Abstract
Emerging evidence suggests that neuronal responses to N-methyl-d-aspartate (NMDAR) activation/inactivation are influenced by subunit composition. For example, activation of synaptic NMDAR (comprised of GluN2A > GluN2B) phosphorylates cAMP-response-element-binding protein (CREB) at Ser 133, induces BDNF expression and promotes neuronal survival. Activation of extrasynaptic NMDAR (comprised of GluN2B>GluN2), dephosphorylates CREB (Ser 133), reduces BDNF expression and triggers neuronal death. These results led us to hypothesize that chronic inhibition of GluN2B-containing NMDAR would increase CREB (Ser 133) phosphorylation, increase BDNF levels and subsequently alter downstream dynorphin (DYN) and neuropeptide Y (NPY) expression. We focused on DYN and NPY because these neuropeptides can decrease excitatory neurotransmission and seizure occurrence and we reported previously that seizure-like events are reduced following chronic treatment with GluN2B antagonists. Consistent with our hypothesis, chronic treatment (17-21 days) of hippocampal slice cultures with the GluN2B-selective antagonists ifenprodil or Ro25,6981 increased both CREB (Ser 133) phosphorylation and granule cell mossy fiber pathway DYN expression. Similar treatment with the non-subtype-selective NMDAR antagonists D-APV or memantine had no significant effect on either CREB (Ser 133) phosphorylation or DYN expression. In contrast to our hypothesis, BDNF levels were decreased following chronic treatment with Ro25,6981, but not ifenprodil, D-APV or memantine. Blockade of BDNF actions and TrkB activation did not significantly augment hilar DYN expression in vehicle-treated cultures and had no effect in Ro25,6981 treated cultures. These finding suggest that chronic exposure to GluN2B-selective NMDAR antagonists increased DYN expression through a putatively pCREB-dependent, but BDNF/TrkB-independent mechanism.
Keywords: hippocampus; ifenprodil; memantine; neuropeptide Y; N-Methyl-D-Aspartate; Ro25,6981
Introduction1
N-methyl-D-aspartate receptors (NMDAR) are heteromeric ionotropic glutamate receptors composed of obligatory GluN1 and variable, modulatory GluN2A-D subunits. GluN2A and GluN2B are the primary GluN2 subunits in hippocampus and cortex (Monyer et al., 1994; Yamakura and Shimoji, 1999) and differ in their subcellular localization, biophysical properties, signaling pathway coupling, and brain function contributions (Vicini et al., 1998; Barria and Malinow, 2005; Flint et al., 1997; Lavezzari et al., 2004; Monyer et al., 1994). Data emerging over the past decade suggest that neuronal responses to NMDAR activation/inactivation are influenced by receptor subunit composition and subcellular localization (Wyllie et al., 2013; Zhou and Sheng 2013). For example, activation of synaptic NMDAR (comprised of GluN2A > GluN2B) phosphorylates cAMP-response-element-binding protein (CREB) at Ser 133, induces BDNF expression and promotes neuronal survival. Conversely, activation of extrasynaptic NMDAR (comprised of GluN2B > GluN2A) dephosphorylates CREB (Ser 133), reduces BDNF expression and triggers neuronal death (Hardingham et al., 2002; Liu et al., 2004, Tovar and Westbrook 1999).
Based upon these previous findings we hypothesized that chronic inhibition with GluN2B antagonists would increase CREB (Ser 133) phosphorylation and increase BDNF expression. We further hypothesized that chronic inhibition with GluN2B antagonists would alter dynorphin A (DYN) and neuropeptide Y (NPY) expression because CREB (Ser 133) phosphorylation can directly increase expression of DYN and NPY (Higuchi et al., 1988; Cole et al., 1995; Carlezon et al., 1998; Chance et al., 2000) and indirectly regulate their expression through altered BDNF levels (Nawa et al., 1993; Nawa et al., 1993; Croll et al., 1994; Tao et al., 1998; Shieh et al., 1998). We focused on DYN and NPY because these neuropeptides can decrease excitatory neurotransmission and seizure occurrence (Baraban 2004; Bausch et. al., 1998; Chen et al., 1995; Chen and Huang 1998, Simonato and Romualdi 1996; Colmers et al., 1987; Colmers et al., 1988; Klapstein and Colmers 1997; Patrylo et al., 1999; Richichi et al., 2004; Wagner et al., 1993) and seizure-like events were decreased following chronic treatment with GluN2B antagonists (Wang and Bausch 2004).
Results
Chronic treatment with GluN2B-selective, but not non-subunit-selective NMDAR antagonists, increased CREB phosphorylation
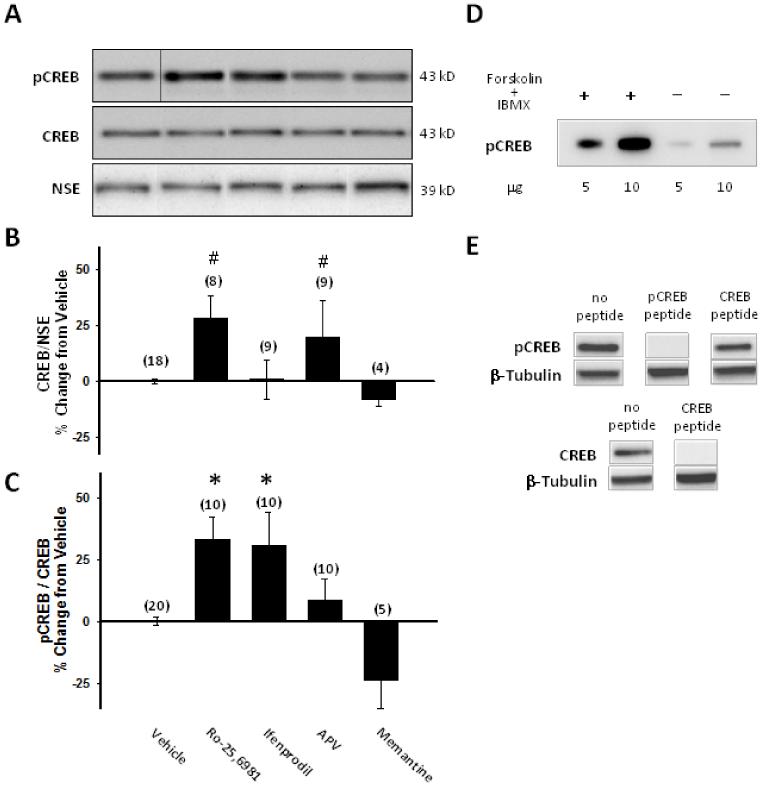
To test our hypothesis, we first performed Western blot analysis for CREB and CREB (Ser 133) phosphorylation on hippocampal slice culture homogenates. Chronic treatment with the GluN2B-selective NMDAR antagonist Ro25,6981, but not ifendprodil, as well as the non-subtype-selective NMDAR antagonist, APV increased CREB levels relative to NSE loading controls (Fig. 1A, B). However, chronic treatment with the GluN2B-selective NMDAR antagonists, Ro25,6981 and ifenprodil, but not the non-subunit-selective NMDAR antagonists, D-APV or memantine significantly increased CREB (Ser 133) phosphorylation relative to CREB levels (Fig. 1A,C). Homogenates prepared from vehicle-treated slice cultures displayed moderate levels of CREB (Ser 133) phosphorylation (Fig. 1A).
Fig. 1.
