Abstract
Human respiratory syncytial virus (RSV) is a leading cause of severe respiratory disease and hospitalizations in infants and young children. It also causes significant morbidity and mortality in elderly and immune compromised individuals. No licensed vaccine currently exists. Parainfluenza virus 5 (PIV5) is a paramyxovirus that causes no known human illness and has been used as a platform for vector-based vaccine development. To evaluate the efficacy of PIV5 as a RSV vaccine vector, we generated two recombinant PIV5 viruses - one expressing the fusion (F) protein and the other expressing the attachment glycoprotein (G) of RSV strain A2 (RSV A2). The vaccine strains were used separately for single-dose vaccinations in BALB/c mice. The results showed that both vaccines induced RSV antigen-specific antibody responses, with IgG2a/IgG1 ratios similar to those seen in wild-type RSV A2 infection. After challenging the vaccinated mice with RSV A2, histopathology of lung sections showed that the vaccines did not exacerbate lung lesions relative to RSV A2-immunized mice. Importantly, both F and G vaccines induced protective immunity. Therefore, PIV5 presents an attractive platform for vector-based vaccines against RSV infection.
INTRODUCTION
Respiratory syncytial virus is the most important cause of pediatric respiratory virus infection, and is a major cause of morbidity and mortality among infants, immune compromised individuals, and the elderly [1]. In the early 1960s, vaccination of infants with a formalin-inactivated RSV vaccine not only failed to protect against RSV disease during the following RSV season, but some vaccinees developed enhanced disease upon natural infection, resulting in increased rates of severe pneumonia and two deaths [2]. In the intervening years, a number of different approaches have been evaluated, including subunit vaccines, vectored vaccines, and live attenuated vaccines. However, there remains no licensed RSV vaccine. Therefore, there is a pressing need for a safe and effective vaccine for RSV.
Parainfluenza virus 5 (PIV5), a negative-sense, non-segmented, single-stranded RNA virus, is a good viral vector for vaccine development. PIV5 is safe, as it infects a large number of mammals without being associated with any disease except canine kennel cough [3–7]. Humans have been exposed to PIV5 [8–10], likely due to the wide use of kennel cough vaccines containing live PIV5, which dogs can shed after vaccination [11]. Given anti-PIV5 immunity in humans, anti-vector immunity may be a problem. Our recent studies indicate that pre-existing immunity to PIV5 does not negatively affect immunogenicity of a PIV5-based vaccine in dogs, demonstrating that pre-existing immunity is not a concern for using PIV5 as a vector [12]. This result is consistent with the report that neutralizing antibodies against PIV5 do not prevent PIV5 infection in mice [13].
PIV5 has been used as a platform for developing vector-based vaccines against other viruses. A single-dose immunization of PIV5 expressing the rabies virus glycoprotein G protects mice against lethal rabies virus challenge [14]. Additionally, a single-dose inoculation of PIV5 expressing hemagglutinin (HA) or the NP protein of influenza virus protects against lethal H5N1 challenge in mice [15, 16]. Importantly, intranasal administration of PIV5 is effective for eliciting robust mucosal immune responses [17], and is therefore ideal for vaccinating against respiratory pathogens.
Since an anti-RSV-F monoclonal antibody has been used to control RSV infection, it may be possible to develop an RSV vaccine by targeting RSV-F. Although several studies have implicated the G protein in RSV disease pathogenesis [18–21], prophylactic or therapeutic treatment with a monoclonal antibody (mAb 131-2G) specific to RSV-G mediates virus clearance and decreases leukocyte trafficking and IFN-production in the lungs of RSV-infected mice [22–26]. In this study, we have tested the efficacies of recombinant PIV5 expressing RSV-F (rPIV5-RSV-F) or RSV-G (rPIV5-RSV-G) as potential vaccines in mice.
MATERIALS AND METHODS
Cells and viruses
BSR-T7 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 10% tryptose phosphate broth (TPB), 100 IU/mL penicillin, 100 μg/mL streptomycin (1% P/S; Mediatech Inc., Manassas, VA), and 400 μg/mL G418 sulfate (Mediatech, Inc.). MDBK, BHK21, and Vero cells were maintained in the same media without TPB or G418.
To construct the plasmids for rescuing rPIV5-RSV-F or rPIV5-RSV-G, the coding sequence of the green fluorescent protein (GFP) gene in the BH311 plasmid [27], containing GFP between HN and L of the full-length PIV5 genome, was replaced with the RSV-F or RSV-G gene, respectively. rPIV5-RSV-F and rPIV5-RSV-G were rescued as described previously [27]. PIV5, rPIV5-RSV-F and rPIV5-RSV-G were grown in in MDBK cells described previously [27]. RSV A2 and rA2-Luc (RSV A2 expressing Renilla luciferase) were grown in Vero cells as previously described [28].
