Abstract
Though only a decade has elapsed since the default network was first emphasized as being a large-scale brain system, recent years have brought great insight into the network’s adaptive functions. A growing theme highlights the default network as playing a key role in internally-directed—or self-generated—thought. Here, we synthesize recent findings from cognitive science, neuroscience, and clinical psychology to focus attention on two emerging topics as current and future directions surrounding the default network. First, we present evidence that self-generated thought is a multi-faceted construct whose component processes are supported by different subsystems within the network. Second, we highlight the dynamic nature of the default network, emphasizing its interaction with executive control systems when regulating aspects of internal thought. We conclude by discussing clinical implications of disruptions to the integrity of the network, and consider disorders when thought content becomes polarized or network interactions become disrupted or imbalanced.
Keywords: default, autobiographical, social, self, mind-wandering, psychopathology
Introduction
Despite constant sensory stimulation from the busy world surrounding us, the human mind has the capacity to overcome external constraints in favor of a different time, place, or mental perspective. Whether commuting to work or trying unsuccessfully to concentrate during a long meeting, we often find ourselves simulating past experiences, planning upcoming activities, and reflecting on the lives of other people. Characterized by their independence from external stimuli, these self-generated thoughts (Box 1) are a complex and heterogeneous class of cognition. Sometimes they are formed with effort and purpose, and can be directly related to personal goals and aspirations; other times they unfold without our intent, hijacking attention until a salient stimulus or intermittent moment of awareness alerts us to the present moment.1,2 Self-generated thoughts can also be a source of creative insight, facilitating novel solutions to ongoing problems.3 At the same time, such thoughts can lead to distress and unhappiness,4 disrupting task performance and preventing us from dealing with immediate concerns.5 Understanding the psychological and neural mechanisms underlying self-generated thoughts, including their adaptive and maladaptive functional outcomes, has been a key aim of cognitive and neuroscientific research in recent years.
Box 1. Self-generated thought and related terms.
Thoughts and feeling can arise that are only loosely related to ongoing sensory input. In the literature, these experiences have been described using a wide range of terms. Some capture their independence from ongoing events such as task-unrelated thought or stimulus-independent thought. Others capture their internal rather than external focus: internally-directed, spontaneous, or autobiographical thought. One term that captures both their active nature and their relative independence from ongoing sensory input is self-generated thought.2 These experiences can occur as part of a task if a decision must be made that depends on an internal representation to reconstruct or imagine a situation, understand a stimulus, or generate an answer to a question. They can also occur independently from an external task, such as when individuals daydream or mind-wander when performing a task, or while resting with no explicit task to perform. Mental content during self-generated experiences depend to a large extent upon associative and constructive processes that take place within an individual and can be contrasted with thoughts whose primary referent can be derived simply from immediate perceptual input (perceptually-generated thought).
The studies considered in this review, as well as several meta-analyses, demonstrate that the DN is active during both task-relevant and task-irrelevant examples of self-generated thought. These findings demonstrate that the DN is characterized not by its opposition to a task but by the type of self-generated mental content it supports.
Many of the neural systems that support externally focused tasks show coordinated activity at rest (such as the motor network or the visual network). One important question that these observations raise is whether spontaneous changes in regions outside of the DN contribute to an individual’s self-generated experiences, and if so what cognitive or experiential properties they represent.
An important avenue for future work will be a clearer delineation of brain regions involved in the mechanisms driving self-generated thought on the one hand, and the content of self-generated thought on the other.
An established body of research over the last decade has pinpointed a large-scale brain system referred to as the default networka (DN; Box 2; Fig. 1) as supporting several aspects of spontaneous and deliberate self-generated thought.6–8 The DN has received widespread interest from several sub-disciplines in the social and biological sciences for its psychological and clinical relevance. When Buckner, Andrews-Hanna, and Schacter published their initial review of the network in the Year in Cognitive Neuroscience in 2008,7 several questions remained unanswered. What do people think about when left to their own musings? Are different aspects of self-generated thought supported by distinct components within the DN? How does the DN interact with other large-scale brain systems when maintaining an internal train of thought? Though recent years have contributed substantial progress towards answering these questions, much still remains to be understood. Here, we synthesize this research, drawing parallels with a growing psychological literature on mind-wandering and highlighting several avenues for future research.
Box 2. Defining the default network.
Although the regions that compose the DN were originally defined by patterns of deactivation during goal-directed tasks compared to passive control conditions, this pattern breaks down when goal-directed tasks are of an internal nature (see main text). Because of this task-related variability, we instead define the DN based on its patterns of temporal correlations using resting-state functional connectivity MRI (RSFC).14,51,242 In a comprehensive set of studies, Yeo, Choi, Buckner, and colleagues applied clustering techniques to RSFC data collected from 1,000 participants to partition the cortex, striatum, and cerebellum into seven intrinsic large-scale brain systems.51,52,53 As shown in Figure 1a, the DN includes voxels spanning the mPFC (the dmPFC, the rostral anterior cingulate, and parts of the anterior and ventral mPFC), lateral frontal cortex (the superior frontal cortex and the inferior frontal gyrus), medial parietal cortex (the posterior cingulate and retrosplenial cortex), medial temporal lobe (the hippocampus and parahippocampal cortices), lateral parietal cortex (spanning the angular gyrus and the posterior supramarginal gyrus/TPJ), and lateral temporal cortex (extending anteriorly to the temporal poles). In addition to these cortical regions, the DN also includes large areas of the cerebellum (including Crus I and Crus II subdivisions) and the striatum (the medial wall of the caudate and the posterior putamen). Interestingly, there exists substantial convergence between the spatial extent of the DN as defined with RSFC and with large-scale meta-analyses of functional neuroimaging data using NeuroSynth65 (Fig. 1b).
Figure 1.
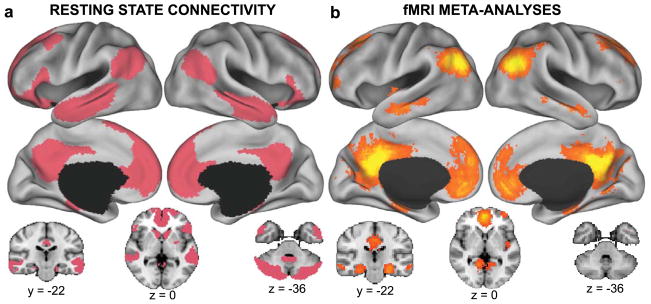
The default network. (A) The default network as revealed by resting-state functional connectivity MRI of the cortex, striatum, and cerebellum. Figure created using data from Yeo et al.,51 Choi et al.,53 and Buckner et al.52 (B) The default network revealed by a meta-analysis of functional neuroimaging data using NeuroSynth software.65 Shown are false discovery rate–corrected reverse inference statistical maps (P term|activation) for meta-analyses corresponding to default.mode, default.network, or default.mode.network.
We first challenge common notions that the DN is a passive brain network by reviewing evidence suggesting it contributes to several active forms of internally directed cognition. Next, we examine self-generated thought and the DN on a finer scale, synthesizing recent findings that self-generated thought is composed of multiple component processes partly supported by distinct subsystems within the DN. In light of research examining the DN within a larger connectome of interacting brain systems, we suggest that the DN does not operate in isolation, but rather interacts with other brain systems when maintaining or inhibiting an internal train of thought. Finally, we propose that the DN and self-generated thought is directly relevant to psychopathology and disease. One paradox about self-generated thoughts is that they can be associated with both costs and benefits, and this trade-off varies widely across individuals.10–13 In light of this observation, we highlight the content of self-generated thought and the context under which it occurs as two key factors underlying why it can be detrimental for some, yet beneficial for others.
The default network plays an active role in self-generated cognition
When the DN was first characterized, it was mainly appreciated for its elevated activity during passive-control conditions, and its relative absence during externally focused goal-directed tasks.9 For what are likely historical reasons, the notion that the DN exhibited task-induced deactivations led to its description as a “task-negative network” and the idea that it supports passive rather than active mental states.14 We suggest that this view of the DN is erroneous, and fails to acknowledge that the goals of an individual almost always extend beyond the here and now.6,15,16 The paradigms favored by most cognitive scientists define goal states as performing cognitive operations on external sensory input, a definition that has obscured the possibility that the DN serves important psychological functions.
A review of the literature on the DN reveals that it increases its activity during goal-directed cognitive tasks, as long as experimental conditions require participants to engage in directed forms of self-generated thought. Tasks that activate the network often require participants to retrieve episodic, autobiographical, or semantic information, think about or plan aspects of their personal future, imagine novel scenes, infer the mental states of other people, reason about moral dilemmas or other scenarios, comprehend narratives, self-reflect, reference information to one’s self, appraise or reappraise emotional information, and so on.6–8 As with most experiments, many of these tasks involve an external stimulus and require a motor or vocal response. However, what seems unique to conditions that recruit the DN is their need to actively self-generate mental contents in order to arrive at the desired goal.
The DN is sometimes transiently engaged during externally focused tasks, particularly those that are easy, boring, or highly practiced. This activity can signify the presence of mind-wandering, a term that refers to a shift in attentional focus towards unrelated self-generated information at the cost of task-relevant perceptual stimuli.17–20 Participants’ spontaneous self-generated thoughts may also contribute to the DN’s high metabolic activity during unconstrained periods of passive rest (often referred to as the resting state). This idea was initially highlighted by Andreasen and colleagues,21 who reasoned that similar patterns of regional blood flow between autobiographical memory and rest tasks were attributable to the presence of spontaneous thoughts that consisted of “a mixture of freely wandering past recollection, future plans, and other personal thoughts and experiences.” Recent studies employing experience-sampling methods or retrospective self-report questionnaires support these findings, revealing that participants spend a considerable amount of time engaged in self-generated thoughts during periods of awake rest.22–24 Below, we examine the phenomenological characteristics of these thoughts, revealing that they often reflect an active mental process.