CREB (Ser 133) phosphorylation was increased following chronic treatment with GluN2B-selective, but not non-subunit-selective NMDAR antagonists. Western blots probed for CREB, pCREB (Ser133) and NSE (A) or β-tubulin (E) were generated using (A-C, E) homogenates prepared from hippocampal slice cultures treated with vehicle or the stated NMDAR antagonists for the entire 17-21 DIV culture period or (D) purchased extracts prepared from untreated SK-N-MC cells or SK-N-MC cells treated with forskolin and IBMX as described in the Materials and Methods. A) shows a representative Western blot for pCREB, CREB, and neuron specific enolase (NSE) (all obtained from the same blot). B) quantitative Western blot analysis showed increased CREB levels relative to NSE loading controls in organotypic hippocampal slice cultures treated chronically with the GluN2B-selective NMDAR antagonist Ro25,6981, but not ifendprodil, as well as the non-subtype-selective NMDAR antagonist, APV. C) quantitative Western blot analysis showed increased CREB (Ser133) phosphorylation relative to CREB levels in slice cultures treated with the GluN2B-selective NMDAR antagonists Ro25,6981 or ifenprodil, but not in cultures treated with the non-subtype-selective NMDAR antagonists APV or memantine. D) Specificity of the anti-pCREB antibody for pCREB is illustrated by increased pCREB following forskolin and IBMX treatment compared to untreated controls. E) The specificities of anti-pCREB and anti-CREB antibodies are shown by peptide block. β-tubulin was used as a loading control. Bars represent the mean ± SEM (normalized to vehicle mean/experiment). X-axis label in C is for A-C. Numbers in parentheses indicate the number of slice cultures. *, p<0.05, different than vehicle; #, p<0.001, different than vehicle, ifenprodil, APV; ANOVA with Holm-Sidak posthoc comparison.
Chronic treatment with GluN2B-selective, but not non-subunit-selective NMDAR antagonists, increased DYN, but not NPY expression
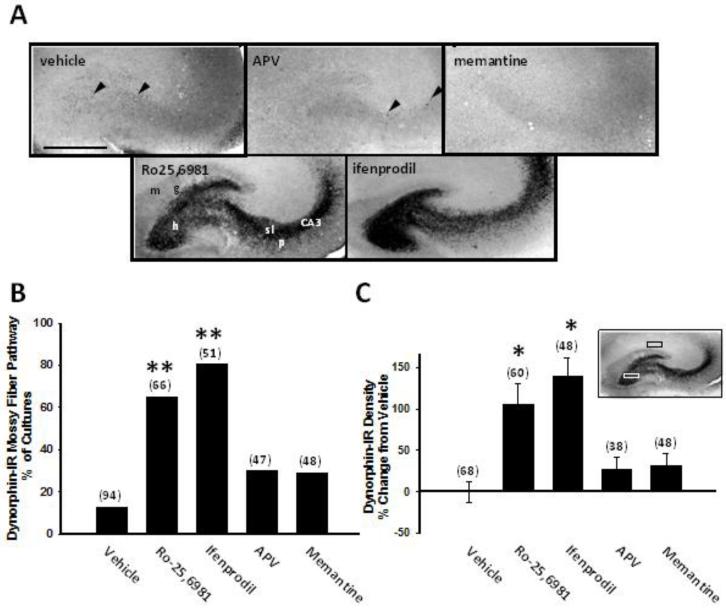
To examine the potential down-stream consequences of CREB (Ser 133) phosphorylation on neuropeptide expression, we first examined effects of NMDAR antagonist treatment on DYN peptide levels using immunohistochemistry. Vehicle-treated hippocampal slice cultures displayed DYN immunoreactivity in puncta localized sparsely in the hilus and more prominently in CA3 stratum lucidum (Fig. 2A, vehicle), consistent with a previous report in neonatal, prepubescent rats (Shirayama et al., 2005). Given the documented expression of DYN in granule cells and their mossy fiber axons (McGinty et al., 1994; Pierce et al., 1999), these puncta most likely represent DYN immunoreactivity in dentate granule cell mossy fiber terminals. Chronic treatment of cultures with the non-subunit-selective NMDAR antagonists, APV or memantine, did not significantly affect DYN immunoreactivity (Fig. 2A-C). In contrast, chronic treatment with the GluN2B-selective NMDAR antagonists, Ro25,6981 or ifenprodil, significantly increased both the percentage of cultures exhibiting DYN immunoreactivity throughout the mossy fiber pathway (Fig. 2A,B) and the density of hilar DYN immunoreactivity (Fig. 2A,C).
Fig. 2.
DYN expression in the dentate granule cell mossy fiber pathway was increased following chronic treatment with GluN2B-selective, but not non-subtype-selective NMDAR antagonists. Hippocampal slice cultures treated for the entire 17-21 day culture period with different classes of NMDAR antagonists were stained immunohistochemically with anti-DYN A antiserum using the ABC method and analyzed as described in the Materials and Methods. A) Representative hippocampal slice cultures as well as B) subjective analysis and C) quantitative densitometry revealed increased mossy fiber DYN immunoreactivity associated with the dentate granule cell mossy fiber pathway in organotypic hippocampal slice cultures treated with the GluN2B-selective NMDAR antagonists Ro25,6981 or ifenprodil, but not in cultures treated with the non-subtype-selective NMDAR antagonists APV or memantine. C inset) Hilar DYN immunoreactivity density (white box, 200 × 100 μm) was subtracted from background density in CA1 (black box, 200 × 100 μm). Abbreviations: g, dentate granule cell layer; h, hilus; m, molecular layer; sl, stratum lucidum; p, pyramidal cell layer. Arrowheads in A point to DYN-IR puncta. Bars indicate B, percentages or C, means ± SEM. The number of slice cultures is indicated in parentheses. Scale bar is for all panels in A, 500 μm. *, different than vehicle, p<0.05, ANOVA with Holm-Sidak post hoc comparison; **, different than vehicle, p<0.001, z-test.
