of this meta-analysis should be interpreted with caution. We minimized the likelihood of bias by developing a detailed protocol before initiating the study, by performing a meticulous search of published studies and by using explicit methods for study selection, data extraction and data analysis.
Conclusion
Sensitivity and specificity of the urine cytology and BTA stat tests are the 2 of the most critical factors in the diagnosis of bladder cancer. In this meta-analysis, we evaluated the pooled data of sensitivity, specificity, positive LR, negative LR, DOR, AUC and Q index from 8 clinical trials. We found that urine cytology test was the most specific and the BTA stat test was the most sensitive in detecting bladder cancer. Due to the limited sample in our meta-analysis, prospective studies with larger samples are warranted.
Acknowledgments
We would like to thank all respondents of the study and all the people who give the help for this study.
Footnotes
Competing interests: Dr. Guo, Dr. Wang, Dr. Gao, Dr. Shi, Dr. Sun and Dr. Wan all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Sartini D, Muzzonigro G, Milanese G, et al. Upregulation of tissue and urinary nicotinamide N-methyltransferase in bladder cancer: Potential for the development of a urine-based diagnostic test. Cell Biochem Biophys. 2013;65:473–83. doi: 10.1007/s12013-012-9451-1. [DOI] [PubMed] [Google Scholar]
- 2.Zlotta AR, Roumeguere T, Kuk C, et al. Select screening in a specific high-risk population of patients suggests a stage migration toward detection of non-muscle-invasive bladder cancer. Eur Urol. 2011;59:1026–31. doi: 10.1016/j.eururo.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava AK, Singh PK, Srivastava K, et al. Diagnostic role of survivin in urinary bladder cancer. Asian Pac J Cancer Prev. 2013;14:81–5. doi: 10.7314/APJCP.2013.14.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Takashi M, Schenck U, Kissel K, et al. Use of diagnostic categories in urinary cytology in comparison with the bladder tumour antigen (BTA) test in bladder cancer patients. Int Urol Nephrol. 1999;31:189–96. doi: 10.1023/A:1007124724817. [DOI] [PubMed] [Google Scholar]
- 5.Turco P, Houssami N, Bulgaresi P, et al. Is conventional urinary cytology still reliable for diagnosis of primary bladder carcinoma? Accuracy based on data linkage of a consecutive clinical series and cancer registry. Acta cytologica. 2011;55:193–6. doi: 10.1159/000320861. [DOI] [PubMed] [Google Scholar]
- 6.Raitanen M-P, Marttila T, Kaasinen E, et al. Sensitivity of human complement factor H related protein (BTA stat) test and voided urine cytology in the diagnosis of bladder cancer. J Urol. 2000;163:1689–92. doi: 10.1016/S0022-5347(05)67521-0. [DOI] [PubMed] [Google Scholar]
- 7.Raitanen M-P, Marttila T, Nurmi M, et al. Human complement factor H related protein test for monitoring bladder cancer. J Urol. 2001;165:374–7. doi: 10.1097/00005392-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez Baños JL, Martín García B, Hernández Rodríguez R, et al. Usefulness of BTA Stat test (Bard) in the diagnosis of bladder cancer. Preliminary results and comparison with cytology and cystoscopy [in Spanish] Arch Esp Urol. 1998;51:778–82. [PubMed] [Google Scholar]
- 9.Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22* and BTA stat [dagger] J Urol. 1999;162:53–7. doi: 10.1097/00005392-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Morgan R, Bryan RT, Javed S, et al. Expression of Engrailed-2 (EN2) protein in bladder cancer and its potential utility as a urinary diagnostic biomarker. Eur J Cancer. 2013;49:2214–22. doi: 10.1016/j.ejca.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Leyh H, Marberger M, Conort P, et al. Comparison of the BTA statTM test with voided urine cytology and bladder wash cytology in the diagnosis and monitoring of bladder cancer. Eur Urol. 1999;35:52–6. doi: 10.1159/000019819. [DOI] [PubMed] [Google Scholar]
- 12.Wiener H, Mian C, Haitel A, et al. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. 1998;159:1876–80. doi: 10.1016/S0022-5347(01)63184-7. [DOI] [PubMed] [Google Scholar]