Self-generated thought comprises multiple component processes
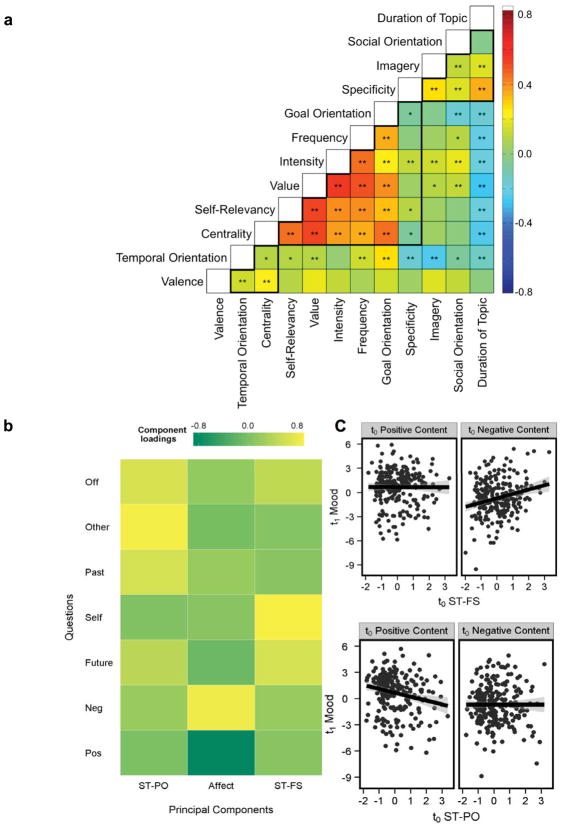
Self-generated thoughts unrelated to external input or immediate tasks are common features of daily life. Experience-sampling studies estimate that adults spend between 30% and 50% of their waking day engaged in thoughts unrelated to ongoing activities,4,25–27 and a close examination of the nature of these thoughts suggests that they are a complex and heterogeneous phenomena.28 Although most studies have focused on one or two aspects of self-generated thought or their interactions,29,30 a few have assessed multiple types of content across large groups of individuals, elucidating their complexity (Fig. 2A–B).12,23,24,31,32 Collectively, these studies suggest that self-generated thoughts can be characterized according to multiple interacting dimensions, including their personal significance, temporal orientation, valence, social orientation, level of specificity/detail, somatosensory awareness, and representational format (inner speech versus visual imagery).12,23,24,31,32
Figure 2.
Heterogeneity of self-generated thought. (A) In this study, Andrews-Hanna and colleagues12 asked 76 participants to recall numerous self-generated thoughts experienced in daily life and rate each thought on a variety of content variables. Within-subject relationships between content variables were averaged across participants, and the results of a hierarchical clustering analysis on the group matrix are shown in boxes. Increases in the content variables correspond to higher ratings on that variable, with these exceptions: duration is reversed such that increases correspond to thoughts rated as shorter duration topics, temporal orientation reflects chronological time such that increases are more future-oriented, and valence ranges from negative to positive such that increases are more positive. Figure adapted from Ref. 12. * = P < 0.05, ** = Bonferroni-corrected. (B) A decomposition of the content of task-unrelated self-generated thoughts while participants performed a simple non-demanding laboratory task.31 This revealed two different components of social thought: one reflecting social thoughts related to the past and others (ST-PO: social temporal past other) and a second relating to the future (ST-FS: social temporal future self). A third nonsocial emotional component was also identified (EMO) (C) Results of a lag analysis exploring the temporal relationship between each component from B. The co-occurrence of positive emotional content with thoughts about the past was followed by more negative mood, whereas negative mental content regarding the future led to a subsequent mood with a more positive tone. For a replication of the two types of social temporal self-generated thoughts, see Ref. 35.
The content of self-generated thoughts suggests that they serve an adaptive purpose by allowing individuals to prepare for upcoming events,33 form a sense of self-identity and continuity across time,30,34 and navigate the social world.35–37 On average, adults tend to rate their thoughts as goal oriented and personally significant,12,13,22,32,38,39 yet thoughts also commonly involve other people.12,31,37 Additionally, self-generated thoughts tend to have a temporal focus, being characterized more by a prospective than a retrospective bias.22,27,30,32,33,40
The content of self-generated thought also evolves in a complex manner with the passage of time. For example, processing negative information increases the frequency of negative and retrospective thoughts,29,40,41 and task-unrelated thoughts can also lead to subsequent unhappiness.4 Thinking about the self increases the frequency of future thinking, and these prospective experiences mediate the memory advantage for self-referential information.30 Using lag analysis, Ruby et al.31 found that social thoughts pertaining to one’s past tend to precede negative mental content, whereas social thoughts pertaining to one’s future are likely to lead to subsequent positive thoughts (Fig. 2C).
Although self-generated thought often involves constructive experiences, there is a considerable degree of within- and between-subject variability in its functional consequences, and we view this topic as an important direction for future research. Self-generated thoughts characterized by polarized content may be symptoms of mental health disorders,42 and the integrity of the internal experience can also break down in a number of neurodegenerative diseases.43 These findings are consistent with the content regulation hypothesis, which proposes that variability in the ability to regulate the content of self-generated thought partly underlies its costs and benefits.10 Links to psychopathology are discussed in below.
The default network comprises multiple interacting subsystems
Although converging evidence reveals that the DN plays an important role in self-generated thought, the heterogeneous nature of the experience suggests a parallel level of complexity in the network’s functional–anatomic organization.6,7,44–49 Here we examine the DN on a finer scale, discussing recent evidence from resting-state functional connectivity MRI (RSFC) and diffusion tensor imaging (DTI) that the DN is composed of distinct yet interacting subsystems.
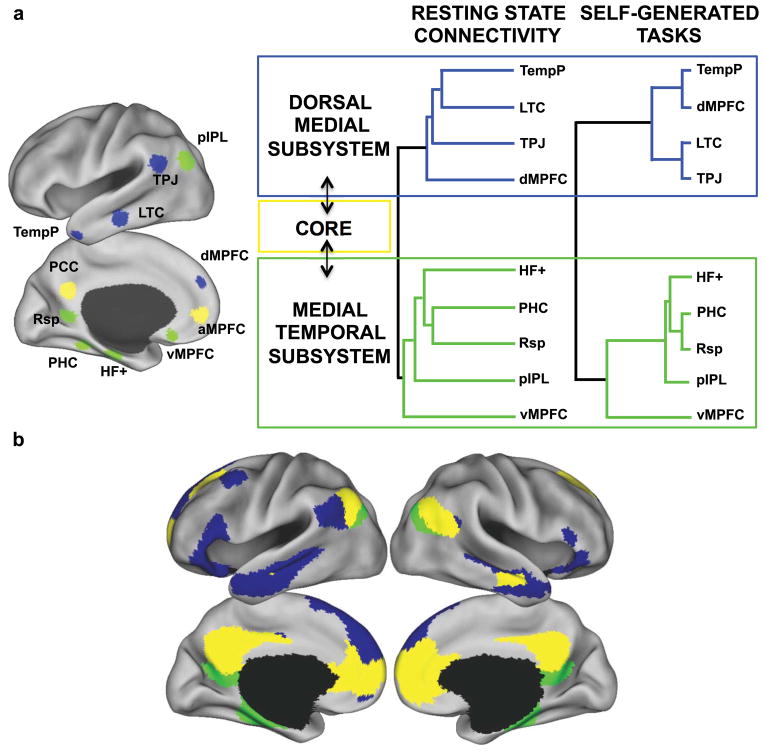
Initial evidence for subsystems within the DN was provided by Andrews-Hanna et al.,50 who used hierarchical clustering analyses to partition RSFC and task-related fMRI data from eleven left-lateralized and midline DN regions into two separable components, each of which were highly correlated with a midline core (Fig. 3A). A medial temporal subsystem comprised the hippocampus, the parahippocampal cortex, the retrosplenial cortex (RSC), the posterior inferior parietal lobe, and the ventromedial PFC (vmPFC), while a dorsal medial subsystem comprised the dorsal medial prefrontal cortex (dmPFC), the temporoparietal junction (TPJ), the lateral temporal cortex, and the temporal pole. Along the cortical midline, the anterior medial prefrontal cortex (amPFC) and the posterior cingulate cortex (PCC) exhibited strong functional coherence with both subsystems and were hypothesized to act as functional hubs, allowing information to transfer between subsystems.
Figure 3.
Heterogeneity of the default network. (A) Graph and clustering analysis of 11 DN regions during passive rest and active self-generated tasks reveal the presence of distinct medial temporal and dorsal medial subsystems that converge on the amPFC and PCC core network. Figure adapted from Andrews-Hanna and colleagues.6,50 amPFC = anterior medial prefrontal cortex; dmPFC = dorsal medial prefrontal cortex; HF = hippocampal formation; LTC = lateral temporal cortex; MTL = medial temporal lobe; PCC = posterior cingulate cortex; PHC = parahippocampal cortex; pIPL = posterior inferior parietal lobule; RSC = retrosplenial cortex; TempP = temporal pole; TPJ = temporoparietal junction; vmPFC = ventral medial prefrontal cortex. (B) DN components as revealed by an unbiased, whole-brain parcellation of resting-state fMRI data from 1,000 participants are broadly consistent with panel A. Note the additional involvement of lateral prefrontal regions with the dorsal medial subsystem, and the addition of the superior part of the angular gyrus in the DN core. Figure created using data from Yeo and colleagues.51
These findings have since been replicated and extended using unbiased whole-brain clustering approaches. Yeo, Buckner, Choi, and colleagues applied clustering algorithms to resting state activity from 1000 participants, partitioning more than 1000 uniformly spaced regions spanning the cortex,51 the cerebellum,52 and the striatum53 into seven correlated networks of intrinsic activity. This coarse analysis was followed by a finer parcellation subdividing the DN into three bilateral subsystems similar to those identified by Andrews-Hanna and colleagues (Fig. 3B). Important differences between the two analyses also emerged. The whole-brain clustering approach revealed the medial temporal subsystem lacked the vmPFC, which in turn clustered into a separate limbic network; the dorsal medial subsystem was largely left-lateralized and also encompassed lateral prefrontal regions including the lateral superior frontal cortex, the ventrolateral PFC, and the inferior frontal gyrus; and the amPFC-PCC core included additional regions within the bilateral angular gyrus, the anterior temporal lobes, and the superior frontal gyrus. Providing further support for these DN components Doucet and colleagues54 observed strong temporal correlations between resting-state components overlapping with the three subsystems identified above. However, the dorsal medial subsystem clustered into a distinct module along with several additional frontoparietal regions, consistent with a more complex attentional role in self-generated thought.