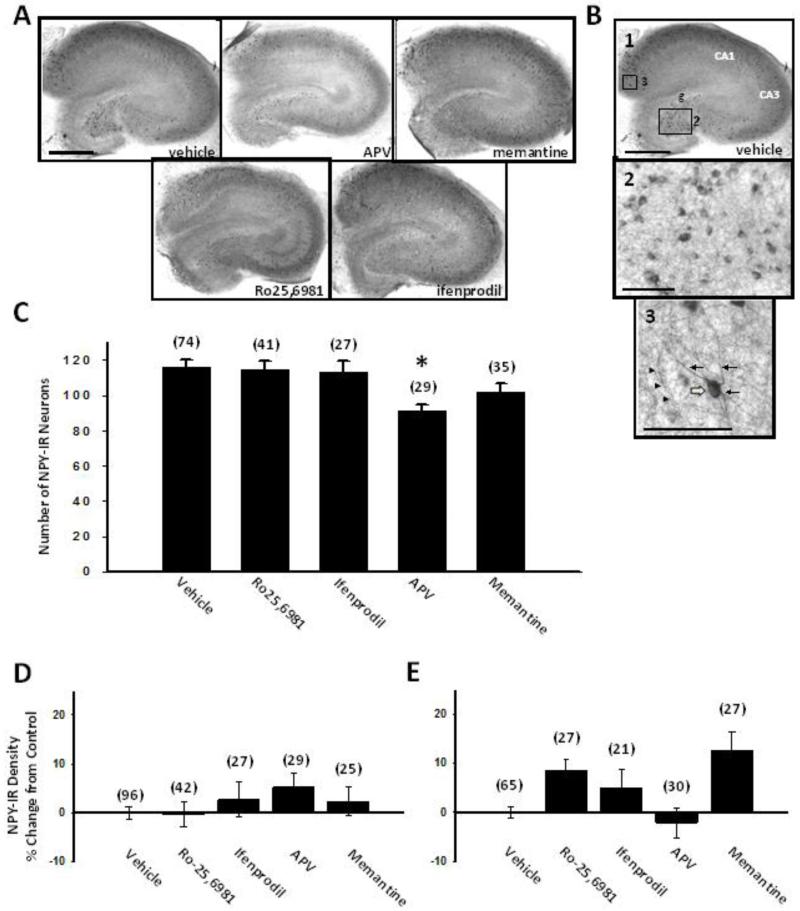
We next examined the effects of chronic NMDAR antagonist treatment on NPY expression using immunohistochemistry. Vehicle-treated hippocampal slice cultures showed NPY immunoreactivity throughout the slice culture (Fig. 3A, vehicle) in scattered, non-principal neuronal somata and dendrites (Fig. 3B1-3). NMDAR antagonists did not significantly affect the density of NPY immunoreactivity (Fig. 3D,E) or the number of hilar/granule cell layer NPY-immunoreactive neurons (Fig. 3C). The exception was D-APV, which significantly reduced the number of hilar/granule cell layer NPY-immunoreactive neurons (Fig. 3C). Reduced neuronal survival following chronic D-APV treatment (Wang and Bausch 2006) is likely to account for decreased numbers of hilar NPY-immunoreactive neurons.
Fig. 3.
NPY expression was not significantly affected by chronic treatment with NMDAR antagonists. Organotypic hippocampal slice cultures treated for the entire 17-21 day culture period with different classes of NMDAR antagonists were stained immunohistochemically with anti-NPY IgG using the ABC method and analyzed as described in the Materials and Methods. A) Representative low power images of hippocampal slice cultures showed NPY immunoreactivity in scattered neurons and neuropil throughout the slice culture. B) Representative higher power images revealed NPY immunoreactivity in neuronal somata (open arrow), dendrites (arrows) and thin neuronal processes (arrowheads). C) Quantitative neuron counts in the granule cell layer and hilus showed that chronic treatment with D-APV decreased the number of NPY-IR neurons. Quantitative densitometry D) in the hilus and E) in the entire hippocampal slice culture illustrated no effect of chronic NMDAR antagonist treatment on NPY immunoreactivity. Abbreviations: g, dentate granule cell layer. Bars indicate means ± SEM. The number of slice cultures is indicated in parentheses. Scale bar in A applies to all panels in A, 500 μm; in B1, 500 μm; in B2, B3, 100 μm. *, p<0.05, different than vehicle, ANOVA on Ranks with Dunn’s posthoc comparison.
Dynorphin up-regulation by chronic treatment with GluN2B-selective NMDAR antagonists was independent of BDNF/TrkB signaling pathways
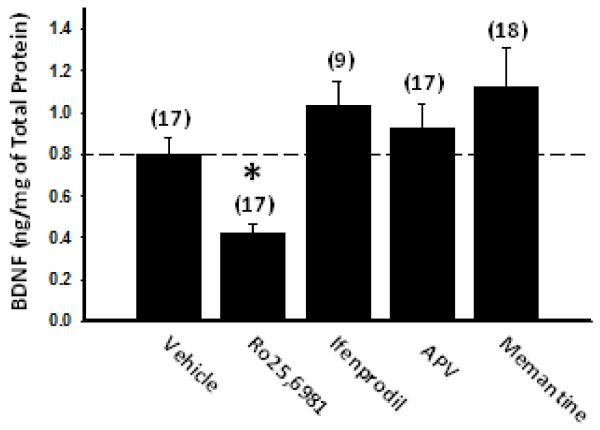
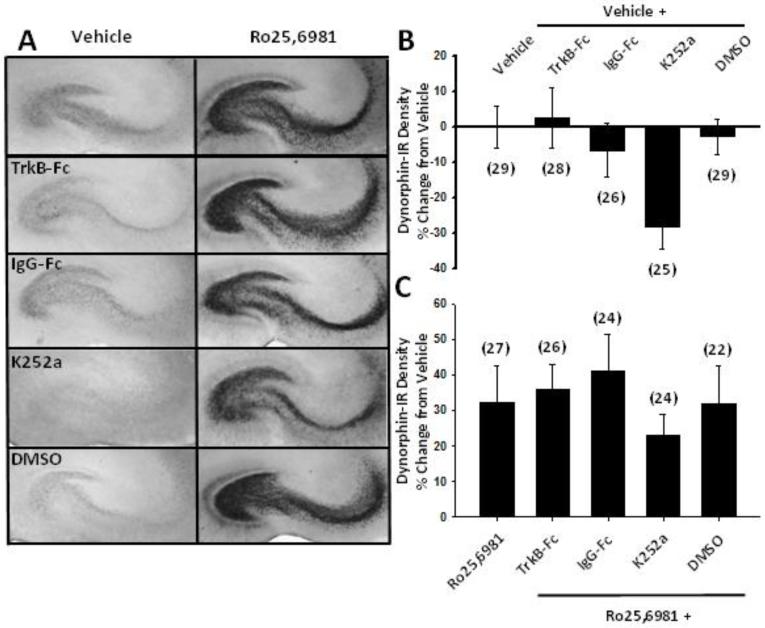
We next investigated the effects of chronic NMDAR antagonist treatment on BDNF and its effects on DYN expression. We first examined BDNF levels using ELISA. Chronic treatment of cultures with the non-subunit-selective NMDAR antagonists, D-APV or memantine, did not significantly alter BDNF levels (Fig. 4), consistent with their lack of effect on CREB (Ser 133) phosphorylation. Treatment of cultures with GluN2B-selective NMDAR antagonists showed differential effects on BDNF. Compared to vehicle, chronic Ro25,6981 decreased, while ifenprodil, had no significant effect on BDNF levels (Fig. 4). The lack of association between BDNF levels and DYN expression in both Ro25,6981- and ifenprodil-treated cultures suggests that altered BDNF expression did not underlie GluN2B antagonist-induced increases in DYN expression. However, BDNF was measured in whole slice cultures, while increased DYN occurred only in the dentate gyrus/hilar mossy fiber pathway. Therefore, we directly examined the influence of BDNF and activation of its receptor, TrkB on DYN immunoreactivity. Vehicle- and Ro25,6981-treated cultures were treated concomitantly with the BDNF scavenger, TrkB-Fc or the broad spectrum Trk tyrosine kinase inhibitor, K252a. If decreased BDNF contributed to increased DYN expression, then TrkB-Fc and/or K252a should increase DYN expression in vehicle-treated cultures. Conversely, if increased BDNF expression and subsequent TrkB activation/phosphorylation lead to increased DYN expression, then TrkB-Fc and/or K252a co-incubation should decrease DYN expression in Ro25,6981-treated cultures. In vehicle-treated cultures neither TrkB-Fc nor its respective control, IgG-Fc significantly affected hilar DYN immunoreactivity (Fig. 5A,B). Opposite to what we expected, K252a caused a strong trend toward reduced hilar DYN immunoreactivity compared to both chronic vehicle treatment alone and its respective DMSO control (Fig. 5A,B). However, in Ro25,6981-treated cultures neither TrkB-Fc nor K252a significantly affected hilar DYN immunoreactivity (Fig. 5A, C). Ro25,6981 did not increase hilar DYN levels by the same magnitude (Fig. 5C) as seen in Fig. 2C due to the higher basal levels of hilar DYN expression in vehicle-treated cultures for this subset of experiments (compare Fig. 2A top to Fig. 5A left). However, the effect of Ro25,6981 on hilar DYN expression was still significant (p=0.012, Mann-Whitney Rank Sum test). Taken together these data suggest that BDNF/TrkB receptor activation may contribute partially to basal DYN expression levels, but that alterations in BDNF and TrkB activation do not underlie chronic GluN2B antagonist-mediated increases in DYN expression.
