Abstract
The corneal stroma is enriched in small leucine-rich proteoglycans (SLRPs), including both class I (decorin and biglycan) and class II (lumican, keratocan and fibromodulin). Transparency is dependent on the assembly and maintenance of a hierarchical stromal organization and SLRPs are critical regulatory molecules. We hypothesize that cooperative interclass SLRP interactions are involved in the regulation of stromal matrix assembly. We test this hypothesis using a compound Bgn−/o/Lum−/− mouse model and single Lum−/− or Bgn−/o mouse models and wild type controls. SLRP expression was investigated using immuno-localization and immuno-blots. Structural relationships were defined using ultrastructural and morphometric approaches while transparency was analyzed using in vivo confocal microscopy. The compound Bgn−/o/Lum−/− corneas demonstrated gross opacity that was not seen in the Bgn−/o or wild type corneas and greater than that in the Lum−/− mice. The Bgn−/o/Lum−/− corneas exhibited significantly increased opacity throughout the stroma compared to posterior opacity in the Lum−/− and no opacity in Bgn−/o or wild type corneas. In the Bgn−/o/Lum−/− corneas there was abnormal lamellar and fibril structure consistent with the functional deficit in transparency. Lamellar structure was disrupted across the stroma with disorganized fibrils, and altered fibril packing. In addition, fibrils had larger and more heterogeneous diameters with an abnormal structure consistent with abnormal fibril growth. This was not observed in the Bgn−/o or wild type corneas and was restricted to the posterior stroma in Lum−/− mice. The data demonstrate synergistic interclass regulatory interactions between lumican and biglycan. These interactions are involved in regulating both lamellar structure as well as collagen fibrillogenesis and therefore, corneal transparency.
Keywords: Corneal Stroma, Biglycan, Lumican, Collagen, Fibrillogenesis, Stromal Organization
1. Introduction
The corneal stroma is enriched in small leucine-rich proteoglycans (SLRPs). There are 2 major classes of SLRPs in the corneal stroma. Decorin and biglycan, are class I SLRPs and lumican, keratocan and fibomodulin are class II SLRPs (Chen and Birk, 2011, 2013; Schaefer and Iozzo, 2008). These SLRPs demonstrate differences in their expression patterns in the developing and mature corneal stroma (Chen and Birk, 2011; Schaefer and Iozzo, 2008). Expression of both class I SRRPs is homogenous across the corneal stroma. However, biglycan expression is low in the mature stroma while decorin expression remains high (Zhang et al., 2009). Class II SLRPs show both temporal and spatial differences. Lumican and keratocan are homogeneous across the cornea stroma at birth, but the expression of lumican is restricted to the posterior stroma after maturation (Chakravarti et al., 2006; Chen et al., 2010; Zhang et al., 2009). Fibromodulin expression in the corneal stroma is strongest at P14, but is decreased and restricted to the peripheral cornea with maturation (Chen et al., 2010). In addition, class I SLRPs have chondroitin sulfate/dermatan sulfate glycosaminoglycan (GAG) chains while class II SLRPs have keratan sulfate GAGs (Chen and Birk, 2013; Schaefer and Iozzo, 2008).
Human congenital corneal stromal dystrophy is the consequence of mutations in class I decorin that result in an altered protein core associated with abnormal stromal structure and function (Kim et al., 2011; Lee et al., 2012; Rodahl et al., 2006). Mutations in keratocan result in cornea plana with a change in corneal curvature resulting in altered refraction, but no significant fibril structural defects (Liu et al., 2003; Pellegata et al., 2000). Data has suggested a linkage between altered lumican expression and high myopia (Feng et al., 2013; Liao et al., 2013). Genetic mouse models support a role for SLRPs in theses human conditions. In addition, the mouse models have demonstrated that SLRPs are critical regulators of collagen fibrillogenesis particularly the linear and lateral growth of protofibrils into mature fibrils(Chakravarti et al., 1998; Chakravarti et al., 2003; Chakravarti et al., 2006; Chen and Birk, 2013; Chen et al., 2010; Ezura et al., 2000; Zhang et al., 2009). In a decorin-null corneal stroma there is a severe disruption of fibril structure and corneal function while the biglycan-null stroma has a phenotype comparable to wild type stromas (Zhang et al., 2009). Corneal stromas deficient in class II, lumican have a fibril phenotype restricted to the posterior stromal with both fibril structure and transparency disrupted in this region (Chakravarti et al., 2000; Chakravarti et al., 2006). In contrast, deficiency in keratocan or fibromodulin is not associated with a fibril phenotype (Chen et al., 2010; Liu et al., 2003). These data suggested that there are dominant regulators and modulatory SLRPs in each class. We suggest that in the corneal stroma, decorin and lumican are dominant regulators while biglycan and keratocan/fibromodulin fine tune the regulatory activity both spatially and temporally.
Expression of SLRPs can be coordinated via gene clustering with class I decorin and class II lumican and keratocan cis-clustered on mouse chromosome 10 (Chakravarti and Magnuson, 1995; Danielson et al., 1999) and human chromosome 12 (Schaefer and Iozzo, 2008). SLRPs also have the potential to alter expression of the other class members, for instance, in the absence of decorin, biglycan expression can be increased (Zhang et al., 2009). In addition, the expression of lumican has been shown to drive the expression of keratocan (Carlson et al., 2005). Altered expression of both class I and class II SLRPs also was observed in a mutant decorin transgenic mouse model (Chen et al., 2011). Regulatory interactions across classes have been demonstrated by an increased severity of the tendon fibril structural phenotype in the absence of both biglycan and fibromodulin compared to either one alone (Ameye et al., 2002). Decorin and biglycan compete for a binding site on collagen I while fibromodulin and lumican compete for a different binding site (Pringle and Dodd, 1990; Svensson et al., 2000). This supports an interclass cooperativity in the regulation of fibril growth. Defining the network of regulatory SLRP interactions is essential to elucidate the roles in development, maintenance and regeneration of tissue-specific structures and functions.