Patterns of anatomical connectivity in humans and macaques are broadly consistent with the presence of anatomical heterogeneity within the DN, although human cortical expansion makes direct comparison between species difficult.55 While the PCC and amPFC are connected by the cingulum bundle56,57 and exhibit widespread connections with additional regions throughout the DN,56,58–60 the medial temporal subsystem is supported by white matter tracts connecting the medial temporal lobe (MTL), RSC, and angular gyrus (area 7a in the macaque).56,58,61,62 Supporting the anatomical basis for the dorsal medial subsystem, the inferior parietal lobe connects to the lateral temporal lobe via the middle longitudinal fasciculus56,63 and to the lateral PFC via the arcuate fasciculus.56 The macaque dmPFC (Brodmann area 9) is also connected to the dorsal and ventrolateral PFC, the superior temporal sulcus, and the temporal pole, whereas connections with the MTL are sparse.59,64
In summary, the functional and anatomical properties of the DN suggest a heterogeneous brain system comprised of at least three separable components. Below, we explore the possible functions of the subsystems by way of both a meta-analytic and narrative synthesis of recent work on cognitive, social, and affective neuroscience.
Default network subsystems support component processes of self-generated thought
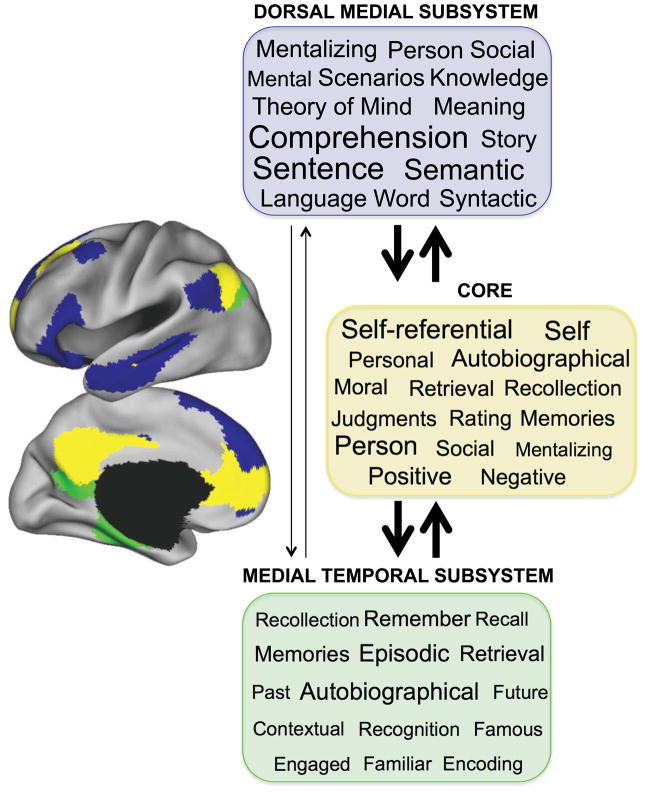
Given the functional–anatomic heterogeneity within the DN, combined with the complexity of the self-generated experience, a logical question to pursue is whether the DN components contribute to different aspects of self-generated thought. We first explored this question by conducting large-scale meta-analyses using NeuroSynth65 to decode the functional properties of the three default subsystem masks in Figure 3B from Yeo and colleagues.51 Out of 526 meta-analyses in the NeuroSynth database, the dorsal medial subsystem corresponded most strongly with meta-analytic maps pertaining to mentalizing and social cognition (i.e., mentalizing, social, person, theory of mind, mental, scenarios), as well as story comprehension and semantic/conceptual processing (i.e., comprehension, semantic, sentence, story, meaning, knowledge, language, word, syntactic) (Fig. 4). The medial temporal subsystem corresponded most strongly with meta-analytic maps pertaining to past and future autobiographical thought (i.e., autobiographical, past, future), episodic memory (i.e., episodic, memories, remember, recollection, recall) and contextual retrieval (i.e., contextual, retrieval) (Fig. 4). Finally, the core network associated with self-related processes (i.e., self-referential, self, autobiographical, personal), emotion/evaluation (i.e., positive, negative, moral), and social and mnemonic processes shared by the dorsal medial and medial temporal subsystem (i.e., social, person, mentalizing, recollection, retrieval, memories) (Fig. 4). These findings provide initial evidence of functional dissociation and interactions among the DN components. Below we interpret these results in the context of existing literature and propose a functional model of the DN.
Figure 4.
Decoding the functions of default network components using automated fMRI meta-analyses. Automated meta-analytic software (NeuroSynth65) was used to compute the spatial correlation between each DN component mask (shown on the left, see Fig. 3B) and every other meta-analytic map (n = 526) for each term/concept stored in the database (i.e., memory, attention, emotion, sensory, etc.). The 15 meta-analytic maps exhibiting the highest correlations for each subsystem mask were extracted, and the term corresponding to each of these meta-analyses is shown in each colored box. The font size reflects the size of the correlation (ranging from r = 0.05–0.35 in increments of 0.05).
Core regions may allow individuals to construct personal meaning from salient information
The PCC, angular gyrus, and amPFC are the most consistently engaged regions within the DN. Recent studies suggest that the PCC is a heterogeneous brain structure, with subdivisions characterized by distinct patterns of structural and functional connectivity, echoing neural signals from several additional large-scale brain networks.66 The PCC can be broadly subdivided into ventral and dorsal components, with further subdivisions in the dorsal PCC.66–68 Consistent with the meta-analytic results, the ventral PCC functionally correlates with the rest of the DN67 and activates across nearly all self-generated tasks, including tasks of self-referential processing, episodic or autobiographical memory, future thinking, mentalizing/theory of mind, spatial navigation, and conceptual processing (Fig. 4).8,69–71 The dorsal PCC functionally correlates with many other brain systems and has been linked to autonomic arousal and awareness72,73 and monitoring for behaviorally relevant stimuli and environmental changes.66,74 Both dorsal and ventral subdivisions are strongly anatomically connected with each other and the adjacent precuneus.61 These observations suggest that the broader PCC can be viewed as an important zone of integration supporting bottom-up attention to behaviorally relevant sources of information drawn from memory and/or perception.73
The anterior lateral temporal cortex and the angular gyrus are additional zones of integration within the DN that activate across a variety of tasks ranging from semantic processing to memory retrieval and theory of mind.75–77 The anterior lateral temporal cortex plays a key role in conceptual processing78 and may store semantic knowledge of items and other concrete conceptual information,79 supported by connections to the ventral visual and auditory “what” processing streams.80,81 In contrast, the angular gyrus exhibits widespread patterns of connectivity with the anterior lateral temporal cortex, remaining DN regions, and with additional regions involved in perception, attention, spatial cognition, and action.75 The angular gyrus may therefore function as a cross-modal hub, allowing internal and perceptual sources of information access conceptual representations about events or items in their spatiotemporal context.75,79
The amPFC is characterized by extensive patterns of connectivity with the PCC, the dorsal medial and medial temporal subsystems, the ventrally-positioned limbic network (including the medial orbitofrontal cortex), and subcortical regions involved in affect and autonomic arousal/regulation.50,82 Consistent with the meta-analysis (Fig. 4), the amPFC is most appreciated for its role in self-related processing, including when individuals reference information to themselves, retrieve personal knowledge, recall autobiographical memories, consider their future goals or mental states, and simulate personal future events or social interactions.6,8,83–85 Personal information is often attributed high value, and perceived value elicits overlapping responses in the amPFC,83,86 often extending more ventrally.87 The amPFC also becomes engaged when making decisions pertaining to other people we value, including our friends and relatives,88–90 as well as those we deem similar91 (but see Ref. 89). Though the amPFC has been most robustly linked to positive emotional material and reward,87 negative emotional material can also engage the amPFC, especially when such material is attributed high personal significance, as when one anticipates or evaluates physical pain92,93 or social threat.94 Through its widespread connectivity with mnemonic, limbic, autonomic, and semantic structures (including the lateral parietal cortex, which is also considered part of this subsystem), the amPFC is well positioned to integrate salient external or internal information (perhaps relayed from the PCC) with one’s current affective experience and prior conceptual or episodic knowledge. An emergent outcome of these associations might be the mental construction of an overarching personal meaning, which can subsequently update existing representations and guide thoughts and behavior over longer time scales.82
The role of the medial temporal subsystem in constructive mental simulation
Results of the meta-analysis suggest that the medial temporal subsystem may play an important role in episodic/contextual retrieval and simulating one’s future. Though these proposed functions are consistent with the literature, recent studies also implicate the medial temporal subsystem in broader aspects of mental simulation, including associative or constructive processes.95–98 Damage to the hippocampus often leads to parallel deficits in remembering and imaginin,99,100 despite preserved narrative processing101 and an intact ability to infer the mental states of other people.102 By contrast, parahippocampal cortex damage leads to broad deficits in spatial and scene recognition103,104 and lesions to the medial temporal lobe alter functional coupling with the medial temporal system.105 RSC lesions often lead to a deficit in spatial navigation known as topographical amnesia,106 while lesions to the angular gyrus can impair recollective aspects of episodic memory.107,108 Consistent with these findings, the medial temporal subsystem is reliably activated when individuals engage in autobiographical memory and episodic future thought,6,7,98 and individual differences in RSFC within the medial temporal subsystem relate to the degree of spontaneous past and future thought experienced during the resting state.22 Furthermore, task strategies involving the use of imagery-based construction account for a large portion of trial-by-trial variability within the medial temporal subsystem,50 supporting theories implicating the DN in scene construction96 or constructive episodic simulation.98 In a related fashion, Ranganath and Ritchey77 proposed that a posterior medial memory system, which closely overlaps with the medial temporal subsystem, functions to integrate an object or an individual into a situation model, including a particular time, place, and context.