Fig. 4.
BDNF levels were unaltered by chronic treatment with GluN2B-selective NMDAR antagonists. ELISA was used to assay BDNF levels in hippocampal slice cultures treated for the entire 17-21 day culture period with different classes of NMDAR antagonists as described in the Materials and Methods. BDNF levels were decreased in organotypic hippocampal slice cultures treated with the GluN2B-selective NMDAR antagonist Ro25,6981, but not in cultures treated with the GluN2B-selective NMDAR antagonist ifenprodil or the non-subtype-selective NMDAR antagonists APV or memantine. Bars represent mean ± SEM. Numbers in parentheses indicate the number of slice cultures. *, p<0.05, different than vehicle, ANOVA on Ranks with Dunn’s posthoc comparison.
Fig 5.
Increased DYN expression following chronic treatment with GluN2B-selective NMDAR antagonists was independent of TrkB-mediated signaling pathways. Organotypic hippocampal slice cultures were treated for the entire 17-21 day culture period with either vehicle or the GluN2B-selective NMDAR antagonist Ro25,6981 alone or in combination with the TrkB/human IgG Fc region chimera TrkB-Fc, the tyrosine kinase inhibitor K252a or their respective human IgG-Fc or DMSO controls. Cultures were then stained immunohistochemically with anti-DYN A antiserum using the ABC method and analyzed as described in the Materials and Methods and depicted in Fig. 2. A) Representative hippocampal slice cultures as well as B), C) quantitative densitometry show increased hilar DYN immunoreactivity following chronic Ro25,6981 treatment (p=0.012, Mann Whitney Rank Sum test), but no significant effect of K252a or TrkB-Fc in either vehicle- or Ro25,6981-treated slice cultures. Bars indicate means ± SEM. Numbers in parentheses indicate the number of slice cultures.
Discussion
In this study we tested the hypothesis that chronic inhibition of GluN2B-containing NMDAR would increase CREB (Ser 133) phosphorylation, increase BDNF levels and alter downstream DYN and NPY expression. Consistent with our hypothesis, chronic treatment with the GluN2B-selective antagonists ifenprodil or Ro25,6981 increased both CREB (Ser 133) phosphorylation relative to CREB levels and granule cell mossy fiber pathway DYN expression. Chronic treatment with the non-subtype-selective NMDAR antagonists D-APV or memantine had no effect on either CREB (Ser 133) phosphorylation or DYN expression. In contrast to our hypothesis, BDNF levels were decreased following chronic treatment with Ro25,6981, but not ifenprodil. Hilar DYN expression was not significantly increased in vehicle-treated cultures or decreased in Ro25,6981-treated cultures following blockade of BDNF actions and TrkB activation. However, the Trk tyrosine kinase inhibitor, K252a caused a strong trend toward reduced hilar DYN immunoreactivity, suggesting that BDNF/TrkB receptor activation may contribute to basal DYN expression levels. Small decreases in CREB (Ser 133) phosphorylation by K252a (Pizzorusso et al., 2000) may underlie this trend. Taken together, our findings suggest that chronic exposure to GluN2B-selective NMDAR antagonists increased DYN expression through a putatively pCREB-dependent, but BDNF/TrkB-independent mechanism.
NMDAR regulation of signaling pathways
NMDAR can be activated by spontaneous glutamate release. Lack of similar effects of chronic treatment with GluN2B- and non-subunit-selective NMDAR antagonists on CREB (Ser 133) phosphorylation is initially counterintuitive since both compounds inhibit GluN2B-containing NMDARs. However, on a simplistic level, inhibition of both GluN2A- and GluN2B-containing NMDAR with non-subunit-selective NMDAR antagonists should block both GluN2A-mediated CREB (Ser 133) phosphorylation (Terasaki et al., 2010) and GluN2B-mediated dephosphorylation/reduced phosphorylation of CREB (Ser 133) and thus result in no significant change in CREB (Ser 133) phosphorylation. Inhibition of GluN2B alone should inhibit the dominant GluN2B-mediated reduction in CREB (Ser 133) phosphorylation (Hardingham et al., 2002), without affecting GluN2A-mediated CREB (Ser 133) phosphorylation and thus lead to increased CREB (Ser 133) phosphorylation. Thus, our data are more consistent with the subunit composition model (Liu et al., 2007; Chen et al., 2008) than the synaptic/extrasynaptic localization model (Hardingham et al., 2002). However, while GluN2B subunits are located at both synaptic and extrasynaptic sites (Harris and Pettit 2007; Gray et al., 2011), they are more concentrated in extrasynaptic sites (Tovar and Westbrook, 1999; Groc et al., 2006) and contained primarily in triheteromeric NMDAR assemblies (GluN1/GluN2A/GluN2B) in synaptic sites (Sheng et al., 1994; Gray et al., 2011; Rauner and Kohr 2011). In triheteromeric NMDAR, Ro25,6986 and ifenprodil still exhibit high affinity, but maximal inhibition is greatly reduced (Chazot et al., 2002; Hatton and Paoletti 2005).
CREB (Ser 133) phosphorylation and BDNF expression
The discrepancy between our hypothesis and BDNF data raises the question as to why increased CREB (Ser 133) phosphorylation did not lead to increased BDNF expression since BDNF is CREB target gene (Shieh et al., 1998; Tao et al., 1998). CREB is a ubiquitously expressed transcription factor that promotes gene transcription from cAMP regulatory elements (CRE) following activation/phosphorylation at Ser 133, subsequent CREB dimerization (Montminy et al., 1986; Gonzalez and Montminy, 1989; Dash et al., 1991; Sheng et al., 1991; Finkbeiner et al., 1997), and association with CREB-binding protein (CBP), which has histone deacetylase activity (Chrivia et al., 1993; Bannister and Kouzarides 1996). Chromatin acetylation facilitates CREB access to DNA (Chrivia et al., 1993; Bannister and Kouzarides 1996), while CRE nucleotide methylation reduces CREB access to DNA. CREB interactions with multiple different transcription factors and coactivators also regulate gene transcription in a context-specific, cell-type dependent, and stimulus driven manner. Therefore, activated CREB may not have promoted BDNF transcription in our experiments due to: 1) lack of CREB access to chromatin in the BDNF promoter; 2) inability to recruit required coactivators or interaction with proteins that lack a CRE-binding domain (Foulkes et al., 1991).; 3) phosphorylation of CREB at alternate sites [i.e. CREB phosphorylation at Ser142 by CaMKII inhibits the CREB-CBP association and represses transcription (Sun et al., 1994)]; and/or 4) lack of cell-type-specific factors (see Lonze and Ginty 2002; Carlezon et al., 2005). Indeed, the rat BDNF gene contains four distinct promoters and at least three Ca2+/cAMP regulatory elements (CRE/CaRE1-3). CREB phosphorylation at Ser 133, binding of CREB to all three CRE/CaRE elements, and binding of other transcription factors, including a calcium-responsive transcription factor (CaRF) and upstream stimulatory factors 1 and 2 (USF1/2) are required for BDNF expression (Timmusk et al., 1993; Tao et al., 1998; Tao et al., 2002; Chen et al., 2003). Therefore, one possible explanation for the discrepancy between previous findings and our BDNF data is that the previous study (Hardingham et al., 2002) used dissociated hippocampal cultures, while we used organotypic hippocampal slice cultures that mimic endogenous neuronal networks. Another difference is the stimulus used to drive NMDAR activation. Previous studies used prolonged pharmacological increases in NMDAR activation, while we blocked spontaneously released neurotransmitter. The same convergence of events required for BDNF expression may not occur in our preparation using a NMDAR inactivation paradigm. Another question that arises is why Ro25,6981, but not ifenprodil decreased BDNF levels. While both Ro26,6981 and ifenprodil target GluN2B-containing NMDAR, ifenprodil is less specific overall. Ifenprodil also interacts with β-adrenergic receptors, serotonin receptors and calcium channels (Chenard et al., 1991; Church et al., 1994; McCool and Lovinger 1995), which may have impacted BDNF expression compared to inhibition of GluN2B-containing NMDAR alone.