Immunoprecipitation and Western Blots
Immunoprecipitation (IP) was performed as previously described [27]. A549 cells were infected with rPIV5-RSV-F or RSV A2 in 6-cm dishes. After 18 to 20 hours, the cells were starved and metabolically labeled with 35S-Met and 35S-Cys for 3 hours. The cells were lysed with whole-cell extraction buffer (WCEB; 50 mM Tris-HCl [pH 8], 280 mM NaCl, 0.5% NP-40, 0.2 mM EDTA, 2 mM EGTA, 10% glycerol) [29] and immunoprecipitated with anti-RSV-F antibody. The IP products were resolved on a 10% SDS-PAGE gel and visualized using a Typhoon 9700 Phosphorimager (GE Healthcare Life Sciences, Piscataway, NJ).
To examine RSV-G protein expression, rPIV5-RSV-G-infected MDBK cells and RSV A2-infected A549 cells were lysed with WCEB. The lysates were processed and resolved by SDS-PAGE as described before. The proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane and detected using mouse anti-RSV-G antibody (1:2,000 dilution) as previously described [14].
Recombinant PIV5 Growth Curves and Plaque Assays
6-well plates of Vero cells were infected with rPIV5-RSV-F, rPIV5-RSV-G, or PIV5 at a MOI = 5 or 0.01. 100 μL samples of supernatant were collected at 0, 24, 48, 72, 96, and 120 hours post-infection. Virus was quantified by plaque assay as described in Chen et al. [14].
Immunization of Mice
All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the University of Georgia. Six-to-eight week-old female BALB/c mice (Harlan Laboratories, Indianapolis, IN) were anesthetized by intraperitoneal injection of 200 μL of 2, 2, 2-tribromoethanol in tert-amyl alcohol (Avertin). Immunization was performed by intranasal administration of 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2 in a 50 μL volume. Negative controls were treated intranasally with 50 μL of PBS.
Three weeks post-immunization, blood was collected via the tail vein for serological analysis. Four weeks post-immunization, all mice were challenged intranasally with 106 PFU of RSV A2 in a 50 μL volume. Four days later, lungs were collected from 5 mice per group to assess viral burden. The lungs of the other 5 mice in each group were perfused with 10% formalin solution and sent for histology. To detect neutralizing antibody titers, mice were immunized as described above and terminally bled 4 weeks post-immunization.
ELISAs measuring total RSV antigen-specific IgG, IgG1 and IgG2a
RSV-F and RSV-G-specific serum antibody titers were measured by ELISA. Immulon® 2HB 96-well microtiter plates were coated with 100 μL of purified RSV-F or G protein at 1 μg/ml in PBS [28] and incubated overnight at 4°C. Two-fold serial dilutions of serum were made in blocking buffer (5% nonfat dry milk, 0.5% BSA in wash buffer; KPL, Inc., Gaithersburg, MD). 100 μL of each dilution was transferred to the plates and incubated for one hour at room temperature. After aspirating the samples, the plates were washed three times with wash buffer. Secondary antibody was diluted 1:1,000 [alkaline phosphatase-labeled goat anti-mouse IgG (KPL, Inc.) or horseradish-peroxidase-labeled goat anti-IgG1 or IgG2a (SouthernBiotech, Birmingham, AL)] in blocking buffer. 100 μL of diluted secondary antibody was added to each well, and the plates were incubated for one hour at room temperature. After aspiration, the plates were washed and developed with 100 μL of pNpp substrate or SureBlue Reserve TMB substrate (KPL, Inc.) at room temperature. The OD was read at 405 nm or 450 nm using a BioTek Epoch microplate reader. The endpoint antibody titer was defined as the highest serum dilution at which the OD was greater than two standard deviations above the mean OD of the naïve serum.
Neutralizing Antibody Assay
Two-fold serial dilutions of serum were made starting at a 1:10 dilution with Opti-MEM supplemented with 1% BSA and 5% guinea pig complement (Sigma-Aldrich, St. Louis, MO). The diluted serum was incubated with 100 TCID50 of RSV A2 expressing Renilla luciferase (rA2-Rluc) for one hour at 37°C, 5% CO2 [30]. The serum and virus mixture was transferred to confluent monolayers of Vero cells in 96-well plates and incubated for 18 hours at 37°C, 5% CO2. The cells were then lysed with 70 μL/well of Renilla lysis buffer for 20 minutes while shaking on an orbital shaker. The lysates were transferred to V-bottom plates and clarified by centrifugation at 2000 × g for 5 minutes. 40 μL of clarified lysate was transferred to Costar® white 96-well assay plates (Corning, Inc., Corning, NY) and read using a GloMax® 96 microplate luminometer (Promega). Neutralizing antibody titers were reported as the highest serum dilution at which the luminescence measurement was lower than that of 50 TCID50 of rA2-Rluc based on a standard curve. Cells treated with 100 TCID50 of UV-inactivated rA2-Luc were the negative control.