In this work, we test the hypothesis that there are interclass regulatory interactions involving dominant and modulatory SLRPs. The roles of lumican and biglycan are investigated using lumican-null, biglycan- and compound lumican/biglycan-null mouse models and an analysis of corneal stromal matrix assembly. The data indicate a synergistic regulatory role for interclass interactions involving biglycan and lumican in regulation of corneal stromal matrix assembly. The interclass cooperation has significant impact on the development of cornea structure and transparency.
2. Results
2.1 Characterization of compound Lum/Bgn-null mice
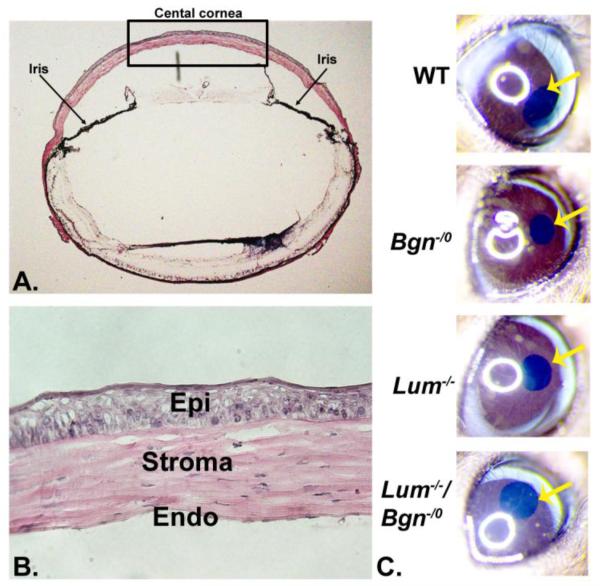
To analyze interclass interactions involving class I biglycan and class II lumican in regulation of stromal matrix assembly, we generated a compound Bgn−/o/Lum−/− mouse model by cross breeding of the single null mice. The mice were viable and demonstrated opacity of the cornea by gross examination (Fig. 1). The wild type and Bgn-/0 corneas were clear while the Lum−/− cornea demonstrated gross opacity that was not as severe as that observed in the compound-null corneas.
Fig. 1. Characterization of the compound Bgn−/o/Lum−/− mouse cornea.
(A) The black rectangle indicates the central cornea where all analyses were carried out. (B) The corneal stroma from the central cornea was analyzed. (C) Compound-null mice demonstrated corneal opacity with no opacity in Bgn−/o and wild type mice. Lum−/− mice showed less opacity than the compound mice. (The yellow arrow indicates the central cornea)
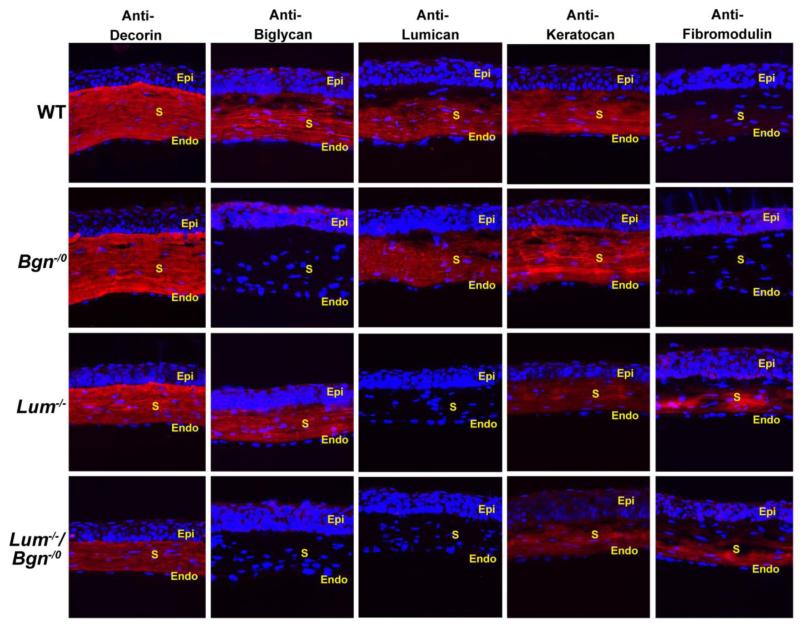
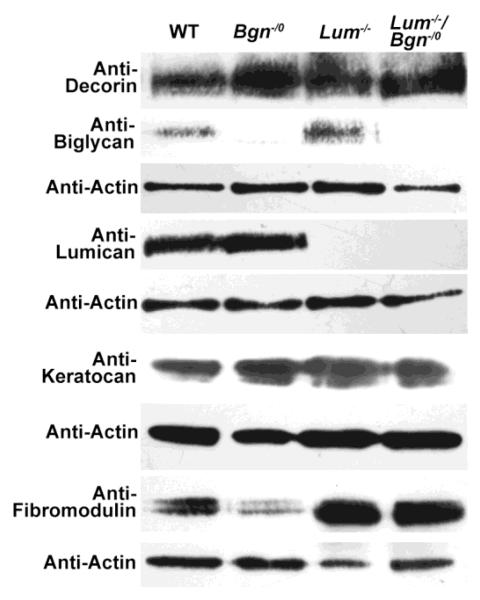
SLRP expression was analyzed in the Bgn−/o/Lum−/− corneas as well as in wild type, Lum−/−,and Bgn−/0 corneas using immuno-localization (Fig. 2) and immuno-blots (Fig. 3). The compound Bgn−/o/Lum−/− P30 corneas were deficient in biglycan and lumican. The wild type corneas demonstrated homogeneous expression of biglycan, lumican and keratocan with an absence of fibromodulin. As expected, the Bgn−/0 and Lum−/− corneas were null for lumican and biglycan respectively (Chakravarti et al., 1998; Xu et al., 1998). The Bgn−/o/Lum−/− and Lum−/− corneas also demonstrated a decrease in keratocan. There was a significant up-regulation of fibromodulin expression in Lum−/− corneas (2.4 fold, p=0.04), and a 1.7 fold increase in Bgn−/o/Lum−/− corneas (p=0.059). Decorin expression was not affected in any of the null mice. These mouse models will be utilized to address our hypothesis that interclass interaction between lumican and biglycan plays important roles in regulating corneal function.