Control of DYN/NPY expression
CREB regulates dynorphin expression through binding to three CRE upstream of the preprodynorphin promoter, which can independently increase transcription. CREB binding to multiple CRE increases the magnitude of DYN transcription (Cole et al., 1995; Kaynard et al., 1992; Douglass, et al., 1994). Thus, our findings showing an association between CREB (Ser 133) phosphorylation and increased dynorphin expression are consistent with a CREB-dependent mechanism. However, thousands of genes contain CREs in their promoter and regulatory regions (see Lonze and Ginty 2002; Carlezon et al., 2005) and whether CREB regulation is direct or indirect remains unclear. For example, BDNF is a CREB target gene that regulates DYN expression. Extended BDNF application into the hippocampus decreased dynorphin peptide and preprodynorphin mRNA (Croll et al., 1994). By analogy, decreased BDNF levels in Ro25,6981-treated cultures could underlie increased DYN expression. However, DYN levels were increased in ifenprodil-treated cultures despite no change in BDNF levels. Lastly, reducing BDNF actions did not result in increased DYN expression in vehicle-treated cultures or block DYN up-regulation in Ro25,6981-treated cultures. Therefore, CREB (Ser 133) phosphorylation, not alterations in BDNF, most likely mediated increased DYN expression following chronic treatment with GluN2B antagonists.
CREB can modulate preproneuropeptide Y gene expression (Higuchi et al., 1988; Chance et al., 2000), so no change in NPY expression despite increased CREB (Ser 133) phosphorylation was somewhat surprising. However, to our knowledge CREB modulation of NPY transcription has not been reported in hippocampal neurons specifically and may require alternate or additional modulators, including non-CRE Ca2+/calmodulin response elements (CaMRE), SP1 family transcription factors, and cooperative interactions between AP-1, AP-2, and SP1 transcription factors (Minth and Dixon, 1990; Andersson et al., 1994; Higuchi et al., 1996). Alternatively, our immunohistochemical methods may not have been sensitive enough to detect modest NPY changes.
Potential involvement of BDNF, DYN, and CREB (Ser 133) phosphorylation on seizure-like activity
We focused on BDNF, DYN, and NPY because each can influence seizures. BDNF and TrkB activation modulate synaptic transmission and enhance seizure expression (Binder et al., 2001). Consistent with previous reports showing that reducing BDNF retards epileptogenesis (Kokaia et al., 1995; Binder et al., 1999; He et al., 2004), decreased BDNF following chronic Ro25,6981 (current study) was associated with reduced expression of seizure-like events (Wang and Bausch, 2004). However, chronic ifenprodil treatment reduced seizure-like events (Wang and Bausch, 2004), but did not affect BDNF levels (current study). Thus, reduced BDNF alone is unlikely to mediate reduced seizure-like events following chronic treatment with GluN2B antagonists, although regional changes in BDNF release cannot be ruled out.
DYN is released from dentate granule cells following high frequency stimulation and reduces excitatory glutamatergic transmission (Wagner et al., 1993; Wagner et al., 1992; Weisskopf et al., 1993; Drake et al., 1994; Shukla and Lemaire, 1994; Simmons and Chavkin 1996a,1996b; Terman et al., 2000). Commensurate with reduced excitatory neurotransmission, DYN reduces seizures in numerous epilepsy models (see Tortella, 1988; Simmons and Chavkin, 1996b; Bausch et al., 1998). Therefore, DYN up-regulation in the dentate granule cell mossy fiber pathway may contribute to the reduced seizure-like events following chronic treatment with GluN2B antagonists (Wang and Bausch, 2004). Further studies are clearly required to mechanistically link increased DYN to reduced seizures following chronic treatment with GluN2B-selective antagonists.
Although this study focused on BDNF, DYN, and NPY, CREB modulates expression of a wide variety of downstream targets, which also may directly or indirectly contribute to reduced seizure expression following chronic treatment with GluN2B-selective antagonists. Indeed, we reported previously that chronic treatment with the GluN2B-selective antagonist, Ro25,6981 down-regulated AMPAR and NMDAR function (He et al., 2013). A separate study showed that Ro25,6981 treatment caused repressive chromatin remodeling via the histone methyltransferase, Setdb1 and reduced GluN2B expression (Jiang et al., 2010). Therefore we speculate that chronic GluN2B antagonist treatment may induce a complex landscape of activity-dependent CREB-modulated transcription as well as repressive epigenetic marks that impact transcription of genes putatively regulated by CREB activation. Future studies investigating the role of permissive and restrictive epigenetic environment regulated by NMDAR activity and their relationship to seizure expression are clearly needed.
