Abstract
Autism spectrum disorder (ASD) is more common in males than females. An underrepresentation of females in the ASD literature has led to limited knowledge of differences in social function across the sexes. Investigations of face perception represent a promising target for understanding variability in social functioning between males and females. The current study analyzed electrophysiological brain recordings during face perception to investigate sex differences in the neural correlates of face perception and their relationship to social function. Event related potentials (ERP) were recorded from children with ASD while viewing faces, inverted faces, and houses. Relative to males, females showed attenuated response at an ERP marker of face perception, the N170. Among females, but not males, atypical face response was associated with symptom severity. Observed sex differences reflect influential differences in social information processing, and impairment in these features correlates with deficits in social information processing in females, but not males, with ASD. These findings hold significance for future treatment protocols, which should account for differences in males and females with ASD in clinical presentation and neural phenotypes.
Keywords: autism spectrum disorder, sex differences, ERP, N170, face perception
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairment in social communication and reciprocal social interactions, as well as restricted and repetitive interests and behaviors. ASD is more common in males than females at a ratio estimated to range from 2.7–7.2:1 (Baio, 2012). Notably, this sex ratio has remained consistent despite significant changes in prevalence and in diagnostic criteria (Bryson & Smith, 1998; McMahon & Ritvo, 1989; Fombonne, 2003; Yeargin-Allsopp et al., 2003). Because ASD is more prevalent in males, the majority of published studies related to autism are composed primarily, and in some cases, exclusively, of males; many studies do not directly compare sexes on variables of interest. For these reasons, a paucity of literature investigating sex differences in ASD exists (Koenig & Tsatanis, 2005). Because an important objective in ASD research is to parse heterogeneity within the disorder, the current study examined two biologically determined subgroups of children with ASD—males and females—to provide information about sources of phenotypic variability in ASD associated with sex.
Sex differences have been observed in the behavioral phenotype in ASD, particularly in social and cognitive functioning. Females with high functioning ASD show fewer social problems early in development but, by adolescence, lack reciprocal friendships and have more social problems according to parent report (McLennan, Lord, & Schopler, 1993; Holtmann, Bolte, & Poustka, 2007). Females also exhibit more associated features of the disorder, such as sleep problems (Hartley & Sikora, 2009; McLennan et al., 1993), sensory sensitivities (Lai et al., 2011), motor difficulties (Carter et al., 2007), and comorbid psychopathology (Hartley & Sikora, 2009) than males. Males tend to exhibit more restrictive and repetitive behaviors than females and perform better than females across domains of cognitive functioning (Mandy et. al., 2011; Lord, Schopler, & Revicki, 1982; Volkmar, Szatmari, & Sparrow, 1993). The ratio of males to females with ASD is less than 2:1 for children with IQs under 55 (Lord & Schopler, 1985; Lord et al., 1982; Tsai & Beisler, 1983; Volkmar et al., 1993; Wing, 1981) but as high as 8:1 for children with average or above average IQ (Scott, Baron-Cohen, Bolton, & Brayne, 2002).
These phenotypic distinctions suggest greater detrimental impact of ASD on function in females; however, several critical issues have not been addressed by prior work. Most of the extant literature on sex differences has included samples ranging widely in age, which may confound true sex differences with developmental effects (McLennan et al., 1993). The few studies that have accounted for age have demonstrated sex differences in functional level, demonstrating the need to also account for and match on intellectual ability (Volkmar et al., 1993). Researchers have not yet investigated phenotypic differences associated with sex in samples well-controlled for both chronological and cognitive development.
Although sex differences in ASD are poorly understood, there are well-documented examples of sex differences in social and communicative function in typical development. Females tend to develop verbal communicative abilities earlier than males and engage in more spontaneous language (Bauer, Goldfield, & Reznick, 2002; Huttenlocher, Haight, Bryk, Seltzer, & Lyons, 1991; Moore, 1967). Socially, school-aged females are often ascribed greater social competence than males and are better at identifying emotions, with higher levels of empathic responses (Eisenberg & Lennon, 1983; Joliffe & Baron-Cohen, 1997; Lang-Takac & Osterweil, 1992) and more rapid theory of mind development (Happé, 1995). Female infants show greater interest in faces (Leeb & Rejskind, 2004), make more eye contact with their mothers (Lutchmaya & Baron-Cohen, 2002), and commonly outperform males on facial recognition tasks (Herlitz & Rehnman, 2008).
Brain activity subserving communicative and social behavior also reveals sex differences in typical development. Tasks of language processing assessed by fMRI have revealed sex differences in lateralization in the superior temporal sulcus (Kansaku, Yamaura, & Kitazawa, 2000) and whole brain activation (Pugh et al., 1996); these findings are independent of behavioral differences in task performance and indicate potentially distinct pathways for communicative processing. Electrophysiological studies demonstrate stronger activation and greater processing efficiency in females when making social judgments about observed events; this difference has been interpreted as reflecting a relative strength in anticipation and prediction of social interactions (Pavlova, Guerreschi, Lutzenberger, Sokolov, & Krageloh-Mann, 2012). Females also display advantages in neural specialization for face processing, with enhanced amplitudes and shorter latencies for face-sensitive event-related potentials, such as the N170 (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Itier & Taylor, 2002; Taylor, Batty, & Itier, 2004; Proverbio, Brignone, Matarazzo, Del Zotto, & Zani, 2006).