Interestingly, mnemonic retrieval of items previously encoded in laboratory settings also activates the medial temporal subsystem, but only if the mnemonic judgment is associated with a subjective sense of recollection or if the task requires participants to retrieve additional contextual details related to how the item was initially encountered, such as the spatial location, the temporal sequence, or the type of judgment in which the item was encoded.77,109,110 Supporting its role in associative aspects of simulation, regions throughout the medial temporal subsystem (in addition to the vmPFC, which clusters into a distinct large-scale brain system51) also become engaged when individuals (1) view objects whose association with a spatial context based on past experiences is strong,111(2) retrieve concrete/perceptual knowledge,69 and (3) acquire and use associative conceptual knowledge to guide decision making.112 Working together, it is possible that the medial temporal subsystem, through its interactions with the vmPFC, plays a broad role in associative or constructive aspects of mental simulation.
The involvement of the dorsal medial subsystem in mentalizing and conceptual processing
Key structures within the dorsal medial subsystem, including the dmPFC and TPJ, are widely appreciated for their role in mentalizing, 6–8,76,84,113–119 the metacognitive process of inferring or reflecting upon the mental states of other people and/or one’s self.113 The False Belief task is a commonly used measure of mentalizing or theory of mind requiring participants to infer the false mental state of a protagonist.120 While these and other theory of mind tasks involve external stimuli, they also rely on self-generated cognition decoupled from the physical world because humans do not have immediate perceptual access to other people’s thoughts.113,114 Social tasks that do not require individuals to process such internal or self-generated information do not tend to activate the dorsal medial subsystem.114,121 Regions throughout this subsystem (with the exception of the right TPJ) also become engaged when individuals are asked to reflect on their own preferences, beliefs, desires, and emotions,84,122,123 though often not as strongly.84 The dorsal medial subsystem also contributes to social and/or self-reflective aspects of autobiographical memory or future thought.124–127
As with the core network, there appear to be important functional differences within the dorsal medial subsystem. While the dmPFC activates during a broad range of social-reflective tasks, including discriminating between thoughts about distinct individuals,128 the right TPJ activates when individuals are asked to reflect on the beliefs of other people.129 It should be noted, however, that the dorsal medial subsystem as defined by Yeo et al.51 encompasses only a small region within the right TPJ, whereas the right TPJ extends more broadly in many theory-of-mind tasks. Other regions within the dorsal medial subsystem have been shown to play a role in more basic (i.e., non-reflective or inferential) aspects of mentalizing. These include regions along the left superior temporal sulcus, extending posteriorly into the angular gyrus, and anteriorly into the temporal poles, as well as lateral prefrontal regions likely involved in executive aspects of mentalizing.114 Consistent with our meta-analysis (Fig. 4), studies suggest these regions may facilitate the retrieval of semantic and conceptual knowledge (including social knowledge), which is a likely prerequisite of more complex and temporally extended social cognitive processes.69,75,79
The specificity of key regions within the dorsal medial subsystem for social information remains a matter of debate. Although several of these regions tend to be engaged during tasks involving narrative comprehension or inductive reasoning, many of the stimuli employed in such tasks tend to be social in nature.76,130 Meta-analyses of both narrative-based and non-narrative theory-of-mind tasks reveal overlap throughout the dorsal medial subsystem,76 and meta-analyses of social and non-social reasoning tasks reveal that social reasoning tasks sometimes engage the dorsal medial subsystem, while non-social reasoning tasks often do not.130 A recent study observed the dorsal medial subsystem when individuals answered reflective compared to descriptive questions about social and non-social scenes,131 but activity was stronger for social stimuli. Synthesizing these findings, it is possible that our evolutionary social nature may predispose us to preference social over non-social information,132 leading to heightened activity for social material within a key network of regions important for more basic conceptual processes. However, it is also likely that regional and/or pattern specificity exists within the dorsal medial subsystem, and this specificity is an important topic for future research.
Interactions among subsystems and implications for the resting state
As the experiments targeting self-generated thought have become more constrained, the roles of the DN subsystems have become clearer. However, it is important to keep in mind that these processes are highly integrated with the function of the network as a whole, and they likely interact or co-occur during many self-generated experiences,6 including when individuals infer the mental states of a familiar other,133 imagine future social interactions,134 or use memory to think about other people.135,136 After all, autobiographical memories and self-generated thoughts are often characterized by their self-referential and social nature,12,34,37,124,127,137 and many of our past and anticipated experiences involve people for whom we care.37,136 Perhaps not surprisingly, unconstrained periods of rest commonly recruit aspects of all three DN subsystems.6
Summary
Our analysis of the functions served by the DN suggests that it comprises multiple subsystems, each contributing specific processes characterizing self-generated thought. We propose that a DN core works to represent information that is personally relevant, the medial temporal subsystem allows an individual to bring associative information to mind to construct coherent mental scenes, and the dorsal medial subsystem allows information related to self and other to be reflected upon in a meta-cognitive manner, likely using stored conceptual knowledge. We argue that acting in combination, these different neural systems support much of the mental content underlying self-generated thought.
Viewing the DN on a finer scale also helps resolve discrepancies among prior theories behind the function of the DN involvement in scene construction,96 associative prediction,95,111 constructive episodic simulation,97,98 self processing,70 and mentalizing.118,119,138 For example, scene construction, constructive episodic simulation, and associative prediction fit well with previous findings regarding the medial temporal subsystem; self-related functions correspond best with proposed functions of the DN core; and mentalizing is likely a function of the dorsal medial subsystem. Of note, our findings also implicate each DN component in aspects of conceptual processing, including the storage, retrieval, and/or integration of conceptual knowledge, consistent with prior theories.69,79 Indeed, concepts form the basic building blocks of more complex self-generated thoughts, including our autobiographical memories and future plans. Whether distinct domains of knowledge are linked to unique DN components, as suggested by the reviewed research, is an important avenue of future research. Future studies should also explore possible functional differences between left- and right-lateralized regions within the DN subsystems, particularly with respect to their role in social cognition and episodic/autobiographical memory.
Dynamic interactions and top-down control of self-generated thought and the default network
Despite the important role of the DN in self-generated thought, the network also exhibits dynamic interactions with a number of other distributed large-scale brain networks (Fig. 5). The most investigated of these interactions include its anticorrelation with the dorsal attention network and its modulation and coupling with the frontoparietal control network to facilitate goal-directed cognition. In this section we consider the possible functions of these network interactions.
Figure 5.
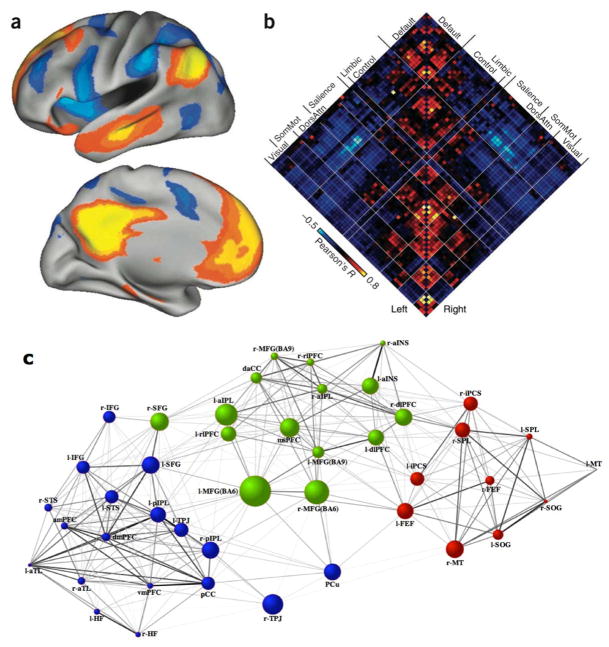
The default network and large-scale network interactions examined using resting-state functional connectivity MRI. (A) RSFC of the DN and anticorrelation with the DAN. Adapted from Ref. 185.(B) A correlation matrix shows the coupling architecture of the cerebral cortex measured at rest. Between-network correlations are characterized by both positive and negative relations, with strong anticorrelation notable between the default and salience/dorsal attention networks. Adapted from Ref. 185. (C) Interregional pairwise connectivity graph within and between the default (blue), dorsal attention (red), and frontoparietal control (green) networks. Line weights represent the magnitude of the positive correlation between nodes. Node size represents the magnitude of betweenness centrality, a graph analytic measure of its contribution as an inter-network connector hub. Adapted from Ref. 163.
Anticorrelations and perceptual decoupling
The DN has an anticorrelated or antiphase relationship with the dorsal attention network (DAN),14,139,140 a brain system consisting of the posterior prefrontal cortex, the inferior precentral sulcus, the superior occipital gyrus, the middle temporal motion complex, and the superior parietal lobule14,51,141 (Fig. 5A). The DAN supports visuospatial processing,141 and its engagement results in the suppression of the DN.142 This dynamic balance emerges in early childhood143 and is reduced with advancing age.144 The magnitude of anticorrelation also relates to cognitive performance145 and certain psychopathological disorders (see below).146–148 In many empirical contexts, suppression of the DN is adaptive and necessary for effective completion of cognitive tasks that require attention to experimental stimuli.