Experimental methods
Organotypic hippocampal slice cultures
Slice cultures were prepared using the Stoppini method (Stoppini et al., 1991) as described previously (Wang and Bausch 2004). Briefly, postnatal day 11 Sprague-Dawley rats (Taconic; Germantown, NY) were anesthetized with pentobarbital and decapitated. The brains were removed, hippocampi were cut into 400 μm transverse slices using a McIlwain tissue chopper and slices were placed into Gey’s balanced salt solution [GBSS; (in mM): 137 NaCl, 5 KCl, 0.25 MgSO4, 1.5 CaCl2, 1.05 MgCl2, 0.84 Na2HPO4, 0.22 K2HPO4, 2.7 NaHCO3, 5.6 glucose] supplemented with an additional 6.5 mg/ml glucose. The entorhinal cortex was removed from the middle 4-6 slices of each hippocampus and slices were placed onto tissue culture membrane inserts (Millipore; Bedford, MA) in a tissue culture dish containing medium consisting of 50% minimum essential medium, 25% Hank’s buffered salt solution, 25% heat-inactivated horse serum, 0.5% GlutaMax, 10 mM HEPES (all from Invitrogen; Carlsbad, CA) and 6.5 mg/ml glucose (pH 7.2). Cultures were maintained at 37°C under room air + 5% CO2 /100% humidity and medium with or without drugs was changed three times per week. Cultures were treated chronically for the entire 17-21 DIV culture period with Ro25,6981 (1 μM; Sigma), ifenprodil (3 μM; Tocris Cookson; Ballwin, MO),D-(−)-2-amino-5-phosphonopentanoic acid (APV, 50 μM; Tocris Cookson) or memantine (1 μM; Tocris Cookson) diluted in medium. Vehicle-treated cultures were treated similarly, but drugs were omitted. Vehicle- and drug-treated cultures were always studied concurrently under identical experimental conditions. In experiments examining the effects of TrkB activation on dynorphin expression cultures also were treated for the full 17-21 day culture period with the TrkB-human IgG-Fc chimera, TrkB-Fc (2 μg/mL; R&D Systems, Minneapolis, MD; Rex et al., 2006; Rex et al., 2007; Xapelli et al., 2008; Lauterborn et al., 2009; Aguirre and Baudry 2009; Gill et al., 2013) diluted in medium, the tyrosine kinase inhibitor K252a (200 nM; EMD Chemicals, Gibbstown, NJ; Tyler and Pozzo-Miller 2001; Koyama rt al., 2004; Pozzo-Miller 2006; Sato et al., 2007) prepared in 0.02% DMSO, or their respective controls human IgG-Fc (2 μg/mL; R&D Systems, Minneapolis, MD) or 0.02% DMSO (Sigma, St. Louis, MO) diluted in medium. Only those hippocampal slice cultures exhibiting distinctive cell layers at the end of the 17-21 day culture period were used in subsequent experiments.
Western blot analyses
Western blot analysis for CREB and pCREB were performed as described previously (Dong et al. 2002) Slice cultures were rinsed in Tyrode solution (in mM): 129 NaCl, 5 KCl, 2 CaCl2, 30 glucose, 25 HEPES (pH 7.35), then frozen on dry ice and stored at −80°C. Frozen slice cultures were homogenized in ice cold 0.1 M Tris buffer [TB (pH 7.4)] containing: 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM dithiothreitol, 0.5mM phenylmethylsulfonyl fluoride (PMSF), 2 mM benzamide HCl, 0.1 mg/ml trypsin inhibitor, and 0.1 mg/ml bacitracin. Homogenate aliquots were stored at −80°C until use. Protein concentrations were determined by BCA assay (Pierce; Rockford, IL) and 7 μg of total homogenates were denatured and separated by 10% SDS-polyacrylamide gel electrophoresis, then transferred to PVDF membranes. After blocking in TB containing 0.15 M NaCl, 5% dried non-fat milk, and 0.1% Tween-20, membranes were incubated with rabbit anti-CREB IgG (Upstate; Lake Placid, NY) diluted 1:1,000 or affinity-purified rabbit anti-phospho-CREB (pCREB; Ser133) IgG (Affinity BioReagents; Golden, CO) diluted 1:1,000 followed by HRP-conjugated goat anti-rabbit IgG (BioRad; Hercules, CA) diluted 1: 3,000 in blocking solution. Protein bands were visualized by ECL chemiluminescence (Pierce, Rockford, IL) according to kit instructions and imaged using a Fujifilm Intelligent Dark Box II system (Fuji Medical systems, Stamford, CT). Background-subtracted protein bands were quantitatively analyzed using Fujifilm software in the linear range. CREB was examined in two separate blots per experiment; one was initially probed for CREB, the other was first probed for pCREB then stripped and reprobed for CREB. Since no significant differences were apparent, CREB densities were represented as the average of the two blots. As loading controls, all blots were stripped with Re-blot Plus (Chemicon; Temecula, CA) and re-probed with rabbit anti-neuron specific enolase (NSE) antiserum (Chemicon) diluted 1:200,000 or mouse monoclonal anti-β-tubulin IgG1 (clone AA2; Upstate) diluted 1:15,000 and processed with HRP-conjugated goat anti-rabbit IgG (BioRad) diluted 1: 6,000 or HRP-conjugated goat anti-mouse IgG (BioRad) diluted 1:5,000, respectively, then visualized and analyzed as described above. Specificity of the CREB and pCREB antibodies was confirmed with control cell extract (Fig 1D) and peptide block (Fig 1E) experiments. Purchased extracts (Cell Signaling; Beverly, MA) prepared from SK-N-MC cells with and without forskolin and IBMX treatment were used to show specificity of the anti-pCREB (Ser133) antibody. Peptide block experiments were used to show specificity of the anti-CREB and anti-pCREB (Ser133) antibodies and were performed by pre-incubating the CREB or pCREB antibodies (10 μg) with pCREB (Ser133) blocking peptide (10 μg; Cell Signaling) or CREB immunizing peptide (10 μg; Upstate) for 2 hr. at room temperature (RT) prior to application to blots of slice culture homogenates.
Immunohistochemistry
Slice cultures were fixed for 20 min. with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for NPY or with 4% paraformaldehyde in PB containing 0.15% glutaraldehyde for dynorphin, removed from the membrane and free-floating slice cultures were processed for immunohistochemistry using the avidin-biotin-peroxidase complex (ABC) method (Hsu et al., 1981) as described previously (Drake et al., 1994; Bausch et al., 1995). All steps were performed at RT unless stated otherwise. Slice cultures fixed with glutaraldehyde were pretreated with 1% sodium borohydride in 0.1M Tris buffer [TB (pH 7.4)]. All slice cultures were pretreated sequentially with 70% ethanol, 0.3% H202 in 100% methanol, 70% ethanol, followed by 0.1 M TBS (dynorphin) or 0.1 M PBS (NPY) (TB/PB containing 0.15 M NaCl and 2.7 mM KCl [pH 7.4]). Cultures were then sequentially pretreated with 7% avidin in TBS/PBS, 7% biotin in TBS/PBS, and blocked with TBS/PBS containing 2% gelatin and 10% normal goat serum for 1 hr at 37°C. Slice cultures were then incubated in rabbit anti-dynorphin A antiserum (Peninsula Laboratories; San Carlos, CA; Drake et al., 1994) diluted 1:3,000 or rabbit anti-NPY IgG (Peninsula Laboratories) diluted 1:2,500 (0.2 ng/ml) for 1 hr at RT followed by 36 hr at 4°C. Specificity of the anti-dynorphin A antiserum was previously documented by (Pickel et al., 1993); specificity of anti-NPY IgG by (Malmberg et al., 1997; Croll et al., 1999). All antibodies were diluted in TBS/PBS containing 2% bovine serum albumin (BSA), 10% normal goat serum and 0.25% Triton X-100. Slice cultures were then processed as follows: rinsed with TBS/PBS containing 0.1% Triton X-100; incubated in biotinylated goat anti-rabbit IgG (Vector; Burlingame, CA) diluted 1:400 in diluent for 1 hr; rinsed; incubated in ABC Elite (Vector) diluted in TBS/PBS containing 1% BSA and 0.1% Triton X-100 according to kit instructions (1×, NPY; 2×, dynorphin) for 1 hr at 37°C; and rinsed. Immunoreactivity was visualized with 0.04% 3,3′-diaminobenzidine (DAB, Sigma) and 0.0024% H202 in TB. Slice cultures were mounted onto subbed glass slides, dehydrated, cleared in xylenes and coverslipped with Permount mounting media (Fisher). Slice cultures were digitally photographed using a Zeiss Axioskop microscope (Carl Zeiss MicroImaging, LLC, Thornwood, NY), Optronics (Goleta, CA) Microfire camera and Picture Frame software (Optronics). The dynorphin-IR mossy fiber pathway was defined subjectively as continuous staining of the entire mossy fiber pathway. The density of dynorphin and NPY immunoreactivities was quantified with MetaMorph software (Universal Imaging / Molecular Devices, Downingtown, PA).