Titration of RSV from mouse lungs
Mouse lungs were harvested aseptically into gentleMACS M tubes (Miltenyi Biotec Inc., Auburn, CA) containing 3 mL of Opti-MEM with 1% BSA and stored on ice. Lungs were homogenized at 4°C using the Protein_01 program of a gentleMACS Dissociator (Miltenyi Biotec Inc.) and then centrifuged at 3000 × g for 10 minutes. RSV titers in the supernatants were determined using plaque assay as described in Johnson et al., except the media was 0.8% methylcellulose in Opti-MEM with 2% FBS, 1% P/S [31].
Histology
Four days post-challenge, the lungs from the mice were perfused with 1 mL of 10% formalin and then immersed in 10% formalin for at least 24 hours. The formalin-fixed lungs were transferred to 70% ethanol, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin. A pathologist scored the sections in a group-blind fashion for perivascular cuffing, interstitial pneumonia, bronchiolitis, alveolitis, vasculitis and pleuritis. The lesions were scored on a scale of 0 to 4, with 0 indicating no lesions and 4 indicating severe lesions.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism software version 5.04 for Windows (Graphpad Software, La Jolla, CA). Analysis of variance (ANOVA) and Tukey multiple comparison tests were used to analyze total serum IgG, IgG1 or IgG2a antibody titers and lung viral loads. Unpaired, two-tailed t-test was used to analyze neutralizing antibody titers. Histology data was analyzed using the Kruskal-Wallis test.
RESULTS
Construction and characterization of recombinant PIV5-based RSV vaccine viruses
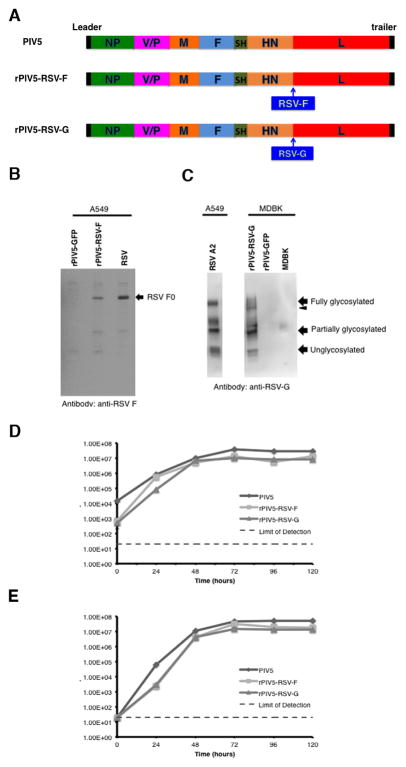
RSV-F and RSV-G genes from RSV A2 were cloned into a plasmid containing the PIV5 backbone. The genes were inserted in between the HN and L genes, and the viruses were rescued by methods previously described (Fig. 1A) [32]. RSV-F expression in rPIV5-RSV-F-infected cells was confirmed by immunoprecipitation with an RSV-F-specific monoclonal antibody (Fig. 1B). Expression of RSV-G in rPIV5-RSV-G-infected cells was shown by Western blot using an RSV-G-specific monoclonal antibody (Fig. 1C). RSV-G expressed in rPIV5-RSV-G-infected cells displayed both wild-type size and glycosylation pattern. RSV-F and RSV-G were detected in rPIV5-RSV-F and rPIV5-G virions respectively (data not shown).
Figure 1. Generation of rPIV5-RSV-F and rPIV5-RSV-G.