Fig. 2. Immunolocaization of SLRPs in the compound Bgn−/o/Lum−/− mouse cornea.
The wild type corneas demonstrated homogeneous expression of biglycan, lumican and keratocan with an absence of fibromodulin. Both lumican and biglycan immuno-reactivity were absent in cornea stromas in compound Bgn−/o/Lum−/− mice as expected. Increased fibromodulin and decreased keratocan expression was observed in Lum−/− and Bgn−/o/Lum−/− mice. There were no significant changes in immuno-reactivity of decorin across all groups. (S: stroma; Epi: epithelium; Endo: endothelium).
Fig. 3. SLRP expression in the compound Bgn−/o/Lum−/− mouse cornea.
SLRP expression was analyzed using immuno-blots with actin expression as a control. As expected, the Bgn−/0 and Lum−/− corneas were null for lumican and biglycan respectively. Both Bgn−/o/Lum−/− and Lum−/− corneas demonstrated a decrease in keratocan and increase in fibromodulin expression. Decorin expression was not affected in any of these mice.
2.2 Increased stromal opacity in compound Bgn−/o/Lum−/− mouse corneas
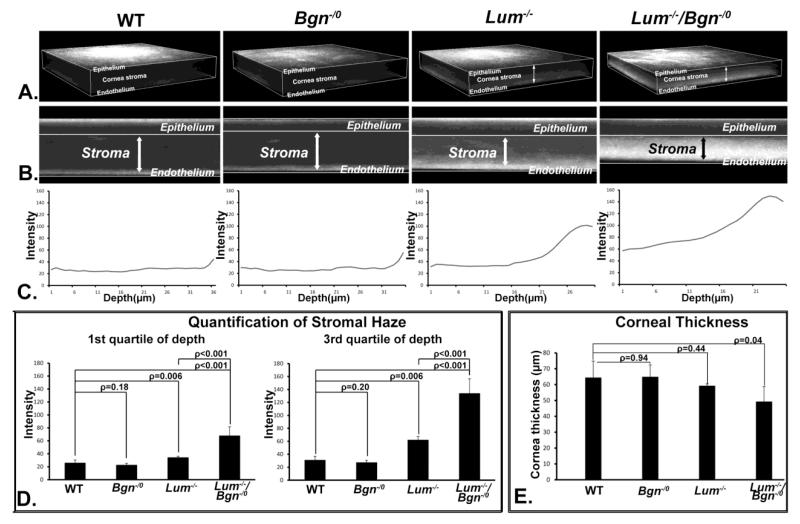
Changes in corneal transparency were analyzed in P30 Bgn−/o/Lum−/− as well as in wild type, Lum−/−,and Bgn−/0 mice using in vivo confocal microscopy (Fig. 4). The Bgn−/0 mouse stroma had virtually no opacity and was comparable to the wild type controls. It has been previously demonstrated that the Lum−/− corneas have increased light scattering in the posterior stroma although its expression is homogeneous across the whole stroma at this stage (P30)(Chakravarti et al., 2000; Chakravarti et al., 2006). The data presented here are consistent with our previous work. In contrast to the Lum−/− mouse model, the compound Bgn−/o/Lum−/− mouse model has a significant increase in stromal opacity compared to the opacity in the posterior stroma of the Lum−/− cornea or the lack of opacity in the Bgn−/o cornea, in both anterior and posterior stroma (Fig. 4). Differences in spatial localization of the functional deficits were also observed with the compound Bgn−/o/Lum−/− stromas showing homogeneous opacity throughout the stroma and the Lum−/− stromas demonstrating a posterior localization of the opacity. The in vivo confocal images from the compound mutant mice also demonstrated substantially and consistently thinner corneas compared to the single null or wild type corneas (Fig. 4, p=0.04). The observed defects in the compound mutant corneas are not the additive combination of the Bgn−/o and Lum−/− phenotypes. These data suggest a synergistic role for class I biglycan and class II lumican in the regulation of corneal stromal transparency.
Fig. 4. Compound Bgn−/o/Lum−/− mice demonstrate severe corneal opacity.
Corneal transparency was analyzed using In vivo confocal. (A) Three dimensional renderings (B) and representative sections demonstrated that corneas from wild type and Bgn−/o mice were comparable with no light scattering in the stroma. Lum−/− mice exhibited light scattering in the stroma with the opacity restricted mostly in the posterior stroma, where the majority of the lumican is located after P30. Compared to the Lum−/− mice; Bgn−/o/Lum−/− mice demonstrated increased opacity. (C) The stromal haze for each genotype was presented as a depth intensity profile and (D) quantitated for both the anterior (1st quartile) and posterior (3rd quartile) stroma. (E) The thickness of the cornea stroma was significantly decreased in the compound Bgn−/o/Lum−/− mice compared to wild type controls (ρ=0.04).