Patterns of anticorrelation between the DN and the DAN are thought to support competitive relationships between an internal focus of attention that occurs during self-generated thought and attention to concurrent environmental stimuli. Task-unrelated thoughts are often associated with a reduction in the evoked response in the EEG that reflects the processing of external information,149–152 even when such information is irrelevant to the task.153 Importantly, the reduction in evoked response during mind wandering has been observed for early EEG components that are thought to index perceptual processing.149 Altogether these studies provide experiential evidence that the occurrence of self-generated thought reduces the processing of sensory information, a phenomenon which has been termed perceptual decoupling.1,2,154,155 One question raised by these findings is whether the competition between the DN and DAN reflects a situation that is necessary for the integrity of self-generated thought processes. It is possible, for example, that perceptual decoupling has adaptive value because it corresponds to a situation in which the competition of perceptual input for attention is reduced, allowing self-generated thought to persist with fewer interruptions.2 This is an important question for future research.
Default and frontoparietal control network interactions
Many higher-order cognitive functions depend on top-down regulatory processes to ensure that relevant goals are achieved, and the personal and social goals that the DN serves are no exception. It is now widely established that the frontoparietal control network (FPCN) plays an important role in executive control of attention.156–159 Central regions include dorsal lateral PFC (dlPFC) and anterior inferior parietal lobe, yet RSFC investigations have revealed an extended system additionally including the rostral PFC, the dACC, the frontal operculum/anterior insula, the precuneus, and the posterior inferior lateral temporal cortex.51,160 This system can be further subdivided into two subsystems: the frontal-parietal and cingulo-opercular networks.161 Although spatially distinct, the FPCN is anatomically interposed between the default and dorsal attention networks,51,160,162 suggesting it may play an important modulatory role in the activation and suppression of these other networks based on switching goal states. Consistent with this notion, there is a high level of intrinsic functional integration among the default and frontoparietal control systems.54,163 Moreover, all of these networks appear to be functionally segregated from sensory motor systems,54 suggesting a dedicated role for these regions and their dynamic interactions in the implementation of higher cognitive processes.
Most studies investigating the FPCN have done so in the context of externally-directed tasks where task goals depend on different stimulus–response contingencies. However, the FPCN has also been shown to play an important role in regulating self-generated mnemonic, social, and emotional information, and does so by varying its functional connectivity with the DAN or the DN, respectively. Spreng and colleagues162 scanned participants while completing two different planning tasks: visuospatial planning, as assessed by the well-established Tower of London task; and autobiographical planning, as assessed by a novel task that required participants to devise personal plans, such as how to get out of debt, in order to meet specific goals. Visuospatial planning engaged the DAN, whereas autobiographical planning engaged the DN. Both engaged the FPCN and, critically, task-related functional connectivity analyses indicated that this network was coupled with the DN during autobiographical planning and with the dorsal attention network during visuospatial planning. Gao et al.143 observed a similar pattern of large-scale network interactivity as participants alternated between a motor sequence task and watching a movie.
There is now a relatively extensive body of work suggesting that default and control networks can cooperate to perform particular mental operations. For example, Gerlach et al.164 examined brain activity during a problem-solving task involving mental simulations. Relative to a semantic processing control task, problem solving engaged several key regions within the DN, including the MPFC and PCC, as well as a region of lateral prefrontal cortex linked to executive processing. In the context of autobiographical planning, DN to FPCN coupling was found to be specific to actively imagining the steps necessary to attain a personal goal, not imagining events associated with the achieved outcome.165 Similarly, a parametric modulation of keeping track of person information provides evidence for the coactivation of the DN and FPCN during social working memory,166,167 while another study showed that DN regions coactivated with regions of the FPCN when participants evaluated creative ideas.168 All of these tasks depend on the maintenance or extended evaluation of internal information in the service of a goal, leading to the suggestion that these forms of large-scale interactions reflect temporally extended evaluation of self-generated thoughts. The dynamic interactions between the FPCN and the default and dorsal attention networks may also account for the involvement of the FPCN in certain forms of self-regulation,169,170 emotion regulation,171,172 memory suppression,173 and task-unrelated thought.19
The flexible modulation hypothesis receives additional support from Spreng and colleagues,163 who used measures of RSFC MRI and graph analyses to further examine relations among the DN, FPCN, and DAN in the absence of an overt task (Fig. 5C). Converging with findings from task-based fMRI, the authors observed little positive connectivity between default and dorsal attention networks, accompanied by a high degree of connectivity between each of these networks and the FPCN, with preferential patterns of pairwise connectivity amongst core network nodes (Fig. 5). Additionally, Chang and Glover174 used a sliding-window correlation approach to demonstrate that temporal relationships between the DN and the FPCN dynamically fluctuate across short time scales. Whether this temporal variability parallels dynamic shifts in attentional focus remains an interesting avenue for future research.
Within the behavioral literature on self-generated thought, multiple lines of research support the notion that internally guided thought can depend upon executive processes. For example, studies routinely show that the occurrence of self-generated thought is reduced by tasks with a working-memory component.18,175 This working-memory suppression of self-generated thought is especially true of experiences focused on the future.30,40 Such evidence is consistent with the notion that at least some forms of self-generated thought require executive resources. Participants who generate more task-unrelated thoughts during less demanding conditions have a higher working memory capacity,176 show less impulsive economic decision making,177,178 and tend to engage in self-generated thoughts pertaining to autobiographical plans.33 By contrast, under more demanding task conditions, individuals with greater cognitive control generate fewer thoughts that are unrelated to the task at hand.26,179,180 Together these findings have been suggested to reflect the experiential equivalent of the contextual changes in the correlation between the frontoparietal and default networks,2 and provide support for the context regulation hypothesis10 (see below).
Summary
The reviewed evidence suggests that the DN exhibits complex interactions with several additional large-scale networks, and one open question is what function these interactions serve. One possibility is that together they reflect the neural processes that allow individuals to make progress on goals that depend upon input from the present moment as well as goals that do not. Both exogenous and endogenous loci of information processing are necessary for navigating the complex environment in which we live, and each is likely to contribute to adaptive behavior under different situations. The anticorrelation between perceptual and internal systems may reflect a necessary condition for the brain to focus in a detailed manner on one stream to the exclusion of the other.2,181,182 The FPCN (as well as the salience network; Box 3) may in turn, mediate internally and externally directed cognition by maintaining a dynamic balance between the default and attention networks.15,155,160,162,163
Box 3. The salience network: toggling between external perception and self-generated thoughts.
The salience network encompasses the dorsal anterior cingulate cortex (dACC), the anterior insulae (aINS), the supramarginal gyrus extending ventrally into the superior temporal sulcus, and the posterior dorsal cingulate sulcus.51 Evidence suggests that the salience network is involved in the detection of behaviorally significant stimuli in the external environment243 and plays a key role in dynamically switching between external and internal modes of attention. The right aINS has previously been identified as a critical node for suppressing DN activity and reallocating attentional resources to salient events.244,245 In a compelling study of traumatic brain injury, the structural integrity of the white matter tracts linking the dACC and aINS predicted the degree of suppression of the DN during a stop-signal task, a measure of inhibitory control.246 To date, the evidence in favor of the salience network’s role in dynamic switching and re-orienting of attention comes from salient external tasks that lead to the suppression of the DN. It is therefore an open question whether this system can enhance activity in the DN, potentially in response to a salient internal thought. However, there is little positive RSFC between the default and salience networks, and key regions of the salience network are more aligned with the DAN (Fig. 5b–c). It is possible that the ventral PCC subserves this function for salient internal representations.
Disruption of the default network and self-generated thought
Thus far, we have synthesized evidence suggesting that the DN contributes to adaptive forms of self-generated cognition. A clear prediction from these findings is that anatomical or functional disruptions of the network will have severe consequences for normal psychological functioning. Indeed, DN alterations have been reported in numerous mental health disorders and neurological diseases, including depression, anxiety, schizophrenia, obsessive compulsive disorder, psychopathy, substance abuse, attention deficit hyperactivity disorder (ADHD), autism, Tourette’s syndrome, Alzheimer’s disease, semantic dementia, and chronic pain (among others).7,43,183,184 Based on the conceptual framework outlined above, a closer look into these patient populations reveals that both the nature and topographical locations of DN alterations often differ across disorders, paralleling varied symptom profiles. While disorders of integrity (e.g.., Alzheimer’s disease) are often associated with hypo-activation or connectivity of a particular DN component and impairments in specific aspects of self-generated cognition, disorders of content (e.g., depression) and regulation (e.g., ADHD) are typically associated with hyperactivation and hyperconnectivity, paralleled by polarized or excessive forms of self-generated thought. Below, we propose three different mechanisms that explain how psychopathological states can be linked to variations in the function of the DN. However, it is important to note that many studies do not assess whether group differences in motion, respiration, and other confounds can explain observed differences in activity or connectivity.185 Further consideration of these possible confounds will be necessary moving forward.
The integrity hypothesis of default network function
If the network’s adaptive value arises because it supports self-generated goal states, diseases targeting the integrity of this system—leading to hypoactivation and hypoconnectivity—should have catastrophic consequences on self-generated cognition. We refer to this idea as the integrity hypothesis of default network function.