The current study applied insights from these studies of the brain bases of face processing in typical development to elucidate sex differences in the neural correlates of social perception in ASD. Individuals with ASD exhibit delayed and attenuated electrophysiological brain responses to faces (O'Connor, Hamm, & Kirk, 2005), but little is known about sex differences in this brain response. Because regional brain activation associated with face perception is already well understood (Kanwisher, McDermott, & Chun, 1997), we applied electrophysiological methods to study sex differences in terms of both strength and timing of neural response. In the current study, we followed up on a recent study of face perception in ASD (McPartland et al., 2011) by recruiting an ancillary sample of females with ASD to supplement the predominantly male clinical sample and permit sex comparisons within the ASD group. As in the previous study, we compared brain response to faces versus houses and upright versus inverted faces.
We examined two hypotheses. First, because previous studies in ASD suggest more profound impact on females, we explored the possibility that females would show more pronounced dysfunction in face processing than males (i.e., slower, attenuated N170 to faces; attenuated inversion effect). Alternatively, because sex differences in typical development suggest stronger social brain function in females, we investigated whether females with ASD would display more robust markers of social information processing than males (i.e., faster, enhanced N170 to faces; more pronounced inversion effect). We also explored relationships among adaptive behavior and ASD symptomology with neural metrics of face perception, predicting that these neural responses would associate with increased ASD symptomology and decreased adaptive functioning.
METHODS
Participants
Participants included 12 males with ASD included in a prior study (McPartland et al., 2011) and 12 females with ASD selected as a comparison group to enable comparison by sex. Males and females were individually matched on age and IQ. Diagnosis was confirmed with gold standard diagnostic assessments for research: a combination of parent interview (Autism Diagnostic Interview-Revised; ADI-R (Lord, Rutter, & Le Couteur, 1994)), semi-structured social behavior and communication assessment (Autism Diagnostic Observation Schedule; ADOS (Lord et al., 2000)), and clinical diagnosis based on DSM-IV-TR (American Psychiatric Association, 2000) criteria by an expert clinician. Exclusionary criteria for participants included seizures, history of serious head injury, sensory or motor impairment that would impede completion of the study protocol, neurological disease, active psychiatric disorder (other than ASD; screened with the Child Symptom Inventory: Fourth Edition (Gadow & Sprafkin, 1994)), medication known to affect brain electrophysiology, or Full Scale IQ scores lower than 60 (measured by the Differential Ability Scales: Second Edition (Elliott, 2007) or the Wechsler Abbreviated Scales of Intelligence (Wechsler, 1997)). Groups did not significantly differ in terms of sex, ethnicity, handedness, chronological age, or Full Scale IQ. Table 1 displays demographic and behavioral data for the final sample. All procedures were approved by the Human Investigation Committee at Yale School of Medicine and were carried out in accordance with the Declaration of Helsinki (1975/1983).
Table 1.
Mean characteristic information, standard deviation (in italics), and range (in parenthesis) for males and females.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | p value | ||
| Demographics | Age (in years) | 10.25 (1.82) | 8.3–14.0 | 10.08 (1.48) | 8.3–13 | .797 |
| IQ | 98.82 (19.00) | 75–139 | 100.17 (20.06) | 64–129 | .869 | |
| Benton | 40.92 (8.98) | 27–42 | 35.92 (4.29) | 25–44 | .383 | |
| ADOS | Total | 11.64 (5.57) | 1–20 | 10.80 (3.39) | 6–16 | .686 |
| Social affect | 9.64 (4.98) | 1–17 | 8.20 (2.44) | 5–13 | .420 | |
| Restrictive and repetitive behaviors | 2.00 (1.26) | 0–4 | 2.64 (1.50) | 0–5 | .295 | |
| ADI | Communication | 18.82 (2.89) | 9–24 | 17.08 (4.36) | 10–30 | .630 |
| Reciprocal social interaction | 23.36 (3.61) | 18–29 | 20.00 (6.65) | 7–27 | .122 | |
| Restrictive and repetitive behaviors | 6.82 (2.32) | 4–11 | 4.58 (2.19) | 2–8 | .022* | |
| Age of onset | 3.64 (1.29) | 1–5 | 3.58 (0.99) | 0–5 | .632 | |
| Vineland | Communication | 84.33 (11.73) | 65–112 | 85.50 (11.82) | 74–106 | .819 |
| Social | 77.33 (13.52) | 57–104 | 80.50 (10.43) | 62–96 | .552 | |
p <.05
Behavioral procedures
Face perception
Face recognition was measured with the Benton Facial Recognition Test (Benton, Sivan, Hamsher, Varney & Spreen, 1994). Participants viewed grayscale images of faces and specified one or three matches from a selection of six faces, varying in lighting conditions and orientation.
Autism severity
To provide a measure of symptom severity, we examined summary and total scores on the ADOS algorithm and algorithm subscales (social affect, restrictive and repetitive behaviors) and ADI subscales (communication, reciprocal social interaction, restrictive and repetitive behaviors, age of onset). One male and two females were excluded from correlational analysis involving the ADOS and one male was excluded from the ADI data analysis because the ADOS and ADI data were administered more than 12 months prior to EEG recording for these participants.
Vineland Scales of Adaptive Behavior
Adaptive function was measured via a parent interview using the Vineland Scales of Adaptive Behavior – Survey Interview, 2nd Edition (Sparrow, Cicchetti, & Balla, 2005). We examined standard scores for the social and communication domains. Two females did not receive a Vineland within 12 months of EEG session and were therefore excluded from these analyses.