(A) Schematic of rPIV5-RSV-F and rPIV5-RSV-G. NP, nucleoprotein; V, V protein; P, phosphoprotein; M, matrix protein; F, fusion protein; SH, small hydrophobic protein; HN, hemagglutinin-neuraminidase protein; L, RNA-dependent RNA polymerase; RSV-F, respiratory syncytial virus fusion protein; RSV-G, respiratory syncytial virus G attachment glycoprotein. (B) RSV-F expression in rPIV5-RSV-F-infected A549 cells was detected by immunoprecipitation. rPIV5-RSV-F-infected cells were starved and labeled with 35S-Met and 35S-Cys at 18 to 20 hours post-infection. Proteins from cell lysate were immunoprecipitated with mouse anti-RSV-F antibody, and expression was visualized by 35S incorporation. Lysate from RSV A2-infected A549 cells was the positive control. Lysate from rPIV5-GFP-infected A549 cells was the negative control. (C) Detection of RSV-G expression by Western blot. MDBK cells were infected with rPIV5-RSV-G and lysate was immunoblotted with mouse anti-RSV-G antibody. Lysate from RSV A2-infected A549 cells was the positive control. Lysate from rPIV5-GFP-infected MDBK cells was the negative control. (D) Single-cycle growth curves of rPIV5-RSV-F and rPIV5-RSV-G in Vero cells. Vero cells were infected at a MOI = 5. Aliquots of cell culture supernatant were collected at 24-hour intervals for 120 hours. (E) Multi-cycle growth curves of rPIV5-RSV-F and rPIV5-RSV-G in Vero cells. Vero cells were infected at a MOI = 0.01. Aliquots of cell culture supernatant were collected at 24-hour intervals for 120 hours. Plaque assays were performed in BHK21 cells to determine the virus titer at each time point. Growth curve samples were assayed in duplicate. Error bars represent the standard error of the mean.
Single-step and multi-step growth rates of rPIV5-RSV-F, rPIV5-RSV-G and PIV5 were compared. In the single-step growth curve, both rPIV5-RSV-F and rPIV5-RSV-G displayed slightly delayed growth kinetics at 24 hours compared to PIV5, and grew to similar, though slightly decreased, titers by 48 hours (Fig. 1D). This growth delay was also evident in the multi-step growth curve at 24 hours, but both the rPIV5-RSV-F and rPIV5-RSV-G grew to titers similar to PIV5 by 48 hours (Fig. 1E). Therefore, growth kinetics of the rPIV5-RSV-F and rPIV5-RSV-G were similar to that of PIV5, although with a slight delay at early time points and a slight decrease in final viral titer.
Immunization with recombinant PIV5-based RSV vaccine viruses induces RSV antigen-specific antibody responses
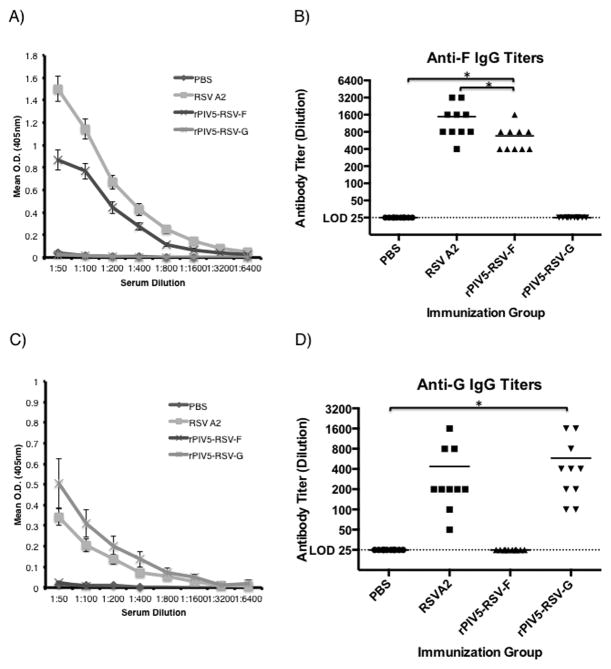
Total serum IgG antibody titers to RSV were measured 21 days post-vaccination. Mice immunized with rPIV5-RSV-F developed F-specific serum IgG antibodies, although to a lesser degree (~ 2-fold lower) than RSV A2-immunized mice (Fig. 2A and 2B). Interestingly, mice vaccinated with rPIV5-RSV-G developed G-specific antibody titers slightly higher (~ 2-fold) than those seen in mice immunized with RSV A2 (Fig. 2C and 2D). Mice treated with PBS had no detectable RSV-specific antibodies (Fig. 2A–D).
Figure 2. Immunization with rPIV5-RSV-F and rPIV5-RSV-G induces RSV antigen-specific IgG antibody responses in mice.