In healthy older adults, the integrity of the DN is diminished both in function186,187 and structure,188 and observed declines are associated with impairments in memory function. Further, social cognitive deficits in aging have been associated with reductions in activity within the dmPFC.189 More dramatic changes emerge in the context of pathological aging. Alzheimer’s disease (AD) and forms of frontotemporal lobar degeneration (FTLD), including semantic dementia (SD) and behavioral variant frontotemporal dementia (bv-FTD), are neurodegenerative disorders that target relatively distinct regions within and outside the DN.190–193 The pathology of AD primarily affects the PCC and the medial temporal subsystem.191,192,194 Consistent with their hypothesized functions, atrophy of these regions predicts severe impairments in episodic/autobiographical memory and episodic future thought.193,195,196,197 However, as anatomical and functional disruption extends more broadly with disease progression, individuals with AD exhibit noticeable impairments in self-reflective, social, and executive aspects of self-generated thought.191,198–200,199
In contrast to AD, FTLD more specifically affects the dorsal medial subsystem, the amPFC, and the salience network, with degeneration extending from dorsal to ventral prefrontal regions and into the lateral temporal cortex.43,192 Consistent with this degeneration, symptoms include alterations in personality and impairments in social functioning, self-reflection, emotional processing, and autobiographical memory/future thought.43,195–197,201
Finally, SD is associated with degeneration of the lateral temporal lobes extending into MTL structures.43,192 SD typically presents with impairments in semantic knowledge, language, emotion processing, autobiographical memory, and construction of future scenarios—possibly due to the insidious deterioration of conceptual knowledge.43,202–204 Deficits in episodic future thinking in SD also correlate with the degree of atrophy in temporal structures.196
In summary, disorders that affect the integrity of the default network’s structural or functional neuroanatomy (e.g., aging, neurodegenerative disease, focal lesions,105 or disorders of consciousness205) will produce marked impairments in the quality of self-generated thought and may compromise the production mechanism altogether.
The content regulation hypothesis of adaptive DN function
Although self-generated thought and DN activity has an adaptive potential, it does not necessarily follow that all attempts at self-generated thought improve cognitive functioning or psychological well-being. Polarized or excessive self-generated thoughts may signify serious mental health problems, delusions, and chronic distraction, and these symptoms may be associated with heightened patterns of DN activity or connectivity. Below, we propose two additional mechanisms to explain why self-generated thoughts might be beneficial for some yet harmful for others (see also Ref. 10).
Our first hypothesis proposes that harnessing the beneficial aspects of self-generated thought and associated DN activity requires the ability to adaptively regulate the content underlying this internal experience. Impairments in content regulation often manifest as polarized forms of internal thinking (i.e., most thoughts pertain to similar content), with difficulty flexibly shifting between different types of self-generated thought. While disorders of content are often linked to broad negative consequences for cognitive functioning and well-being, the precise nature of the consequences are likely to vary as a function of the content itself.42,206 For example, excessive focus on negatively valenced or past-related thoughts may be a signature of depression,207,208 while excessive focus on overly confident, positive, and grandiose thoughts may be indicative of disorders involving manic states.209 Forms of thinking characterized as being too focal or specific could signify autism spectrum disorders,210 while rumination has been linked to styles of thinking characterized as too general, often with an elevated self-focus.211,212 Self-generated thoughts characterized by an exaggerated likelihood and severity of personal harm are associated with many anxiety disorders, including phobias and obsessive–compulsive disorder (OCD).213,214 Interestingly, recent findings suggest that thought content relates to psychological well-being even in non-clinical samples. For example, individuals who report a predominance of negative and personally significant thoughts score higher on depressive questionnaires, while those who characterize their thoughts as overly general/abstract also tend to be higher in trait rumination.12
Given the role of distinct DN components in different aspects of self-generated thought, the polarized content apparent in mental health disorders could manifest as tradeoffs between hyper- and hypoactivity or connectivity among DN subsystems, or with other large-scale brain systems contributing to self-generated thought. For example, mood disorders including major depressive disorder and bipolar disorder are associated with enhanced activity and connectivity of DN structures with key regions of the limbic and/or salience networks.147,148,215–219 This pattern may reflect the fact that depressed individuals tend to exhibit a perseverative focus on unachievable or failed goal states, which could maintain, if not exacerbate, states of negative affect—a process that has been termed depressive interlock.220 Individuals with OCD exhibit enhanced connectivity between the mPFC and the ventral striatum,221 while individuals with chronic pain exhibit enhanced connectivity between the mPFC and the insula, a region important for the perception of pain.222 Finally, alterations in self-referential thought and social cognition in schizophrenia manifest as increased PCC activation during social reflection and reduced vmPFC activation.223
A major theme of psychological interventions such as cognitive behavior therapy involves altering the content of self-generated thoughts through a process known as cognitive restructuring.224 Similarly, mindfulness and/or acceptance interventions that seek to alter an individual’s relationship with his/her internal thoughts through the practice of decentering or defusion may have adaptive downstream effects on the regulation of thought content.225–227 The neurocognitive effects of these therapeutic interventions with respect to the DN mark an important avenue for future research.
The context regulation hypothesis of adaptive default network function
A focus on self-generated thoughts is most likely appropriate when the external environment is relatively non-demanding; in these contexts the perceptual neglect that accompanies self-generated thought is less likely to undermine the integrity of external goals. A final form of psychopathology could, therefore, be a failure to regulate self-generated thought to a context when it does not interfere with ongoing tasks. We refer to this idea as the context regulation hypothesis of adaptive DN function. Whereas the content regulation hypothesis refers to which topics self-generated thoughts typically concern, the context regulation hypothesis refers to when such thoughts occur. Many disorders are characterized by dysfunctional regulation of both content and context, and alterations in both processes are likely to yield devastating consequences on cognitive functioning and well-being.
Many disorders including ADHD, schizophrenia, depression, rumination, and obsessive–compulsive disorder (OCD) have problems regulating the occurrence of self-generated thoughts. These impairments often manifest as increased distractibility or elevated levels of mind-wandering,212,228–231 as well as hyperactivity of the DN and weaker anticorrelations with networks involved in external attention.146–148,229,232–235 By contrast, individuals with improved executive control are able to limit their self-generated thought to non-demanding or unimportant contexts.10,176,178 In addition to these manifestations, individuals with ADHD also exhibit elevated response-time variability,236 supporting theories that the DN interferes with maintenance of external task goals by periodically disrupting on-task attention.146,232 Depressed individuals, particularly those who ruminate, have “sticky thoughts” and problems with updating the contents of working memory and task switching such that prior goal states exert a stronger influence on on-going mental processes than normal.237,238 Depressed individuals who ruminate also exhibit greater dominance of the DN compared to the DAN during rest.239 Finally, disruptions in the functional integrity of frontoparietal control networks240 may result in a hyperactive DN147,148,235 and may be an important source of blurred boundaries between internal thoughts and the external world.241 Together these studies suggest that for many psychopathological conditions, an inability to control the occurrence of excessive or distracting self-generated thoughts in a context-dependent manner may lead to impairments in ongoing tasks, and so provides basic support for the context regulation hypothesis.
Summary
The last decade has seen an increase in our understanding of both the DN and its capacity to engage in thoughts that do not arise directly from perception. These complex mental processes are a central aspect of normal neurocognitive functioning, and are supported, in part, by specific subsystems within the DN and dynamic interactions with other large-scale systems involved in cognitive control and attention. These observations explain why the network is both an essential part of normal human functioning and can be a critical element of poor psychological well-being. Although some states of psychopathology result from compromised integrity of the DN, others reflect a failure to regulate DN activity and self-generated thoughts to contexts when its psychological functions are appropriate, or to content that is adaptive in nature. Despite this progress, several important questions remain for future research (Box 4).
Box 4. Outstanding questions on the role of the default network in self-generated experiences.
In this review, we have focused on the idea that the DN plays an important role in many aspects of self-generated thought. Despite support for this general principle, several key questions remain (see main text for additional questions):
Is self-generated thought restricted to the DN? As reviewed in the main text, the rich variety of self-generated experiences reflects one of the more complex aspects of human cognition. Given its intricate nature, the full extent of self-generated thought is unlikely to be attributable to a single network of regions, and the brain regions involved in self-generated thought may critically depend upon both the content underlying the experience (see also Ref. 247) and the precise process by which the experience occurs.2 The DN exhibits complex temporal interactions with other neural systems, including the anticorrelation between the DN and the dorsal attention network, as well as its cooperation with the FPCN in ensuring integrity in a self-generated train of thought. Furthermore, coordinated neural process that occur during periods of unconstrained rest are not limited to the DN, but are present in almost all of the networks activated by a task. Together, these different lines of evidence converge on the notion that the DN’s coordination and competition with many other large-scale networks may be important in dictating the variety of human self-generated experiences that we experience in daily life.
What are the specific computational processes subserved by the DN? The DN is a large-scale distributed network of brain regions. Spatial distribution is a common and defining feature of neural networks and affords computational complexity because complex cognitive states emerge through the interaction of different lower-level component processes. One important question, therefore, is which level can describe the computations performed by different regions within the DN. It seems unlikely that spatially distinct regions (such as the PCC or mPFC) support computations that are redundant with one another; rather, these different regions likely serve complementary functions that, in combination, allow more complex phenomena (such as autobiographical thought) to emerge. However, to fully describe the functions of the network, it will be necessary to devise experiments that target lower-level processes and use neuroimaging techniques that allow faster temporal resolution, finer spatial resolution, and extraction of specific patterns of activity within subsystems.
Acknowledgments
The authors wish to thank several people for providing helpful discussion or feedback on prior versions of the manuscript: Randy Buckner, Daniel Margulies, Gary Turner, Marina Lopez-Sola, Roselinde Kaiser, Mathieu Roy, Yoni Ashar, Muireann Irish, Scott Barry Kaufman, Andrew Reineberg, and Leonie Koban. Additionally, we would like to thank Tal Yarkoni for providing NeuroSynth software tools. This work was supported by the National Institutes of Health: F32MH093985 (J.A.) and the Office of Education (J.S.).