EEG procedures
Stimuli
Stimuli were administered in pseudorandom sequence and consisted of gray-scale digitized images of neutral faces, houses, inverted faces, and inverted houses (not included in current analyses), all displayed from a direct frontal perspective, as per (McPartland et al., 2011). Subjects were presented with 23 stimuli from each category four times, for a total of 92 stimuli per category. Attention was monitored by the experimenter through a live-feed closed circuit video camera and by instructing participants to press a button whenever a stimulus repeated. Stimuli were standardized in terms of size (visual angle of approximately five degrees high), background color (gray), and average luminance.
Data collection
Stimuli were presented on a Pentium-IV computer controlling a 51 cm color monitor (75-Hz, 1024x768 resolution) running E-Prime 2.0 software (Schneider, Eschman, & Zuccolotto, 2002). Participants viewed stimuli at a distance of 90 cm in a sound attenuated room with low ambient illumination. EEG was recorded using NetStation 4.3. A 256 lead Geodesic sensor net (Electrical Geodesics Incorporated; (Tucker, 1993) was fitted according to the manufacturer’s specifications, dampened with potassium-chloride electrolyte solution, and placed on the participant’s head. Impedances were kept below 40 kilo-ohms. ERP was recorded continuously throughout each stimulus presentation trial, consisting of a fixation cross (randomly varying from 250–750 ms), stimulus (500 ms), and blank screen (500 ms). The EEG signal was amplified (×1000) and filtered (0.1 Hz high-pass filter and 100 Hz elliptical low-pass filter) via a preamplifier system (Electrical Geodesics Incorporated). The conditioned signal was multiplexed and digitized at 250 Hz using an analog-to-digital converter (National Instruments PCI-1200) and a dedicated Macintosh computer. The vertex electrode was used as a reference, and data were re-referenced to an average reference after data collection.
Data editing and reduction
Data were averaged for each subject by stimulus type across trials. Averaged data were digitally filtered with a 30 Hz low-pass filter. The window for segmentation of the ERP extended from 100 ms before to 400 ms after stimulus onset. NetStation artifact detection settings were set to 200 v for bad channels, 150 µv for eye blinks, and 150 v for eye movements. Channels with artifacts for more than 50 percent of trials were marked as bad channels and replaced through spline interpolation. Segments that contained eye blinks, eye movement, and those with more than 20 bad channels were also excluded. One subject’s data required manual removal of artifact due to malfunctioning eye-electrodes. Participants with less than 29% good trials for any stimulus category were excluded from analysis, a cut-off that all participants met. Electrodes of interest were selected based on maximal observed amplitude of the N170 to faces in grand averaged data and to conform to those used in previous research (J. C. McPartland et al., 2011). Data were averaged across eight electrodes over the left (95, 96, 97, 106, 107, 108, 116, 117) and right (151, 152, 153, 160, 161, 162, 170, 171) lateral posterior scalp. The time windows for P1 and N170 analysis, extending from 55 ms to 167 ms and 123 ms to 311 ms post-stimulus onset, respectively, were chosen by visual inspection of grand averaged data and confirmed in individual averages. Peak amplitude and latency to peak were averaged across each electrode group within the specified time window and were extracted for each participant for each stimulus category.
Data analysis
P1 and N170 amplitudes and latencies to peak for faces vs. houses and faces vs. inverted faces were separately analyzed using univariate repeated measures analyses of variance (ANOVA) with two within-subject factors, condition (face/house; face/inverted face) and hemisphere (left/right). The between subjects factor was group (males/females). A planned comparison using a one-way ANOVA was employed to test the specific hypothesis that the N170 in females would be faster and enhanced to faces compared to houses, and slower and enhanced for inverted faces compared to faces. To examine N170 amplitude independent of low-level perceptual processes (P1), we performed a peak-to-trough analysis in which we subtracted the P1 amplitude from N170 amplitude. Finally, to examine the relationship between brain function and behavior in males and females, we calculated an ERP index of sensitivity to social information by subtracting N170 amplitude to face from houses and correlating it with adaptive behavior (Vineland) and severity of ASD (ADOS and ADI). We predicted that behavior would be related to brain function such that more normative neural responses to social stimuli would be indicative of more adaptive and better real-world functioning.