2.3 Abnormal lamella structure in the Bgn−/o/Lum−/− corneal stroma
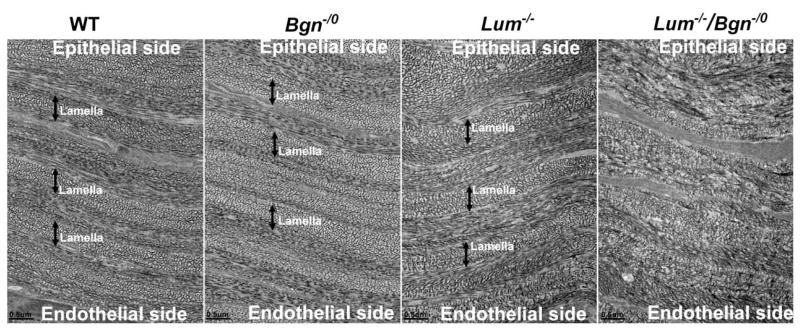
The effects of altered interclass SLRP interactions on stromal structure were examined. Orthogonally organized lamellae composed of regularly packed small diameter fibrils are essential for corneal transparency (Maurice, 1957). The compound Bgn−/o/Lum−/− mouse model demonstrated a significant disruption of lamellar structure with disorganized intra-lamella fibril packing across the entire cornea stroma. This was in marked contrast to that the normal organization observed in wild type and Bgn−/o corneas that were comparable (Fig. 5). The Lum−/− stromas demonstrated significant disruption of lamellae in the posterior stroma (Chakravarti et al., 2000; Chakravarti et al., 2006) with only a modest disruption in the mid stroma (Fig. 5). As described for the functional data above, these data suggest a synergistic interaction between biglycan and lumican in the regulation of lamellar –stromal organization. This influence on higher order structure within the structural hierarchy required for transparency suggests keratocyte involvement in determination of long range order.
Fig. 5. Disorganized lamellar structure in compound Bgn−/o/Lum−/− mice.
Compound Bgn−/o/Lum−/− mice demonstrated a significant disruption of lamellar structure with disorganized intra-lamella fibril packing across the entire cornea stroma. This was in marked contrast to the normal organization observed in wild type and Bgn−/o corneas. The Lum−/− stromas demonstrated significant disruption of lamellae in the posterior stroma (supplemental Fig 1) with only a modest disruption in the mid stroma. (Mid stroma sections from P30 corneas; double headed arrows indicate lamella. Bar=0.5μm)
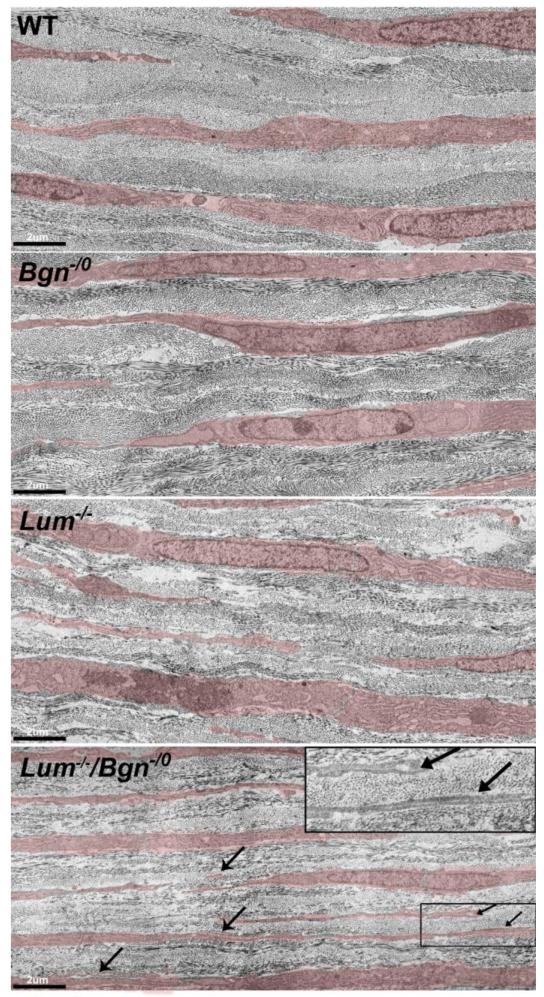
The influence of biglycan and lumican on stroma keratocytes was examined using the SLRP-null mouse models. Keratocytes synthesize, secrete and direct assembly of the extracellular macromolecules required for corneal stromal assembly. During development, keratocytes transit from active proliferating status to a quiescent status where keratocytes are compact with abundant rough endoplasmic reticulum and lamellipodia interspersed in the extracellular lamellae. It has been suggested that perpendicular keratocyte lamellipodia direct collagen fibril deposition resulting in lamellar assembly and orthogonal organization of the stroma (Birk and Trelstad, 1984; Doane and Birk, 1991; Trelstad and Coulombre, 1971). Abnormal keratocyte organization was observed in the P4 Bgn−/o/Lum−/− stroma. Compared to wild type, Bgn−/o and Lum−/− mouse, keratocytes in the compound Bgn−/o/Lum−/− mouse corneal stroma exhibit slender cell bodies with lengthy lamellipodia that often are overlap with each other in disorganized lamellae (Fig. 6). The data suggest a role for lumican and biglycan in the regulation of keratocytes organization.
Fig. 6. Altered keratocyte organization in compound Bgn−/o/Lum−/− mice.