Footnotes
References
- 1.Schooler JW, et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci. 2011;15:319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Smallwood J. Distinguishing how from why the mind wanders: A process–occurrence framework for self-generated mental activity. Psychol Bull. 2013;139:519–535. doi: 10.1037/a0030010. [DOI] [PubMed] [Google Scholar]
- 3.Baird B, et al. Inspired by distraction: Mind wandering facilitates creative incubation. Psychol Sci. 2012;23:1117–1122. doi: 10.1177/0956797612446024. [DOI] [PubMed] [Google Scholar]
- 4.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 5.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 6.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 8.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 9.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smallwood J, Andrews-Hanna J. Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Front Psychol. 2013;4:1–6. doi: 10.3389/fpsyg.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mooneyham BW, Schooler JW. The costs and benefits of mind-wandering: A review. Can J Exp Psychol. 2013;67:11–18. doi: 10.1037/a0031569. [DOI] [PubMed] [Google Scholar]
- 12.Andrews-Hanna JR, et al. A penny for your thoughts: dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Frontiers in Psychology. 2013;4:1–13. doi: 10.3389/fpsyg.2013.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMillan RL, Kaufman SB, Singer JL. Ode to positive constructive daydreaming. Frontiers in psychology. 2013;4:626. doi: 10.3389/fpsyg.2013.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreng RN. The fallacy of a ‘task-negative’ network. Front Cogn. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callard F, Smallwood J, Margulies DS. Default Positions: How Neuroscience’s Historical Legacy has Hampered Investigation of the Resting Mind. Front Psychol. 2012;3:321. doi: 10.3389/fpsyg.2012.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. 1996;7:2095–2099. [PubMed] [Google Scholar]
- 18.Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PloS One. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreasen N, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 22.Andrews-Hanna JR, Reidler JS, Huang C, Randy L, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delamillieure P, et al. The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res Bull. 2010;81:565–573. doi: 10.1016/j.brainresbull.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Diaz BA, et al. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Front Hum Neurosci. 2013;7:1–15. doi: 10.3389/fnhum.2013.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinger E, Cox W. Dimensions of thought flow in everyday life. Imagin Cogn Pers. 1987;7:105–128. [Google Scholar]
- 26.Kane MJ, et al. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 27.Song X, Wang X. Mind wandering in Chinese daily lives–an experience sampling study. PloS One. 2012;7:e44423. doi: 10.1371/journal.pone.0044423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox KCR, Nijeboer S, Solomonova E, Domhoff GW, Christoff K. Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Front Hum Neurosci. 2013;7:1–18. doi: 10.3389/fnhum.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smallwood J, O’Connor RC. Imprisoned by the past: unhappy moods lead to a retrospective bias to mind wandering. Cogn Emot. 2011;25:1481–1490. doi: 10.1080/02699931.2010.545263. [DOI] [PubMed] [Google Scholar]
- 30.Smallwood J, et al. Self-reflection and the temporal focus of the wandering mind. Conscious Cogn. 2011;4:1120–1126. doi: 10.1016/j.concog.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Ruby F, Smallwood J, Engen, Singer T. How self-generated thought shapes mood. PLoS ONE. doi: 10.1371/journal.pone.0077554. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stawarczyk D, Cassol H, D’Argembeau A. Phenomenology of future-oriented mind-wandering episodes. Front Psychol. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baird B, Smallwood J, Schooler JW. Back to the future: Autobiographical planning and the functionality of mind-wandering. Conscious Cogn. 2011;20:1604–1611. doi: 10.1016/j.concog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Prebble SC, Addis DR, Tippett LJ. Autobiographical memory and sense of self. Psychological bulletin. 2013;139:815–40. doi: 10.1037/a0030146. [DOI] [PubMed] [Google Scholar]
- 35.Ruby FJ, Smallwood J, Sackur J, Singer T. Is Self-Generated Thought a means of Social Problem Solving? Front Psychol. 2013;4:962. doi: 10.3389/fpsyg.2013.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Immordino-Yang MH, Christodoulou Ja, Singh V. Rest Is not idleness: implications of the brain’s default mode for human development and education. Perspectives on Psychological Science. 2012;7:352–364. doi: 10.1177/1745691612447308. [DOI] [PubMed] [Google Scholar]
- 37.Mar RA, Mason MF, Litvack A. How daydreaming relates to life satisfaction, loneliness, and social support: the importance of gender and daydream content. Conscious Cogn. 2012;21:401–407. doi: 10.1016/j.concog.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Klinger E. In: Stimul Handb Imagin Ment. Markman KD, Klein WMP, Suhr JA, editors. Psychology Press: Taylor & Francis Group; 2009. pp. 225–239. [Google Scholar]
- 39.Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychol. 2011;136:370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Smallwood J, Nind L, O’Connor RC. When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Conscious Cogn. 2009;18:118–125. doi: 10.1016/j.concog.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: negative moods lead the mind to wander. Emot. 2009;9:271–276. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- 42.Harvey A, Watkins E, Mansell W, Shafran R. Cognitive Behavioural Processes across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford University Press; 2004. [Google Scholar]
- 43.Irish M, Piguet O, Hodges JR. Self-projection and the default network in frontotemporal dementia. Nat Rev Neurol. 2011;8:152–161. doi: 10.1038/nrneurol.2012.11. [DOI] [PubMed] [Google Scholar]
- 44.Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uddin LQ, Kelly aM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human brain mapping. 2009;30:625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H. A dual-subsystem model of the brain’s default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage. 2012;61:966–77. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Seghier ML, Price CJ. Functional Heterogeneity within the Default Network during Semantic Processing and Speech Production. Frontiers in psychology. 2012;3:281. doi: 10.3389/fpsyg.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salomon R, Levy DR, Malach R. Deconstructing the default: Cortical subdivision of the default mode/intrinsic system during self-related processing. Human brain mapping. 2013 doi: 10.1002/hbm.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bzdok D, et al. Segregation of the human medial prefrontal cortex in social cognition. Frontiers in human neuroscience. 2013;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1265. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doucet G, et al. Brain activity at rest: a multiscale hierarchical functional organization Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- 55.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Schmahmann JD, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 57.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 59.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H, et al. Connectivity-Based Parcellation of the Human Frontal Pole with Diffusion Tensor Imaging. J Neurosci. 2013;33:6782–6790. doi: 10.1523/JNEUROSCI.4882-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parvizi J, Hoesen GWV, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uddin LQ, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makris N, et al. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19:777–785. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Yarkoni T, Poldrack RA, Nichols TE, Essen DCV, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binder JR, Desai RH, Graves WW, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 71.Brewer JA, Garrison KA, Whitfield-gabrieli S. What about the “ self ” is processed in the posterior cingulate cortex? Frontiers in Human Neuroscience. 2013;7:1–7. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baars BJ, Ramsøy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain J Neurol. doi: 10.1093/brain/awt162. in press. [DOI] [PMC free article] [PubMed]
- 74.Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seghier Mohamed L. The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist. 2012;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- 77.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 78.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature reviews Neuroscience. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 79.Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ungerleider LG, Haxby JV. “What” and “where” in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 81.Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci U S A. 2000;97:11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moran JM, Kelley WM, Heatherton TF. What can the organization of the brain’s default mode network tell us about self-knowledge? Front Hum Neurosci. 2013;7:391. doi: 10.3389/fnhum.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Argembeau A, et al. Valuing one’s self: Medial prefrontal involvement in epistemic and emotive investments in self-views. Cereb Cortex. 2011;22:659–667. doi: 10.1093/cercor/bhr144. [DOI] [PubMed] [Google Scholar]
- 87.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50:1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- 89.Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray RJ, Schaer M, Debbané M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 91.Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 92.Ochsner KN, et al. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atlas LY, Bolger N, Lindquist Ma, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wager TD, et al. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Schacter DL, et al. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 99.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47:2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 101.Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- 103.Ploner CJ, et al. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex. 2000;10:1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi N, Kawamura M. Pure topographical disorientation–the anatomical basis of landmark agnosia. Cortex. 2002;38:717–725. doi: 10.1016/s0010-9452(08)70039-x. [DOI] [PubMed] [Google Scholar]
- 105.Hayes SM, Salat DH, Verfaellie M. Default network connectivity in medial temporal lobe amnesia. J Neurosci. 2012;32:14622–14629. doi: 10.1523/JNEUROSCI.0700-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain J Neurol. 1999;122:1613–28. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 107.Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal Lobe and Episodic Memory: Bilateral Damage Causes Impaired Free Recall of Autobiographical Memory. J Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davidson PSR, et al. Does lateral parietal cortex support episodic memory? Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim H. Differential neural activity in the recognition of old versus new events: An Activation Likelihood Estimation Meta-Analysis. Human Brain Mapping. 2011;34:814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology. 2013;23:255–60. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 112.Kumaran D, Summerfield JJ, Hassabis D, Maguire Ea. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 115.Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 117.Overwalle FV. Social cognition and the brain: A meta-analysis. Hum Brain Mapp. 2009;858:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the ‘default system’ of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Schilbach L, et al. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PloS one. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 121.Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 122.Ochsner KN, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–17472. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 123.Van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 124.D’Argembeau A, et al. Brains creating stories of selves: the neural basis of autobiographical reasoning. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nst028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and Unique Neural Correlates of Autobiographical Memory and Theory of Mind. J Cogn Neurosci. 2009;22:1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- 126.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 127.Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. doi: 10.1016/j.neuroimage.2014.01.032. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hassabis D, et al. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb Cortex. doi: 10.1093/cercor/bht042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saxe R. Handb Theory Mind. Psychology Press: Taylor & Francis Group; 2010. [Google Scholar]