RESULTS
Faces vs. houses
P1 latency
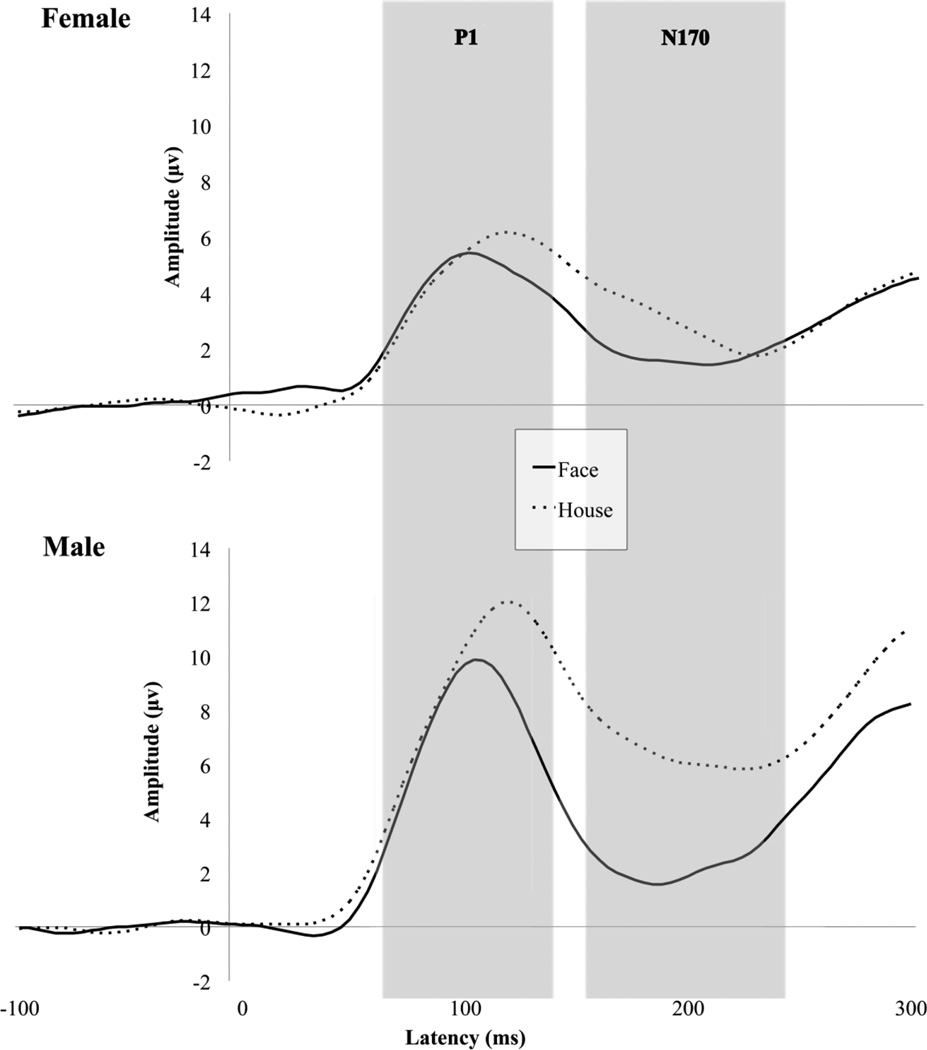
Figure 1 displays grand averaged waveforms elicited by faces and houses for both males and females averaged across hemisphere. A main effect of condition [F(1,22) = 20.61, p < .001] indicated that faces elicited shorter P1 latencies across both hemisphere and group. A main effect of hemisphere indicated that P1 latency was significantly faster in the right hemisphere [F(1,22) = 5.01, p < .05].
Fig. 1.
Grand average waveforms for P1 and N170 to upright faces and houses in females and males.
P1 amplitude
A main effect of condition [F(1,22) = 17.842, p < .001] indicated that houses elicited a more positive P1 than faces across hemispheres for both groups. A main effect of group [F(1,22) = 5.829, p < .05] indicated that males had a more positive P1 amplitude than females. A group by condition interaction [F(1,22) = 8.157; p < .01] indicated that in males, but not in females, P1 amplitude varied by condition such that males had a more positive P1 to houses than faces.
N170 latency
A main effect of condition [F(1,22) = 15.460; p ≤ .001] indicated that faces elicited shorter N170 latencies across both hemispheres, and a main effect of group [F(1,22) = 6.697; p < .05] indicated that, irrespective of condition, N170 latency was significantly shorter in males than in females.
N170 amplitude
A main effect of condition [F(1,22) = 30.812, p < .001] indicated that faces elicited a more negative N170 than houses across both hemispheres, and a main effect of group [F(1,22) = 4.696, p < .05] indicated that males had reduced N170 amplitude relative to females. A group by condition interaction [F(1,22) = 10.313, p < .01] indicated that males but not females exhibited differential N170 amplitude to faces versus houses.
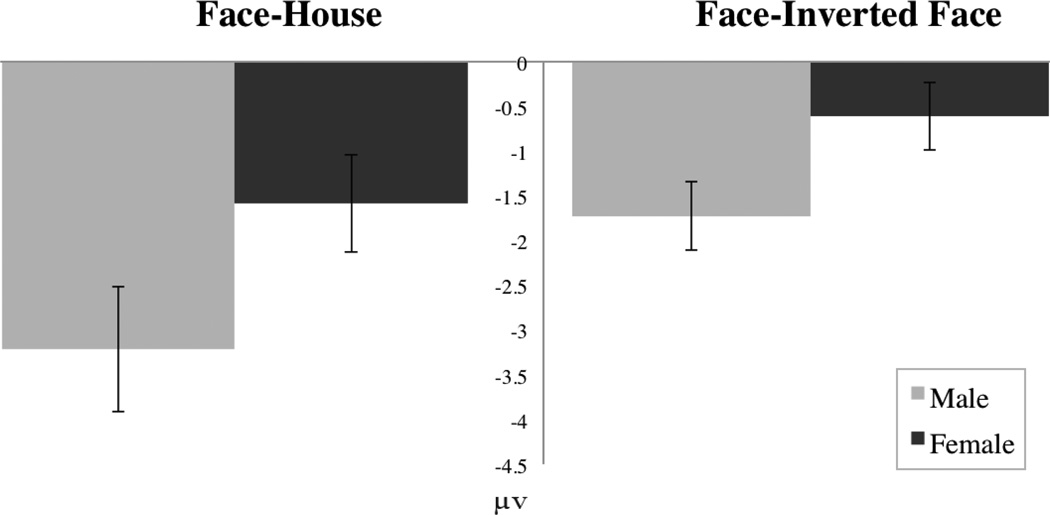
To examine differences observed at the N170 independent of effects at the P1, we performed a peak to trough analysis. Figure 2 displays a bar chart of N170 amplitude difference for males and females. Analysis of amplitude differential between P1 and N170 revealed a main effect of condition [F(1,22) = 26.244, p < .001] such that, across groups, faces elicited an enhanced N170 across hemisphere and group. A main effect of group [F(1,22) = 6.381, p < .05] indicated that the amplitude difference between conditions was larger in males. A group by condition interaction [F(1,22) = 8.136, p < .01] indicated that males but not females discriminated between conditions at the N170, with males showing a more negative N170 to faces than houses.