Keratocytes (transparent red) are compact with abundant rough endoplasmic reticulum and lamellipodia are interspersed within the lamellae. Compared to wild type, Bgn−/o, and Lum−/− mice, compound Bgn−/o/Lum−/− mice exhibited disorganized keratocytes. The keratocytes are flattened and extended with extended lamellipodia (arrows) that often overlap with each other (insert) within the disorganized lamellae. (P4 mice, Bar=2μm)
2.4 Dysfunctional regulation of collagen fibrillogenesis in the Bgn−/o/Lum−/−corneal stroma
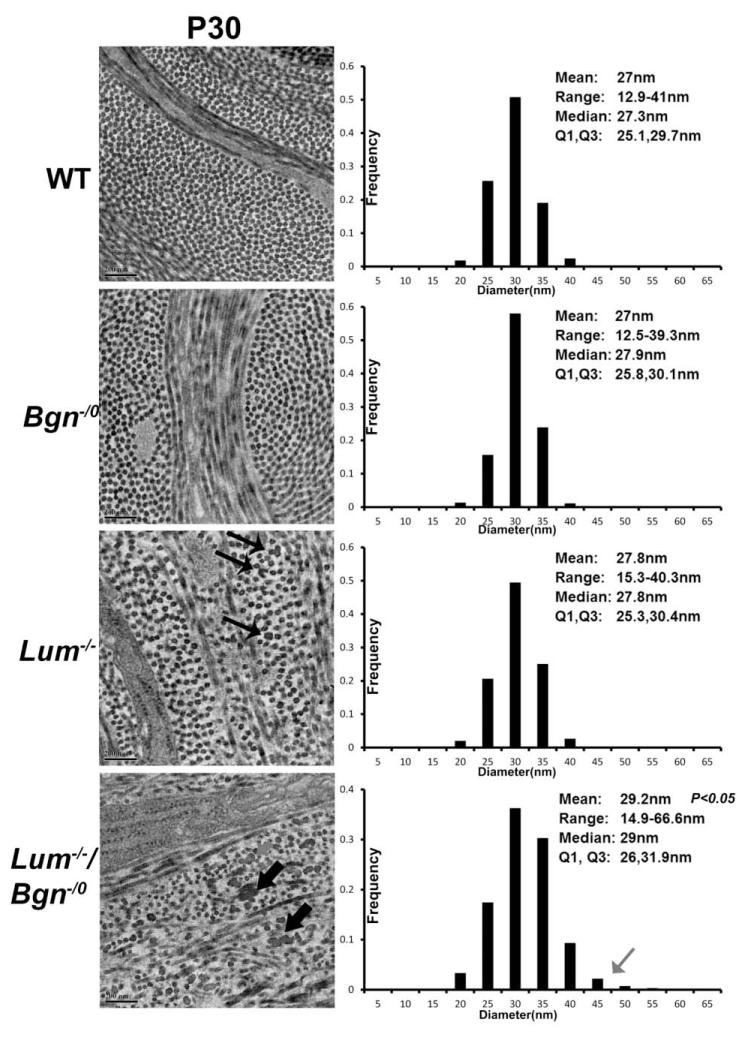
To address interclass SLRP regulatory interactions in the corneal stroma, collagen fibrillogenesis in the SLRP mouse models was analyzed using transmission electron microscopy and morphometric analysis. In the compound Bgn−/o/Lum−/− stroma the lamella were composed of disorganized fibrils; both the regular packing and orientation was disrupted compared to the organization in the wild type and Bgn−/o stromas which were comparable. The mid corneal stroma of Lum−/− mice showed a modest disruption in fibril packing, but no disruption in orientation. However the posterior Lum−/− stroma demonstrated comparable disruption to that observed in the compound Bgn−/o/Lum−/− stroma (Supplemental Fig. 1 (Chakravarti et al., 2000)).
Larger diameter collagen fibrils with irregular contours were observed in compound Bgn−/o/Lum−/− mice compared to Lum−/−, Bgn−/o and wild type mice (Fig. 7). The mean of the fibril diameter in Bgn−/o/Lum−/− mice is 29.2 nm and is significantly greater that than that in Lum−/− (27.8nm, p=0.046), Bgn−/o (27.0nm, p=0.037), and wild type mice (27.0nm, p=0.035). The fibril distribution was heterogeneous in compound-null mice compared to other groups, and a group of larger diameter fibrils was observed, indicating a dysfunctional regulation of lateral fibril growth in the disorganized lamellae.
Fig. 7. Altered fibril structure and organization in compound Bgn−/o/Lum−/− mice.