BDNF ELISA
Homogenates were prepared from slice cultures in a lysis buffer composed of: 137 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin and 0.5 mM sodium vanadate. Aliquots were frozen and stored at −80 °C until use. Total protein concentration was determined using a BCA assay (Pierce). BDNF levels were quantified according to BDNF Emax ® ImmunoAssay System (Promega, Madison WI) instructions.
Statistics
Cultures from experimental and control groups were always treated, processed and analyzed concurrently under identical conditions. Investigators were blinded to experimental groupings for all data analyses. Statistical analyses were performed with Sigma Stat software (SPSS Inc., Chicago, Illinois) and significance was defined as p ≤ 0.05.
Acknowledgements
We thank Mr. Dong Choe for assistance with pCREB control Western blot experiments. All treatment of animals was conducted according to National Institutes of Health, Department of Defense, and institutional guidelines and was approved by the Uniformed Services University Institutional Animal Use and Care (IACUC) committee. Work was supported by the Defense Brain and Spinal Cord Injury Program (S.B.B.); National Institute of Health National Institute of Neurological Disorders and Stroke [Grant NS045964] (S.B.B.); and the American Society for Pharmacology and Experimental Therapeutics Summer Undergraduate Research Fellowship program (USU Dept. Pharmacology) with matching funds from the Henry M. Jackson Foundation for Military Medicine. Some parts of this work were previously presented in abstract form (Barksdale et al., 2004; Rittase et al., 2008). The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University.
Footnotes
Abbreviations: ABC, avidin-biotin-peroxidase complex; APV, D-(−)-2-amino-5-phosphonopentanoic acid; BCA, bicinchoninic acid; BDNF, brain-derived neurotrophic factor; BSA, bovine serum albumin; CREB, cAMP response element-binding protein; DAB, 3,3′-diaminobenzidine; DMSO, dimethylsulfoxide; DYN, dynorphin; ECL, enhanced chemiluminescence; EDTA, ethylenediaminetetraacetic acid; EGTA, ethylene glycol tetraacetic acid; ELISA, enzyme-linked immunosorbent assay; GBSS, Gey’s Balanced Salt solution; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HRP, horse-radish peroxidase; IBMX, 3-isobutyl-1-methylxanthine; IgG, Immunoglobulin G; -IR, immunoreactivity; NMDA, N-methyl-d-aspartate; NMDAR, N-methyl-d-aspartate receptor; NPY, neuropeptide Y; GluN2A, NMDAR 2A subunit; GluN2B, NMDAR 2B subunit; NSE, neuron specific enolase; PB, phosphate buffer; PBS phosphate buffered saline; pCREB, phosphorylated CREB; PMSF, phenylmethanesulfonylfluoride; PVDF, polyvinylidene fluoride; RT, room temperature; SDS, sodium dodecyl sulfate; Ser, serine; TB, Tris buffer; TBS Tris buffered saline; TrkB-Fc, tyrosine kinase, receptor/IgG Fc fragment chimera.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre CC, Baudry M. Progesterone reverses 17β-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur. J. Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G, Påhlman S, Parrow V, Johansson I, Hammerling U. Activation of the human NPY gene during neuroblastoma cell differentiation: induced transcriptional activities of AP-1 and AP-2. Cell Growth Differ. 1994;5:27–36. [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Baraban SC. Neuropeptide Y and epilepsy: recent progress, prospects and controversies. Neuropeptides. 2004;38:261–265. doi: 10.1016/j.npep.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Barksdale DM, Dong Y, Galdzicki Z, Bausch SB. Differential effects of NR2B selective versus general NMDAR antagonists on CREB phosphorylation and downstream expression of neuropeptides. Soc. Neurosci. Abstr. Program. 2004;49:10. [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bausch SB, Patterson TA, Appleyard SM, Chavkin C. Immunocytochemical localization of delta opioid receptors in mouse brain. J. Chem. Neuroanat. 1995;8:175–189. doi: 10.1016/0891-0618(94)00044-t. [DOI] [PubMed] [Google Scholar]
- Bausch SB, Esteb TM, Terman GW, Chavkin C. Administered and endogenously released kappa opioids decrease pilocarpine-induced seizures and seizure-induced histopathology. J. Pharmacol. Exp. Ther. 1998;284:1147–1155. [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, Ryan TE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J. Neurosci. 1999;19:1424–36. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Bonni A, Ginty DD, Dudek H, Greenberg ME. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol. Cell Neurosci. 1995;6:168–183. doi: 10.1006/mcne.1995.1015. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of Cocaine Reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chance WT, Sheriff S, Peng F, Ambikaipakan B. Antagonism of NPY-induced feeding by pretreatment with cyclic AMP response element binding protein antisense oligonucleotide. Neuropeptides. 2000;34:167–172. doi: 10.1054/npep.2000.0807. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology. 2002;42:319–324. doi: 10.1016/s0028-3908(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 2008;39:3042–8. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- Chen WG, West AE, Tao X, Corfas G, Szentirmay MN, Sawadogo M, Vinson C, Greenberg ME. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J. Neurosci. 2003;23:2572–81. doi: 10.1523/JNEUROSCI.23-07-02572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gu Y, Huang L. The Mechanism of Action for the Block of NMDA Receptor Channels by the Opioid Peptide Dynorphin. J. Neurosci. 1995;15:4602–4611. doi: 10.1523/JNEUROSCI.15-06-04602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang L. Dynorphin Block of N-Methyl-D-Aspartate Channels Increases with Peptide Length. J. Pharmacol. Exp. Ther. 1998;284:826–831. [PubMed] [Google Scholar]
- Chenard BL, Shalaby IA, Koe BK, Ronau RT, Butler TW, Prochniak MA, Schmidt AW, Fox CB. Separation of alpha 1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J. Med. Chem. 1991;34:3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Church J, Fletcher EJ, Baxter K, MacDonald JF. Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions. Br. J. Pharmacol. 1994;113:499–507. doi: 10.1111/j.1476-5381.1994.tb17017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–23. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y Action in the Rat Hippocampal Slice: Site and Mechanism of Presynaptic Inhibition. J. Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampus. J. Physiol. 1987;383:285–299. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur. J. Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Croll SD, Chesnutt CR, Greene NA, Linday RM, Wiegand SJ. Peptide Immunoreactivity in Aged Rat Cortex and Hippcampus as a Function of Memory and BDNF Infusion. Pharmacol. Biochem. Behav. 1999;64:625–635. doi: 10.1016/s0091-3057(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. cAMP response element-binding protein is activated by Ca2+/calmodulin-as well as cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Pi X, Rosenberg HC. Seizure induced calcineurin activation and long-lasting reduction of CREB phosphorylation. Soc. Neurosci. Abstr. Program. 2002;791:3. [Google Scholar]
- Douglass J, McKinzie AA, Pollock KM. Identification of multiple DNA elements regulating basal and protein kinase A-induced transcriptional expression of the rat prodynorphin gene. Mol. Endocrinol. 1994;8:333–44. doi: 10.1210/mend.8.3.8015551. [DOI] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. 1994;14:3736–50. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–47. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes NS, Borrelli E, Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Gill R, Chang PK, Prenosil GA, Deane EC, McKinney RA. Blocking brain-derived neurotrophic factor inhibits injury-induced hyperexcitability of hippocampal CA3 neurons. Eur J. Neurosci. 2013;38:3554–3566. doi: 10.1111/ejn.12367. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18769–74. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neuroscience. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J. Physiol. 2007;584:509–19. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- He S, Shao LR, Wang Y, Bausch SB. Synaptic and extrasynaptic plasticity in glutamatergic circuits involving dentate granule cells following chronic N-methyl-D-aspartate receptor inhibition. J. Neurophysiol. 2013;109:1535–1547. doi: 10.1152/jn.00667.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yang HY, Sabol SL. Rat neuropeptide Y precursor gene expression. mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J. Biol. Chem. 1988;263:6288–95. [PubMed] [Google Scholar]