Fig. 2.
Bar chart for differences in N170 amplitude for faces minus houses and faces minus inverted faces.
Faces vs. inverted faces
P1 latency
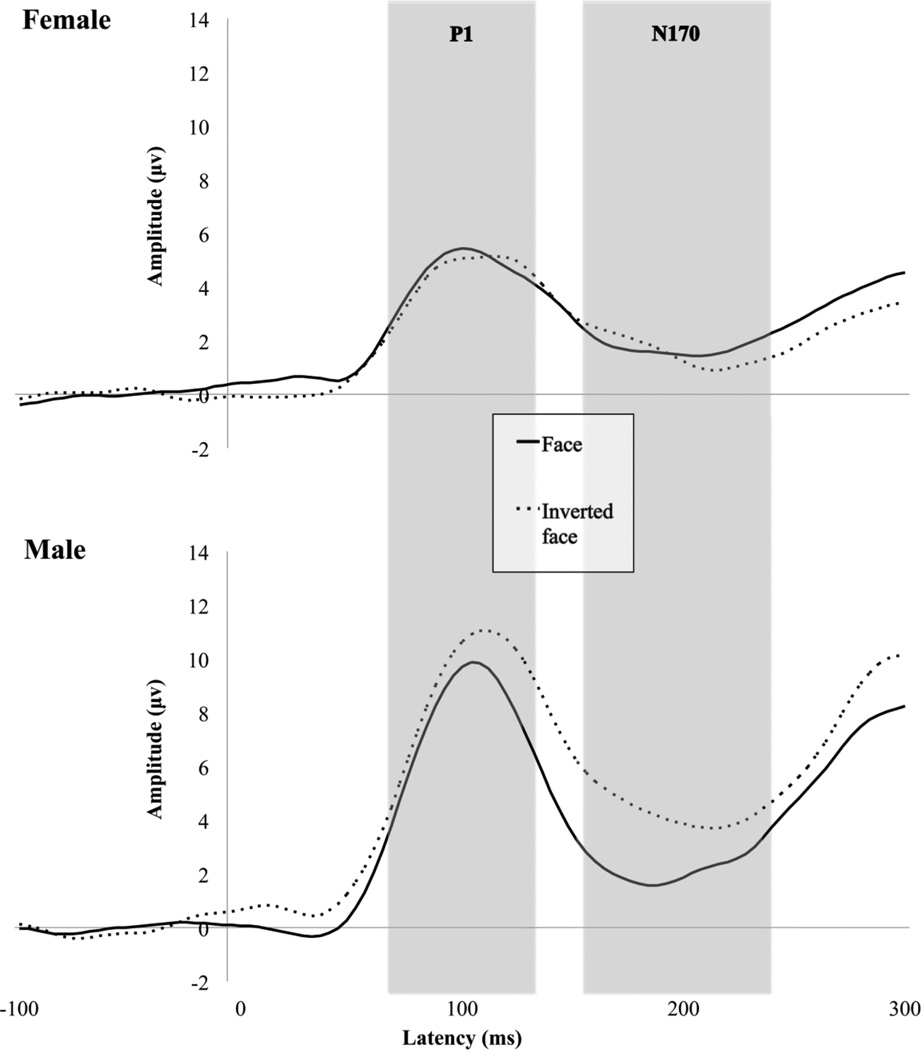
Figure 3 displays grand averaged waveforms elicited by faces and inverted faces for both males and females. A main effect of condition [F(1,22) = 9.329, p < .01] indicated that faces elicited a significantly faster P1 than inverted faces across hemisphere for both groups. A main effect of hemisphere [F(1,22) = 4.665, p < .05] indicated that P1 latency was faster in the right hemisphere than in the left hemisphere across group and condition.
Fig. 3.
Grand average waveforms for P1 and N170 to upright faces and inverted faces in females and males.
P1 amplitude
A main effect of condition [F(1,22) = 6.765, p < .05] indicated that inverted faces elicited a more positive P1 than upright faces across both hemispheres and groups. A main effect of group [F(1,22) = 6.553; p < .05] indicated that males had significantly greater P1 amplitudes than females across both hemispheres and conditions. A group by condition interaction [F(1,22) = 9.828, p < .01] indicated that males but not females discriminated between conditions in P1 response to inverted faces and faces.
N170 latency
A main effect of group [F(1,22) = 4.962, p < .05] indicated that males exhibited significantly faster N170 latency than females. No other significant results were observed in N170 latency.