In the compound Bgn−/o/Lum−/− stroma the lamella were composed of disorganized fibrils; both the regular packing and orientation was disrupted compared to the wild type and Bgn−/o stromas that were comparable. The mid corneal stroma of Lum−/− mice showed a modest disruption in fibril packing (thin arrows), but no disruption in orientation. Larger diameter collagen fibrils with irregular contours were observed in compound Bgn−/o/Lum−/− mice compared to Lum−/−, Bgn−/o and wild type mice (thick arrows). The mean fibril diameter in Bgn−/o/Lum−/− mice is 29.2 nm and is significantly greater that than that in Lum−/− (27.8nm, p=0.046), Bgn−/o (27.0nm, p=0.037), and wild type mice (27.0nm, p=0.035). The fibril distribution was heterogeneous in compound-null mice compared to other groups, and a group of larger diameter fibrils was observed (grey arrow), indicating a dysfunctional regulation of lateral fibril growth in the disorganized lamellae. (M, median; Q1, 25 percentile; Q3, 75 percentile. Bar=200 nm)
3. Discussion
SLRPs are critical in regulating collagen fibrillogenesis and matrix assembly in the corneal stroma (Chakravarti et al., 2000; Chakravarti et al., 2006; Chen et al., 2010; Zhang et al., 2009). In the absence of class I decorin there is a severe disruption of fibril structure and stromal organization while the absence of biglycan has little or no effect on corneal stromal structure (Zhang et al., 2009). However, biglycan has critical roles in regulating collagen fibril structure in other tissues such as aorta and heart (Heegaard et al., 2007; Westermann et al., 2008). Similarly, the absence of class II lumican results in a severe posterior stromal phenotype while keratocan has no effect (Chakravarti et al., 2000; Chakravarti et al., 2006; Liu et al., 2003). This supports our suggestion that SLRP regulation of fibrillogenesis and stromal organization involve the coordinated action of dominant class I and II SLRPs, i.e., decorin and lumican. We also suggest that the regulation is fine-tuned involving modulatory SLRPs in each class, i.e., biglycan and keratocan/fibromodulin. The data support the conclusion that in class I, decorin is the major regulator while biglycan modulates its function. In class II, the regulatory roles of lumican are influenced by keratocan and fibromodulin. In tissues where fibromodulin is the dominant class II SLRP such as tendon and sclera, lumican has been shown to modulate its regulatory activity (Chakravarti et al., 2003; Ezura et al., 2000; Jepsen et al., 2002).
In addition to intraclass regulatory interactions; Collagen fibrillogenesis and matrix assembly involves interclass SLRP regulation. Disruption of tendon-specific fibrillogenesis was demonstrated in the absence of fibromodulin and biglycan with the phenotype being more severe than in the single null mice (Ameye et al., 2002). In the work presented here, regulatory roles of interclass interactions between biglycan and lumican in the regulation of corneal stromal fibrillogenesis and matrix assembly were investigated in the compound Bgn−/o/Lum−/−, Bgn−/o, Lum−/− mouse models and wild type controls. The data demonstrate that compound-null mice lacking both lumican and biglycan exhibited homogeneous corneal opacity indicating a functional deficit. The observed structural abnormalities in stromal fibril structure as well as disrupted lamellar and stromal organization are consistent with abnormal structure-function in the compound mutant corneas. Both the structural and functional alterations were more severe than what would be predicted based on additive effects seen in single-null Bgn−/o, and Lum−/− corneas. This indicates that lumican and biglycan have synergistic regulatory roles in the development of corneal stromal function. Cooperative interactions involving lumican and biglycan are critical in regulating both collagen fibrillogenesis and lamellar organization during stromal matrix assembly. Therefore, interclass interactions involving class I (biglycan) and class II (lumican) SLRPs regulate critical steps in the development of corneal structure and function.
Cornea stroma has a high expression level of a large number of different SLRPs that regulate matrix assembly cooperatively. This provides redundancy and stability of regulation as well as temporal and spatial specificity required for the multiple-steps stromal collagen fibrillogenesis. The cooperation involves coordinated regulation of expression, and synergistic structural interactions with collagen. These SLRPs also can mediate cell-matrix interaction via growth factors or cell surface receptors (Berendsen et al., 2011; Chen and Birk, 2013; Melchior-Becker et al., 2011). Lumican is the major class II SLRP that regulates collagen fibrillogenesis and fibril organization. In the absence of lumican, expression of fibromodulin was up-regulated while keratocan expression was decreased. Compound Bgn−/o/Lum−/− stromas have a SLRP expression pattern similar to that in Lum−/− corneas except for the lack of biglycan, indicating the synergistic functional cooperation rather than the coordinated regulation of expression is involved in the interclass interactions between lumican and biglycan.
In the corneal stroma there are regional differences in fibril packing with the anterior and mid stroma having denser fibril packing than in the posterior stroma (Chakravarti et al., 2000). These spatial differences in fibril packing contribute to cornea curvature. During cornea maturation, the homogenous expression of lumican becomes restricted to the posterior stroma and the absence of lumican results in structural alterations in the posterior stroma with an overlapping loss of function seen as stromal opacity. When both lumican and biglycan are absent, the structural defects are homogeneous across the stroma with overlapping opacity. The absence of biglycan alone generated no structural or functional alterations indicating that the regional differences are regulated via interclass SLRP interactions. There are also regional differences in stromal fibril diameters (Chakravarti et al., 2006). The tight regulation of lateral fibril growth is critical to the development and maintenance of transparency (Hassell and Birk, 2010). Our data indicate a synergistic regulation involving lumican and biglycan of lateral fibril growth.
Our study also demonstrated that inter-class interactions of SLRPs are important in regulation lamellar structure. Collagen protofibrils assemble in keratocyte surface microdomains then undergo linear and in some cases lateral growth into mature fibrils. Keratocyte defined microenvironments are important in regulating the extracellular steps in stromal matrix assembly (Birk and Bruckner, 2011; Birk et al., 1995; Birk et al., 1989). During development and maturation, neural crest cells differentiate into keratocytes, keratocytes then transition from an active proliferating state to a quiescent status. The keratocytes are compact with slender cytoplasmic lamellipodia that form communicating networks (Hassell and Birk, 2010). These dendrite-like lamellipodia are perpendicular to each other, guiding the formation of orthogonal lamellar structure during stromal matrix assembly (Birk and Trelstad, 1984; Doane and Birk, 1991; Trelstad and Coulombre, 1971). Programmed transition from neural crest cells to keratocyte during development is critical to maintain the keratocyte phenotype. Improper activation of keratocytes during wound at later stage transform them into myofibroblasts, which are not compatible with transparency (Hassell and Birk, 2010). Our data indicated that interclass cooperation of lumican and biglycan influence the keratocytes, their organization and resulting stromal matrix assembly. The involvement of communicating keratocyte networks is an essential component critical to the establishment and maintenance of long range order in the corneal stroma. This may also serve to integrate the cellular and extracellular regulatory steps necessary to establish a corneal stroma-specific architecture necessary for function.