- Higuchi H, Nakano K, Kim CH, Li BS, Kuo CH, Taira E, Miki N. Ca2+/calmodulin-dependent transcriptional activation of neuropeptide Y gene induced by membrane depolarization: determination of Ca(2+)- and cyclic AMP/phorbol 12-myristate 13-acetate-responsive elements. J. Neurochem. 1996;66:1802–9. doi: 10.1046/j.1471-4159.1996.66051802.x. [DOI] [PubMed] [Google Scholar]
- Hsu S-M, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, Straubhaar J, Martin G, Akbarian S. Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J. Neurosci. 2010;30:7152–67. doi: 10.1523/JNEUROSCI.1314-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynard AH, McMurray CT, Douglass J, Curry TE, Jr, Melner MH. Regulation of prodynorphin gene expression in the ovary: distal DNA regulatory elements confer gonadotropin regulation of promoter activity. Mol. Endocrinol. 1992;6:2244–56. doi: 10.1210/mend.6.12.1337148. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF. Neuropeptide Y Suppresses epileptiform Activity in Rat Hippocampus In Vitro. J. Neurophysiol. 1997;78:1651–1661. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- Kokaia M, Ernfors P, Kokaia Z, Elmer E, Jaenisch R, Lindvall O. Suppressed epileptogenesis in BDNF mutant mice. Exp. Neurol. 1995;133:215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Koyama R, Yamada MK, Fujisawa S, Katoh-Semba R, Matsuki N, Ikegaya Y. Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J. Neurosci. 2004;24:7215–7224. doi: 10.1523/JNEUROSCI.2045-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience. 2009;159:283–295. doi: 10.1016/j.neuroscience.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-B, Murray KD, Jones EG. Switching of NMDA Receptor 2A and 2B Subunits at thalamic and Cortical Synapses during Early Postnatal Development. J. Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007;27:2846–57. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Malmber AB, Chen C, Tonegawa S, Basbaum AI. Preserved Acute Pain and Reduced Neuropathic Pain in Mice Lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- McCool BA, Lovinger DM. Ifenprodil inhibition of the 5-hydroxytryptamine3 receptor. Neuropharmacology. 1995;34:621–629. doi: 10.1016/0028-3908(95)00030-a. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Bohler WT, Patterson TA, Chavkin C. Kappa receptor immunoreactivity is present in cells and fibers of the rat forebrain. Regul. Pept. 1994;54:185–186. [Google Scholar]
- Minth CD, Dixon JE. Expression of the human neuropeptide Y gene. J. Biol. Chem. 1990;265:12933–9. [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H, Bessho Y, Carnahan J, Nakanishi S, Mizuno K. Regulation of neuropeptide expression in cultured cerebral cortical neurons by brain-derived neurotrophic factor. J. Neurochem. 1993;60:772–5. doi: 10.1111/j.1471-4159.1993.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, van den Pol AN, Spencer DD, Williamson A. NPY Inhibits Glutamatergic Excitation in the Epileptic Human Dentate Gryrus. J. Neurophysiol. 1999;82:478–483. doi: 10.1152/jn.1999.82.1.478. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–8. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J. Neurosci. 2000;20:2809–16. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller L. BDNF enhances dendritic Ca2+ signals evoked by coincident EPSPs and back-propagating action potentials in CA1 pyramidal neurons. Brain Res. 2006;1104:45–54. doi: 10.1016/j.brainres.2006.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J. Biol. Chem. 2011;286:7558–66. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J. Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramár EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richichi C, Lin E-JD, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and Antiepileptogenic Effects mediated by Adeno-Associated Virus Vector Neuropeptide Y Expression in the Rat Hippocampus. J. Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittase WB, Barksdale DM, Bausch SB. Up-regulation of dynorphin in the dentate granule cell mossy fiber pathway following chronic treatment with NR2B-selective NMDAR antagonists is not dependent on BDNF. Soc. Neurosci. Abstr. Program. 2008;129:20. [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–7. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. Creba ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Muneoka KT, Iwata M, Ishida H, Hazama G, Kawahara R. Pregnenolone and dehydroepiandrosterone administration in neonatal rats alters the immunoreactivity of hippocampal synapsin I, neuropeptide Y and glial fibrillary acidic protein at post-puberty. Neuroscience. 2005;133:147–157. doi: 10.1016/j.neuroscience.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol Sci. 1994;15:420–4. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. κ-Opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol. Pharmacol. 1996a;50:80–5. [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int. Rev. Neurobiol. 1996b;39:146–197. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Simonato M, Romualdi P. Dynorphin and Epilepsy. Progress in Neurobiology. 1996;50:557–583. doi: 10.1016/s0301-0082(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–95. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Terasaki Y, Sasaki T, Yagita Y, Okazaki S, Sugiyama Y, Oyama N, Omura-Matsuoka E, Sakoda S, Kitagawa K. Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J. Cereb. Blood Flow Metab. 2010;8:1441–1449. doi: 10.1038/jcbfm.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J. Neurosci. 2000;20:4379–88. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–89. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tortella FC. Endogenous opioid peptides and epilepsy: Quieting the seizing brain. Trends Pharmacol. Sci. 1988;9:366–372. doi: 10.1016/0165-6147(88)90256-8. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The Incorporation of NMDA Receptors with a Distinct Subunit Composition at Nascent Hippocampal Synapses In Vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J. Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998;79:555–66. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Chavkin C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J. Neurosci. 1992;12:132–41. doi: 10.1523/JNEUROSCI.12-01-00132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-M, Bausch SB. Effects of distinct classes of N-methyl-D-aspartate receptor antagonists on seizures, axonal sprouting and neuronal loss in vitro: Suppression by NR2B-selective antagonists. Neuropharmacology. 2004;47:1008–1020. doi: 10.1016/j.neuropharm.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bausch SB. In contrast to NR2B-selective antagonists, chronic treatment with NR2A-selective or non-subunit selective antagonists reduced interneuron survival and GAD65/67 expression. Soc. Neurosci. Abstr. Program. 2006;278:16. [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–7. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Livesey MR, Hardingham GE. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. doi: 10.1016/j.neuropharm.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xapelli S, Bernardino L, Ferreira R, Grade S, Silva AP, Salgado JR, Cavadas C, Grouzmann E, Poulsen FR, Jakobsen B, Oliveira CR, Zimmer J, Malva JO. Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: a role for microglia. Eur. J. Neurosci. 2008;27:2089–2102. doi: 10.1111/j.1460-9568.2008.06172.x. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–50. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]