N170 amplitude
A main effect of condition [F(1,22) = 8.569, p < .01] indicated that inverted faces elicited a more negative N170 than did faces. A main effect of group [F(1,22) = 4.860, p < .05] indicated that males displayed a less negative N170 than did females, and a group by condition interaction [F(1,22) = 14.337, p ≤ .001] indicated that males discriminated between conditions, whereas females did not. To examine differences observed at the N170 independent of effects of the P1, we performed a peak to trough analysis, subtracting N170 amplitude from P1 amplitude. Figure 2 displays a bar chart of N170 amplitude difference for males and females. Analysis of amplitude differential between P1 and N170 revealed a main effect of condition [F(1,22) = 4.813, p < .05] such that, across groups, faces elicited a more negative N170 than inverted faces. A main effect of group [F(1,22) = 6.947, p < .05] indicated that amplitude difference between faces and inverted faces was greater in males. A group by condition interaction [F(1,22) = 4.884, p < .05] indicated that males, but not females, differentiated between conditions at the N170, independent of the P1, with males displaying a larger amplitude difference for faces than inverted faces.
Relationship between brain response and social function
In females, reduced differentiation in N170 amplitude at the right hemisphere between social and non-social stimuli was indicative of higher levels of autism symptomatology (ADOS social affect, communication and social interaction, and total scores; all rs>.65, all ps<.04;). These correlations were not observed in males (all rs<−.537, all ps>.06). Figure 4 displays a scatterplot of ADOS total scores and N170 index of sensitivity to social information.
Fig. 4.
Scatterplot showing differential N170 amplitude of faces and houses to ADOS total scores.
No significant correlations were observed between face recognition and neural markers of face perception (all ps > .05). Males had significantly more restrictive and repetitive behaviors [t(21) = 2.49, p = .028] on the ADI, but did not differ on any other subscales of the ADI [all other t(21) < 1.48, all ps > .152]. Males and females did not differ in levels of autistic symptomatology on any subscales of the ADOS [all t(20) < .1.08, ps > .30]. adaptive social or communication standard scores on the Vineland [all t(20) < .61, ps >.50]; or face recognition on the Benton [t(22) = .89, p = .38].
DISCUSSION
The objective of the current study was to investigate, for the first time, sex differences in electrophysiological markers of social perception in ASD in a sample of males and females individually matched on age and cognitive functioning. Given the observation that females with ASD tend to be more severely impacted by the disorder, we hypothesized that females with ASD would show a relatively delayed and attenuated response to faces and that these neural responses would associate with increased ASD symptomology and decreased adaptive behaviors. We also evaluated the alternative hypothesis that, given female advantage for social perception in typical development, females with ASD would show faster and enhanced response to faces. Results were consistent with the hypothesis that females are more severely affected than males; males, but not females, exhibited modulation of ERP responses by social information. When comparing faces and houses and when comparing faces and inverted faces, females failed to differentiate conditions at the N170. This pattern of results, indicating a lack of discrimination between upright faces and other stimuli in females with ASD, occurred independently of differences at an earlier component (P1) reflecting basic visual perception associated with sensory response.
Although N170 differences were independent of P1 response, sex differences were also observed at this earlier ERP component in females. The N170 is the prototypic ERP index of face perception, but the P1 has been noted to respond selectively to faces (Rossion et al., 1999) and to display face inversion effects (Itier & Taylor, 2002). Males, but not females, demonstrated differential amplitude at this early marker in comparisons of both face and house stimuli and face and inverted face stimuli. Because differential ERP amplitude is interpreted as a developmental marker for face specialization, these results suggest reduced specialization and more severely compromised social information processing in females relative to males.
Correlations among autistic symptomatology and neural response are consistent with the notion that reduced modulation of neural response to faces is associated with more severe social impairment in females with ASD. Among females, but not males, more atypical patterns of neural response (smaller differential amplitude between faces and houses) were associated with greater levels of symptom severity. This was true across measures of social behavior, social communication, and overall autism severity. These relationships exist in spite of more severe restrictive and repetitive behaviors in males, and are independent of other group differences in behavior or autism symptomatology. These correlations therefore suggest that the sex differences observed here reflect influential factors in social information processing and that impairment in these features is associated with deficits in social information processing in females, but not males, with ASD.
Of note, the sex differences observed in the current study for social information processing are primarily related to ERP amplitude. Although attenuated N170 amplitude to faces has been observed in previous studies of ASD (O'Connor et al., 2005), prior studies lacked adequate female sample sizes to explore within-group differences associated with sex. Much prior work in electrophysiological indices of social perception (e.g., McPartland, Dawson, Webb, Panagiotides, & Carver, 2004; McPartland et al., 2011) has indicated that latency, rather than amplitude, most effectively distinguishes atypical brain response to faces in ASD from TD. In the current study, no social stimulus-specific differences between males and females with ASD were observed with respect to latency. We speculate that differences between autistic social dysfunction and typical development may be reflected in latency differences, while more subtle differences associated with sex within a clinical group may be marked by amplitude. This is consistent with findings in typical development demonstrating enhanced ERP amplitude to social information in females (Taylor et al., 2004). Although the sample from which the current study was drawn did not include a sufficient number of typically developing females to permit sex comparisons in typical development, our related work indicates delayed face perception in ASD relative to TD when aggregated across sexes. Replication of these effects in a larger study that includes a typical control sample is an important next step, and we are currently conducting this research in our laboratory.