In summary, synergistic interclass regulatory interactions between lumican and biglycan are important in integration of keratocytes and collagen fibrillogenesis during stroma development. This contributes to the temporal and spatial differences required for the regulation of precisely oriented collagen fibril hierarchy and keratocyte organization. These interactions are involved in regulating both lamellar structure as well as collagen fibrillogenesis and therefore, corneal transparency.
4. Experimental procedures
4.1 Animals
Gene targeted mice null for biglycan (Xu et al., 1998) or lumican (Chakravarti et al., 1998) were bred into a C57BL/6 background for at least 7 generations. However, the C57BL/6 background compound Bgn−/o/Lum−/− mice had decreased viability. In this study, a mixed CD1/C57BL background was used to avoid this issue. In brief, Lum−/− mice in a C57BL/6 background were back crossed with CD1wild type mice to generate Lum−/− mice in a mixed CD1/C57BL background. These mice were then bred with Bgn−/o mice in a C57BL background to generate compound heterozygous mice in a mixed CD1/C57BL background that were bred to generate the mice utilized in this work. All animal studies were performed in compliance with IACUC approved animal protocols.
4.2 Immuno-localization analyses
Eyes from P30 mice were embedded in OCT medium, frozen on dry ice and stored at −80°C. Frozen sections (4μm) were cut using a HM 505E cryostat. The sections were stained with Hematoxylin & Eosin (Sigma-Aldrich Corp., St. Louis, MO, USA) for histological examination. Immunofluorescence localization was done as previously described (Chen et al., 2008). Rabbit anti-decorin (LF113, provided by Dr. L. Fisher, NIH-NICDR) was used at 1:250, both anti-biglycan (LF159, provided by Dr. L. Fisher, NIH-NICDR) and anti-keratocan (provided by Dr. Hassell, USF Tampa, USA) were used at 1:200. Rabbit anti-lumican (provided by Dr. Ake Oldberg, Lund University, Sweden) was used at 1:1000. Rabbit anti-mouse fibromodulin was used at 1:200 (provided by Dr. L. Fisher, NIH-NICDR). The secondary antibody was an Alexa Fluor 568-conjugated goat anti rabbit IgG (Molecular Probes, Eugene, OR) used at 1:100. Vectashield mounting solution with DAPI (Vector Laboratories, Inc., Burlingame, CA) was used as a nuclear marker. Negative controls were sections from null corneas as well as sections incubated identically without the primary antibody. Images were captured using a Leica CTR 5500 microscopy mounted with Leica DFC 340 FX camera identical conditions and set integration times were used to facilitate comparisons between samples.
4.3 Immuno-blots
Corneas were dissected from P30 corneas and SLRPs extracted as previously described (Chen et al., 2010; Zhang et al., 2009). Briefly, corneas were homogenized and extracted in 20 fold excess (weight/volume) of 4 M guanidine-HCl, 50 mM sodium acetate, pH 5.8 with proteinase inhibitors (Thermo Scientific) at 4°C for 48 hours. The extract was clarified by centrifugation and dialyzed against 150 mM Tris-HCl, 150mM NaCl pH 7.3. Samples were digested with chondroitinase ABC (Seikagaku Biobusiness Corporation) or endo-β-galacosidase (Sigma) for 24 hours at 37°C. Total protein was determined using the BCA protein assay kit (Pierce). For semi-quantitative analysis of SLRPs, electrophoresed using 4-20% gels and transferred to Hybond-C extra membranes (GE Health Care) for immuno-blotting. Anti-decorin (LF113) was used at 1:1000, both anti-biglycan (LF159) and anti-fibromodulin (LF-149) was used at 1:200. Secondary goat anti-rabbit IgG-perixodase (Amersham) was used at 1:3000 with an ECL (Pierce) detection system. Actin reactivity in each sample was detected using an anti-actin antibody (Chemicon).
4.4 In vivo confocal microscopy
In vivo corneal haze was analyzed with the Heidelberg Retinal Tomography HRT Rostock Cornea Module (HRT-III; Heidelberg Engineering Inc., Heidelberg, Germany)(Chen et al., 2011). Briefly, four P30 mice from each group of wild-type, Bgn−/0, Lum−/−, and Bgn−/0/Lum−/− were anesthetized and restrained on an adapted stage. GenTeal Gel (Novartis Pharmaceuticals Corp., East Hanover, NJ) was applied to the corneal surface. Images of the apex of the cornea were obtained by mechanically or manually adjusting the CCD camera position. Series of images were sequentially collected from the surface of the epithelium to the endothelium as a z-axis scan at fixed intensity for all of the animals. Images were processed with Metamorph software (Premier Version 7.6.5.0, Molecular Devices, Sunnyvale, CA). A depth intensity profile from the scans was generated by plotting the average pixel intensity per plane as a function of corneal depth. Corneal haze (light scattering) was measured by calculating the total pixel intensity as measured by the area under the curve. Corneal thickness was determined by measuring the axial distance from the epithelium to endothelium.