- 13.Pode D, Shapiro A, Wald M, et al. Noninvasive detection of bladder cancer with the BTA stat test. J Urol. 1999;161:443–6. doi: 10.1016/S0022-5347(01)61918-9. [DOI] [PubMed] [Google Scholar]
- 14.Fukui Y, Samma S, Fujimoto K, et al. Screening methods in the detection of bladder cancer: Comparison of nuclear matrix protein-22, bladder tumor antigen and cytological examinations [in Japanese] Hinyokika Kiyo. 2001;47:311–4. [PubMed] [Google Scholar]
- 15.Poulakis V, Witzsch U, De Vries R, et al. A comparison of urinary nuclear matrix protein-22 and bladder tumour antigen tests with voided urinary cytology in detecting and following bladder cancer: The prognostic value of false-positive results. BJU Int. 2001;88:692–701. doi: 10.1046/j.1464-410X.2001.02355.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, He D-l, Ma Q, et al. Comparison of seven screening methods in the diagnosis of bladder cancer. Chin Med J. 2006;119:1763–71. [PubMed] [Google Scholar]
- 17.Saad A, Hanbury D, McNicholas T, et al. A study comparing various noninvasive methods of detecting bladder cancer in urine. BJU Int. 2002;89:369–73. doi: 10.1046/j.1464-4096.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 18.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Systematic Reviews in Health Care: Meta-Analysis in Context. 2001285-312.
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Li LH, Pan HF, et al. Association of ITGAM polymorphism with systemic lupus erythematosus: A meta-analysis. J Eur Acad Dermatol Venereol. 2011;25:271–5. doi: 10.1111/j.1468-3083.2010.03776.x. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. The Challenge of Epidemiology: Issues and Selected Readings. 2004;1:533–53. [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Walter S. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–56. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Bland JM. Diagnostic tests 3: Receiver operating characteristic plots. BMJ. 1994;309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakumar S, Bhuiyan J, Besse JA, et al. Comparison of screening methods in the detection of bladder cancer. J Urol. 1999;161:388–94. doi: 10.1016/S0022-5347(01)61899-8. [DOI] [PubMed] [Google Scholar]
- 26.Giannopoulos A, Manousakas T, Mitropoulos D, et al. Comparative evaluation of the BTAstat test, NMP22, and voided urine cytology in the detection of primary and recurrent bladder tumors. Urology. 2000;55:871–5. doi: 10.1016/S0090-4295(00)00489-1. [DOI] [PubMed] [Google Scholar]
- 27.van Rhijn BW, Lurkin I, Kirkels WJ, et al. Microsatellite analysis DNA test in urine competes with cystoscopy in follow-up of superficial bladder carcinoma. Cancer. 2001;92:768–75. doi: 10.1002/1097-0142(20010815)92:4<768::AID-CNCR1381>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Boman H, Hedelin H, Holmang S. Four bladder tumor markers have a disappointingly low sensitivity for small size and low grade recurrence. J Urol. 2002;167:80–3. doi: 10.1016/S0022-5347(05)65387-6. [DOI] [PubMed] [Google Scholar]
- 29.Sarosdy MF, Hudson MLA, Ellis WJ, et al. Improved detection of recurrent bladder cancer using the bard BTA stat test. Urology. 1997;50:349–53. doi: 10.1016/S0090-4295(97)00292-6. [DOI] [PubMed] [Google Scholar]
- 30.Vriesema J, Poucki M, Kiemeney L, et al. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: A utility analysis. Urology. 2000;56:793–7. doi: 10.1016/S0090-4295(00)00777-9. [DOI] [PubMed] [Google Scholar]
- 31.Murphy WM, Soloway MS, Jukkola AF, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer. 1984;53:1555–65. doi: 10.1002/1097-0142(19840401)53:7<1555::AID-CNCR2820530723>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–4. doi: 10.1016/S0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 33.Moreno SG, Sutton AJ, Thompson JR, et al. A generalized weighting regression-derived meta-analysis estimator robust to small-study effects and heterogeneity. Stat Med. 2012;31:1407–17. doi: 10.1002/sim.4488. [DOI] [PubMed] [Google Scholar]