In addition to the observed differences in measures of social perception, our results also suggest differences in more basic sensory processing between males and females with ASD. Males displayed an enhanced P1 response across conditions, and females tended to exhibit slower N170 responses across conditions. Differences may reflect distinct mechanisms for sensory and information processing between groups. For example, males with ASD may possess superior sensory processing or more efficient neural responding. Although groups in the present study were matched on IQ, such observations would be consistent with the broader pattern of more significant cognitive impact among females with ASD.
It is noteworthy that observed sex differences in neural response to faces were not paralleled by differences on a behavioral measure of facial recognition. Although clear sex differences in brain structure and function sometimes correlate with behavioral differences, they do not necessitate differences in behavior (Cahill, 2006; Taylor et al., 2004). For example, although typically developing males and females use different brain regions to remember and name images, they do not show behavioral differences in performance (Piefke, Weiss, Markowitsch, & Fink, 2005); Grabowski, 2003). Thus, we found that, while adaptive functioning and autism severity were associated with brain function in females but not in males, neither group demonstrated associations with neural and behavioral measures of face processing.
Because this study lacks a developmental or longitudinal design, the mechanisms behind observed sex differences cannot be clarified. They may reflect innate differences between males and females with ASD, or they may indicate distinct developmental effects. Given the observation that cognitive differences between males and females with ASD vary at different points in development (Carter et al., 2007; Lord & Schopler, 1985); (Volkmar et al., 1993), it will be important to verify the differences observed here in younger and older children with ASD.
It is possible that neural responses in our sample are due to differences in point of gaze to face. Point of gaze to a face has been shown to modulate brain responses (McPartland et al., 2010). Differential neural responses due to point of gaze are evident early in development, with infants at-risk for developing autism showing differential brain responses when fixating on the mouth or eyes compared to low-risk infants, irrespective of looking time (Elsabbagh et al., 2012; Key & Stone, 2012).Understanding differences in ERPs to point of gaze on the face has been limited to date by the lack of integration of eye tracking and EEG technology. An important future direction is to co-register eye-tracking with EEG to investigate and control for effects of gaze on ERPs and to determine whether the identified sex differences in ASD can be attributed to differential scanning patterns.
Given our limited sample size, it is essential to replicate these findings in a larger sample. Furthermore, our sample of convenience did not permit comparison of sex-differences in a typically developing sample with the same paradigm. A critical next step in sex difference literature in ASD is to utilize a well-matched typically developing sample. Although this paradigm and the male portion of this sample have been applied in comparisons with typically developing individuals (McPartland et al., 2011), future research must use larger samples with typical and atypical development to simultaneously study sex differences and differences associated with clinical dysfunction. Our sample consisted of higher-functioning individuals with ASD, and future work should assess effects of social information processing across the IQ range.
The current study provides the first account of distinct neural processing of social information in males and females matched for both chronological development and intellectual function. Consistent with behavioral accounts of more severe symptomatology in females with autism, we demonstrated reduced sensitivity to social information in females with ASD. These differences in social brain response were associated with behavioral measures of social function, and they revealed differential relationships among the sexes that were not evident in behavior alone. The relatively attenuated social perceptual response observed here is especially salient, given effects in the opposite direction in typical development. These findings have significant implications for early intervention and treatment. Given that both the incidence and the nature of ASD differ by sex, understanding behavioral and neural sex differences is an important area for future research, especially given the current lack of literature on the topic. Future treatment protocols should account for differences in clinical presentation and neural phenotypes in males and females with ASD.
ACKNOWLEDGEMENTS
This research was supported by NIMH R03 MH079908, NICHD PO1HD003008, CTSA Grant Number UL1 RR024139, NIMH K23MH086785, NARSAD Atherton Young Investigator Award, NIMH R21 MH091309, NIMH R01 MH100173 and R01 MH100028. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. We gratefully acknowledge the contributions of the funding sources that made this research possible, the parents and children who participated in this study, and several other people who made significant contributions to this research, including Linda Mayes, Robert Schultz, Ami Klin, Danielle Perszyk, Cora Mukerji, Jeffrey Eilbott, Jia Wu, Kevin Pelphrey, and Christopher Bailey.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association.; 2000. [Google Scholar]
- Baio J. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2012;61(3):1546-0738. [PubMed] [Google Scholar]
- Bauer D, Goldfield B, Reznick S. Alternative approaches to analyzing individual differences in the rate of early vocabulary development. Applied Psycholinguistics. 2002;23(3):313–335. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson S, Smith I. Epidemiology of autism: Prevalence, associated characteristics, and implications for research and service delivery. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4(2):97–103. [Google Scholar]
- Cahill L. Why sex matters in neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carter A, Black D, Tewani S, Connolly C, Kadlec M, Tager-Flusberg H. Sex differences in toddlers with autism specrtum disorders. Journal of Autism & Developmental Disorders. 2007;37:86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94(1):100–131. [Google Scholar]