4.5 Transmission electron microscopy
Cornea from wild type, Bgn−/0, Lum−/−, and Bgn−/0/Lum−/− mice at P30 were analyzed by transmission electron microscopy as previously described (Chen et al., 2010). Briefly, the cornea along with anterior sclera were dissected and fixed in 4% paraformaldehyde, 2.5% glutaldehyde, 0.1 M sodium cacodylate pH 7.4, with 8.0 mM CaCl2, post-fixed with 1% osmium tetroxide. They were dehydrated in an ethanol series, followed by propylene oxide. Samples were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride and DMP-30 (Electron Microscopy Sciences, Hatfield, PA.). Sections (70nm) were cut using a Leica ultramicrotome and post-stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined at 80 kV using a JEOL 1400 transmission electron microscope equipped with a Gatan Ultrascan US1000 2K digital camera.
4.6 Fibril diameter distribution
Five corneas from three to five P30 mice were analyzed for Bgn−/0, Lum−/−, Bgn−/0Lum−/− and wild type control mice. Digital images were taken from non-overlapping regions of the mid stroma. Fibril diameters were measured with a RM Biometrics-Bioquant Image Analysis System (Nashville, TN) using randomly chosen and masked digital images. Sample mean, sample median-to-first quartile distance (m-Q1), and sample third quartile distance to median (Q3-m), were analyzed. The median indicates the location of the center of the distribution, while median-to-quartile distances reflect primarily the spread of the data. Distributions of the sample to sample means and both median-to-quartile distances for each deficient group were compared with the wild-type group using the T- test. The null hypothesis of this test was that there is no difference between the two compared distributions.
Supplementary Material
Highlights.
The compound-null mouse model was deficient in the major stromal class II (lumican) and modulatory class I (biglycan) SLRP and demonstrated gross corneal opacity
Interclass lumican and biglycan interactions are required for establishing corneal transparency.
Corneal stromal fibril and lamellar architecture is dependent on lumican and biglycan interactions during matrix assembly
Lumican and biglycan influence corneal keratocyte lamellipodia organization and stromal organization
Synergistic interclass lumican and biglycan interactions are critical in the regulation of corneal stromal collagen fibrillogenesis.
Acknowledgements
Supported by grant EY005129 from NIH NEI. We thank Qingmei Yao for expert technical assistance with the maintenance of the mouse lines. We also thank Sheila Adams and Mei Sun for helpful discussions.
Footnotes
This study was supported by NIH/NEI grant EY005129 and by the Intramural program of the NIDCR, NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Bruckner P. Collagens, Suprastructures, and Collagen Fibril Assembly. In: Mecham RP, editor. The Extracellular matrix: an overview. Biology of Extracellular Matrix. Springer-Verlag; Berlin Heidelberg: 2011. pp. 77–115. [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024–2033. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, Birk DE, Funderburgh JL, Jester JV, Kao WW. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T. Localization of mouse lumican (keratan sulfate proteoglycan) to distal chromosome 10. Mammalian genome: official journal of the International Mammalian Genome Society. 1995;6:367–368. doi: 10.1007/BF00364803. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci. 2003;44:2422–2432. doi: 10.1167/iovs.02-0783. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006;235:2493–2506. doi: 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- Chen S, Birk DE. Focus on molecules: decorin. Experimental eye research. 2011;92:444–445. doi: 10.1016/j.exer.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. The FEBS journal. 2013;280:2120–2137. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Oldberg A, Chakravarti S, Birk DE. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dyn. 2010;239:844–854. doi: 10.1002/dvdy.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sun M, Meng X, Iozzo RV, Kao WW, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy: C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011;179:2409–2419. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008;74:289–299. doi: 10.1038/ki.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KG, Siracusa LD, Donovan PJ, Iozzo RV. Decorin, epiphycan, and lumican genes are closely linked on murine Chromosome 10 and are deleted in lethal steel mutants. Mammalian genome: official journal of the International Mammalian Genome Society. 1999;10:201–203. doi: 10.1007/s003359900971. [DOI] [PubMed] [Google Scholar]
- Doane KJ, Birk DE. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Experimental cell research. 1991;195:432–442. doi: 10.1016/0014-4827(91)90394-a. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YF, Zhang YL, Zha Y, Huang JH, Cai JQ. Association of lumican gene polymorphism with high myopia: a meta-analysis. Optometry and vision science: official publication of the American Academy of Optometry. 2013;90:1321–1326. doi: 10.1097/OPX.0000000000000032. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Experimental eye research. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- Kim J.-h., Ko JM, Lee I, Kim JY, Kim MJ, Tchah H. A novel mutation of the decorin gene identified in a Korean family with congenital hereditary stromal dystrophy. Cornea. 2011;30:1473–1477. doi: 10.1097/ICO.0b013e3182137788. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ki C-S, Chung E-S, Chung T-Y. A novel decorin gene mutation in congenital hereditary stromal dystrophy: a Korean family. Korean J Ophthalmol. 2012;26:301–305. doi: 10.3341/kjo.2012.26.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Yang XB, Liao M, Lan CJ, Liu LQ. Association between lumican gene −1554 T/C polymorphism and high myopia in Asian population: a meta-analysis. International journal of ophthalmology. 2013;6:696–701. doi: 10.3980/j.issn.2222-3959.2013.05.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. The Journal of physiology. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior-Becker A, Dai G, Ding Z, Schafer L, Schrader J, Young MF, Fischer JW. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem. 2011;286:17365–17375. doi: 10.1074/jbc.M110.192682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivela T, Kucherlapati R, Forsius H, de la Chapelle A. Mutations in KERA, encoding keratocan, cause cornea plana. Nature genetics. 2000;25:91–95. doi: 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- Pringle GA, Dodd CM. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- Rodahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am J Ophthalmol. 2006;142:520–521. doi: 10.1016/j.ajo.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Narlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Coulombre AJ. Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol. 1971;50:840–858. doi: 10.1083/jcb.50.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lullmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrmann SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschope C, Fischer JW. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008;117:1269–1276. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.