- 130.Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54:1589–15899. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 131.Baetens K, Ma N, Steen J, Van Overwalle F. Involvement of the mentalizing network in social and non-social high construal. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nst048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dunbar RIM. The social brain hypothesis. Evol Anthr. 1998;6:178–190. [Google Scholar]
- 133.Rabin JS, Rosenbaum RS. Familiarity modulates the functional relationship between theory of mind and autobiographical memory. NeuroImage. 2012;62:520–529. doi: 10.1016/j.neuroimage.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szpunar KK, St Jacques PL, Robbins Ca, Wig GS, Schacter DL. Repetition-related reductions in neural activity reveal component processes of mental simulation. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nst035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perry D, Hendler T, Shamay-Tsoory SG. Projecting memories: The role of the hippocampus in emotional mentalizing. Neuroimage. 2011;54:1669–1676. doi: 10.1016/j.neuroimage.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 136.Spreng RN, Mar RA. I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res. 2012;1428:43–50. doi: 10.1016/j.brainres.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dunbar RIM. Human conversational behavior. Hum Nat. 1997;8:231–246. doi: 10.1007/BF02912493. [DOI] [PubMed] [Google Scholar]
- 138.Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Keller CJ, et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD Signal. J Neurosci. 2013;33:6333–6342. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–53. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 142.Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao W, et al. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 2012;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Sonuga-barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Brain. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 148.Anticevic A, et al. The Role of Default Network Deactivation in Cognition and Disease. Trends in Cognitive Sciences. 2012:1–9. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kam JWY, et al. Slow fluctuations in attentional control of sensory cortex. J Cogn Neurosci. 2011;23:460–470. doi: 10.1162/jocn.2010.21443. [DOI] [PubMed] [Google Scholar]
- 150.Kam JWY, Xu J, Handy TC. I don’t feel your pain (as much): The desensitizing effect of mind wandering on the perception of others’ discomfort. Cogn Affect Behav Neurosci. doi: 10.3758/s13415-013-0197-z. in press. [DOI] [PubMed] [Google Scholar]
- 151.Macdonald JSP, Mathan S, Yeung N. Trial-by-trial variations in subjective attentional state are reflected in ongoing prestimulus EEG alpha oscillations. Front Percept Sci. 2011;2:82. doi: 10.3389/fpsyg.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: mind wandering reduces cortical analysis of external events. J Cogn Neurosci. 2008;20:458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- 153.Barron E, Riby LM, Greer J, Smallwood J. Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol Sci. 2011;22:596–601. doi: 10.1177/0956797611404083. [DOI] [PubMed] [Google Scholar]
- 154.Lenartowicz A, Simpson GV, Cohen MS. Perspective: causes and functional significance of temporal variations in attention control. Front Hum Neurosci. 2013;7:381. doi: 10.3389/fnhum.2013.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 156.Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Banich MT. Executive function: the search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. [Google Scholar]
- 158.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cole MW, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsu001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meyer ML, Spunt RP, Berkman ET, Taylor SE, Lieberman MD. Evidence for social working memory from a parametric functional MRI study. Proc Natl Acad Sci U S A. 2012;109:1883–1888. doi: 10.1073/pnas.1121077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meyer ML, Lieberman MD. Social working memory: neurocognitive networks and directions for future research. Front Psychol. 2012;3:571. doi: 10.3389/fpsyg.2012.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. NeuroImage. 2011;59:1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 169.Hare T, Camerer A, Rangel CF. A Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 170.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–48. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 171.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Buhle JT, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. doi: 10.1093/cercor/bht154. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 174.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Teasdale JD, et al. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 176.Levinson DB, Smallwood J, Davidson RJ. The persistence of thought: Evidence for a role of working memory in the maintenance of task-unrelated thinking. Psychol Sci. 2012;23:375–380. doi: 10.1177/0956797611431465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smallwood J, Ruby FJM, Singer T. Letting go of the present: Mind-wandering is associated with reduced delay discounting. Conscious Cogn. 2013;22:1–7. doi: 10.1016/j.concog.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 178.Bernhardt B, et al. Cortical thickness in medial prefrontal cortex and cingulate cortices supports self-generated thickness and reduced delay discounting. (under review) [Google Scholar]
- 179.McVay JC, Kane MJ. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. J Exp Psychol Learn Mem Cogn. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McVay J, Unsworth N, McMillan BD, Kane MJ. Working memory capacity does not always support future-oriented mind-wandering. Can J Exp Psychol Rev Can Psychol Expérimentale. 2013;67:41–50. doi: 10.1037/a0031252. [DOI] [PubMed] [Google Scholar]
- 181.Franklin MS, et al. The silver lining of a mind in the clouds: interesting musings are associated with positive mood while mind-wandering. Front Percept Sci. 2013;4:583. doi: 10.3389/fpsyg.2013.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50(2):329–39. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 183.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 184.Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues in Clinical Neuroscience. 2013;15:351–358. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature neuroscience. 2013;16:832–7. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 186.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Damoiseaux JS, et al. Reduced resting-state brain activity in the ‘default network’ in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 188.Spreng RN, Turner GR. Structural covariance of the default network in healthy and pathological aging. J Neurosci. 2013;33:15226–15234. doi: 10.1523/JNEUROSCI.2261-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. J Neurosci. 2012;32:5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosen HJ, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 191.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci Off J Soc Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Irish M, et al. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia. 2011;49:2694–702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 194.Zhou J, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain J Neurol. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shany-Ur T, Rankin KP. Personality and social cognition in neurodegenerative disease. Curr Opin Neurol. 2011;24:550–555. doi: 10.1097/WCO.0b013e32834cd42a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain: a journal of neurology. 2012;135:2178–91. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- 197.Irish M, Hodges JR, Piguet O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex. 2013;49:2377–88. doi: 10.1016/j.cortex.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 198.Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moreau N, Viallet F, Champagne-Lavau M. Using memories to understand others: The role of episodic memory in theory of mind impairment in Alzheimer disease. Ageing Res Rev. 2013;12:833–839. doi: 10.1016/j.arr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 200.Mega M, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 201.Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 202.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 203.Maguire Ea, Kumaran D, Hassabis D, Kopelman MD. Autobiographical memory in semantic dementia: a longitudinal fMRI study. Neuropsychologia. 2010;48:123–36. doi: 10.1016/j.neuropsychologia.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McKinnon MC, Black SE, Miller B, Moscovitch M, Levine B. Autobiographical memory in semantic dementia: implication for theories of limbic-neocortical interaction in remote memory. Neuropsychologia. 2006;44:2421–9. doi: 10.1016/j.neuropsychologia.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vanhaudenhuyse A, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing; 2013. [Google Scholar]
- 207.Pyszczynski T, Greenberg J. Self-regulatory perseveration and the depressive self-focusing style: A self-awareness theory of reactive depression. Psychol Bull. 1987;102:122–138. [PubMed] [Google Scholar]
- 208.Larsen RJ, Cowan GS. Internal focus of attention and depression: A study of daily experience. Motiv Emot. 1988;12:237–249. [Google Scholar]
- 209.Gruber J, Johnson SL, Oveis C, Keltner D. Risk for mania and positive emotional responding: Too much of a good thing? Emotion. 2008;8:23–33. doi: 10.1037/1528-3542.8.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 211.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspect Psychol Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 212.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Barlow DH. Anxiety and Its disorders: the nature and treatment of anxiety and panic. The Guilford Press; 2004. [Google Scholar]
- 214.Abramowitz JS. The practice of exposure therapy: relevance of cognitive-behavioral theory and extinction theory. Behavior therapy. 2013;44:548–58. doi: 10.1016/j.beth.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 215.Greicius MD, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological medicine. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- 219.Anticevic A, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biological psychiatry. 2013;73:565–73. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barnard PJ, Teasdale JD. Interacting cognitive subsystems: A systemic approach to cognitive-affective interaction and change. Cogn Emot. 1991;5:1–39. [Google Scholar]
- 221.Harrison BJ, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of general psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 222.Napadow V, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Holt DJ, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Beck JS. Cognitive behavior therapy, second edition: Basics and Beyond. The Guilford Press; 2011. [Google Scholar]
- 225.Frewen Pa, Evans EM, Maraj N, Dozois DJa, Partridge K, Letting Go. Mindfulness and Negative Automatic Thinking. Cognitive Therapy and Research. 2008;32:758–774. [Google Scholar]
- 226.Arch JJ, Craske MG, Angeles L. Acceptance and Commitment Therapy and Cognitive Behavioral Therapy for Anxiety Disorders: Different Treatments, Similar Mechanisms? 2008:263–279. [Google Scholar]
- 227.Crane C, Winder R, Hargus E, Amarasinghe M, Barnhofer T. Effects of Mindfulness-Based Cognitive Therapy on Specificity of Life Goals. Cognitive therapy and research. 2012;36:182–189. doi: 10.1007/s10608-010-9349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 229.Marchetti I, Koster EHW, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- 230.Shaw GA, Giambra LM. Task-unrelated thoughts of college- students diagnosed as hyperactive in childhood. Dev Neuropsychol. 1993;9:17–30. [Google Scholar]
- 231.Smallwood J, O’Connor RC, Sudbery MV, Obonsawin M. Mind-wandering and dysphoria. Cogn Emot. 2007;21:816–842. [Google Scholar]
- 232.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2011;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fassbender C, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liddle EB, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Castellanos FX, et al. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Joormann J, Levens SM, Gotlib IH. Sticky thoughts: depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychol Sci. 2011;22:979–983. doi: 10.1177/0956797611415539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Joormann J. Cognitive inhibition and emotion regulation in depression. Curr Dir Psychol Sci. 2010;19:161–166. [Google Scholar]
- 239.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Baker JT, et al. Disruption of Cortical Association Networks in Schizophrenia and Psychotic Bipolar Disorder. JAMA Psychiatry. 2013:1–10. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 242.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bonnelle V, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Preminger S, Harmelech T, Malach R. Stimulus-free thoughts induce differential activation in the human default network. NeuroImage. 2011;54:1692–702. doi: 10.1016/j.neuroimage.2010.08.036. [DOI] [PubMed] [Google Scholar]