- Elliott CD. Differential Ability Scales: Second Edition (DAS-II) San Antonio, TX: Psychological Corporation; 2007. [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Team B. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22(4):338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological Surveys of Autism and Other Pervasive Developmental Disorders: An Update. Journal of Autism & Developmental Disorders. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Gadow K, Sprafkin J. Child Symptom Inventories manual. Stony Brook, NY: Checkmate Plus; 1994. [Google Scholar]
- Happé F. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Society for Research in Child Development. 1995;66:843–855. [PubMed] [Google Scholar]
- Hartley S, Sikora D. Sex differences in autism spectrum disorder: An examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. Journal of Autism & Developmental Disorders. 2009;39:1715–1722. doi: 10.1007/s10803-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz A, Rehnman J. Sex differences in episodic memory. Current Directions in Psychological Science. 2008;17(1):52–56. [Google Scholar]
- Holtmann M, Bolte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine & Child Neurology. 2007;49:361–366. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27(2):236–248. [Google Scholar]
- Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage. 2002;15(2):353–372. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- Joliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cerebral Cortex. 2000;10:866–872. doi: 10.1093/cercor/10.9.866. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Stone W. Same but different: 9-month-old infants at average and high risk for autism look at the same facial features but process them using different brain mechanisms. Autism Research. 2012;5(4):253–266. doi: 10.1002/aur.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K, Tsatanis K. Pervasive Developmental Disorders in Girls. In: Bell D, Foster E, Mash E, editors. Behavioral and emotional problems in girls. New York: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- Lai M, Lombardo M, Pasco G, Ruigrok A, Wheelwright S, Sadek S, Baron-Cohen S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. Plos One. 2011;6:e20835. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang-Takac E, Osterweil Z. Separateness and connectedness: Differences between the genders. Sex Roles. 1992;27(5/6):277–289. [Google Scholar]
- Leeb R, Rejskind G. Here's looking at you, kid! A longitudinal study of perceived gender differences in mutual gaze behavior in young infants. Sex Roles. 2004;50(1/2):1–14. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Schopler E. Brief Report: Differences in sex ratios in autism as a function of measured intelligence. Journal of Autism & Developmental Disorders. 1985;15(2):185–193. doi: 10.1007/BF01531604. [DOI] [PubMed] [Google Scholar]
- Lord C, Schopler E, Revicki D. Sex differences in autism. Journal of Autism & Developmental Disorders. 1982;12(4):317–330. doi: 10.1007/BF01538320. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S. Human sex differences in social and nonsocial looking preferences, at 12 months of age. Infant Behavior & Development. 2002;25:319–325. [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum diroder: Evidence from a large sample of childrean and adolescents. Journal of Autism & Developmental Disorders. 2011;42(7):1304–1313. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- McLennan J, Lord C, Schopler E. Sex differences in higher functioning people with autism. Journal of Autism & Developmental Disorders. 1993;23(2):217–227. doi: 10.1007/BF01046216. [DOI] [PubMed] [Google Scholar]
- McMahon W, Ritvo A. The UCLA-University of Utah epidemiologic survey of autism: prevalence. American Journal of Psychiatry. 1989;146:194–199. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Eventrelated brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Wu J, Bailey CA, Mayes LC, Schultz RT, Klin A. Atypical neural specialization for social percepts in autism spectrum disorder. Social neuroscience. 2011;6(5–6):436–451. doi: 10.1080/17470919.2011.586880. doi: 10.1080/17470919.2011.586880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. Language and intelligence: A longitudinal study of the first eight years. Human Development. 1967;10(2):86–106. doi: 10.1159/000270561. [DOI] [PubMed] [Google Scholar]
- O'Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger's syndrome. Brain & Cognition. 2005;59:82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Guerreschi M, Lutzenberger W, Sokolov A, Krageloh-Mann I. Cortical response to social interaction is affected by gender. Neuroimage. 2012;50:1327–1332. doi: 10.1016/j.neuroimage.2009.12.096. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss P, Markowitsch H, Fink G. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human brain mapping. 2005;24(4):313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio A, Brignone V, Matarazzo S, Del Zotto M, Zani A. Gender differences in hemispheric asymmetry for face processing. BMC Neuroscience. 2006;7(44) doi: 10.1186/1471-2202-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K, Shaywitz B, Shaywitz S, Constable T, Skudlarski P, Fulbright R, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rossion B, Campanella S, Gomez C, Delinte A, Debatisse D, Liard L, Guerit JM. Task modulation of brain activity related to familiar and unfamiliar face processing: an ERP study. Clinical Neurophysiology. 1999;110:449–462. doi: 10.1016/s1388-2457(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime user's guide. Pittsburg: Psychology Software Tools Inc.; 2002. [Google Scholar]
- Scott F, Baron-Cohen S, Bolton P, Brayne C. Brief report: prevalence of autism spectrum conditions in children aged 5–11 years in Cambridgeshire, UK. Autism. 2002;6(3):231–237. doi: 10.1177/1362361302006003002. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales. Second Edition. Bloomington: Pearson; 2005. [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The Faces of Development: A Review of Early Face Processing over Childhood. Journal of Cognitive Neuroscience. 2004;16(8):1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Tsai B, Beisler M. The development of sex differences in infantile autism. British Journal of Psychiatry. 1983;142(373–378) doi: 10.1192/bjp.142.4.373. [DOI] [PubMed] [Google Scholar]
- Tucker D. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87(3):154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Volkmar F, Szatmari P, Sparrow S. Sex differences in pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1993;23(4):579–591. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale (3rd ed.) 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wing L. Sex ratios in early chilhood autism and related conditions. Psychiatry Research. 1981;5:129–137. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of Autism in a US Metropolitan Area. The Journal of the American Medical Association. 2003;289(1) doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]