Naïve BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. Sera were collected at 3 weeks post-immunization. (A) Anti-RSV-F IgG antibody curves were generated by ELISA using serially diluted sera. (B) RSV-F-specific IgG antibody endpoint titers were determined based on the values in (A). (C) Anti-RSV-G IgG antibody curves were generated by ELISA. (D) RSV-G-specific IgG antibody endpoint titers were determined based on the values in (C). Serum IgG was detected in (A) and (C) using goat anti-mouse IgG antibody conjugated to alkaline phosphatase. Each group consisted of 10 mice. Error bars in (A) and (C) represent standard error of the mean. Horizontal lines in (B and D) represent the mean antibody titer of each group. Statistical significance was determined by ANOVA and Tukey multiple comparison tests. *, P < 0.05. LOD, limit of detection.
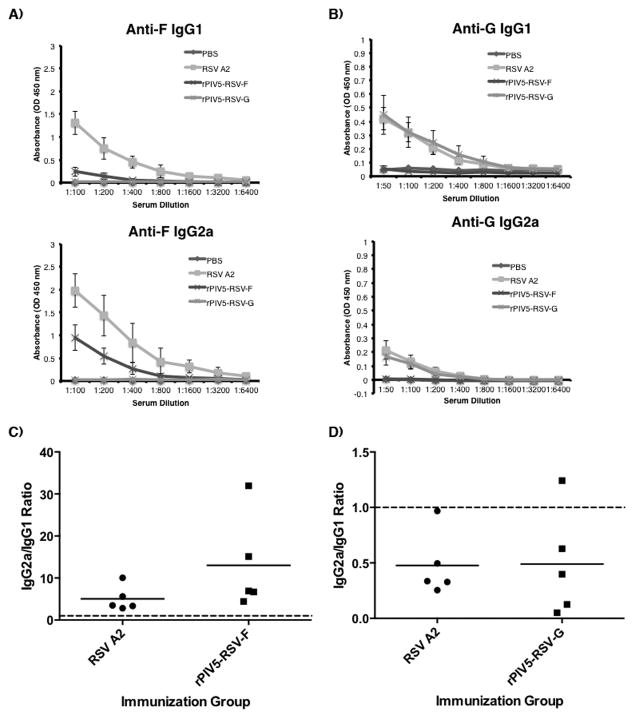
Immunization with the recombinant vaccine viruses induced RSV antigen-specific IgG2a/IgG1 antibody ratios similar to those observed in RSV A2-immunized mice. Overall, RSV-F-specific IgG1 and IgG2a titers were lower in rPIV5-RSV-F-immunized mice compared to the RSV A2-immunized mice (Fig. 3A). RSV-G-specific IgG1 and IgG2a titers in rPIV5-RSV-G and RSV A2-immunized mice were similar (Fig. 3B). Mean RSV-F-specific IgG2a/IgG1 ratios in rPIV5-RSV-F and RSV A2-vaccinated groups were 13 and 5, respectively, with no significant difference between the two groups (Fig. 3C). Mean RSV-G-specific IgG2a/IgG1 ratios of groups vaccinated with rPIV5-RSV-G or RSV A2 were 0.49 and 0.48, respectively (Fig. 3D). The IgG2a/IgG1 ratios induced by the rPIV5 vaccine candidates did not differ significantly from those observed in RSV A2 infection, which is known to generate balanced IgG2a/IgG1 responses.
Figure 3. Immunization with rPIV5-RSV-F and rPIV5-RSV-G induce IgG1 and IgG2a antibody responses similar to RSV A2 infection.
Naïve BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. Sera were collected 3 weeks post-immunization. (A, B) RSV-F (A) and RSV-G-specific (B) IgG1 and IgG2a antibody curves were generated by ELISA using isotype-specific secondary antibodies conjugated to HRP. (C, D) RSV-F (C) and RSV-G-specific (D) IgG2a/IgG1 ratios were calculated using values in (A) and (B), respectively. IgG1 and IgG2a antibodies in the sera were quantified using standard curves to generate IgG2a/IgG1 ratios. Each group consisted of 5 mice. Solid horizontal lines represent the mean of each group. Dotted horizontal lines indicate a ratio of 1.
Immunization with rPIV5-RSV-F generates RSV-neutralizing antibodies
A complement-enhanced microneutralization assay was performed to determine if serum antibodies induced by immunization were able to neutralize RSV A2 expressing Renilla luciferase (rA2-Rluc) in vitro. By 28 days post-immunization, mice immunized with rPIV5-RSV-F or RSV A2 generated neutralizing antibodies against rA2-Rluc. The antibody titers of the rPIV5-RSV-F-immunized group did not differ significantly from the titers of the RSV A2-immunized group. No neutralizing activity was detected in the sera of rPIV5-RSV-G-immunized mice (Fig. 4).
Figure 4. Immunization with rPIV5-RSV-F generates RSV-neutralizing antibodies.
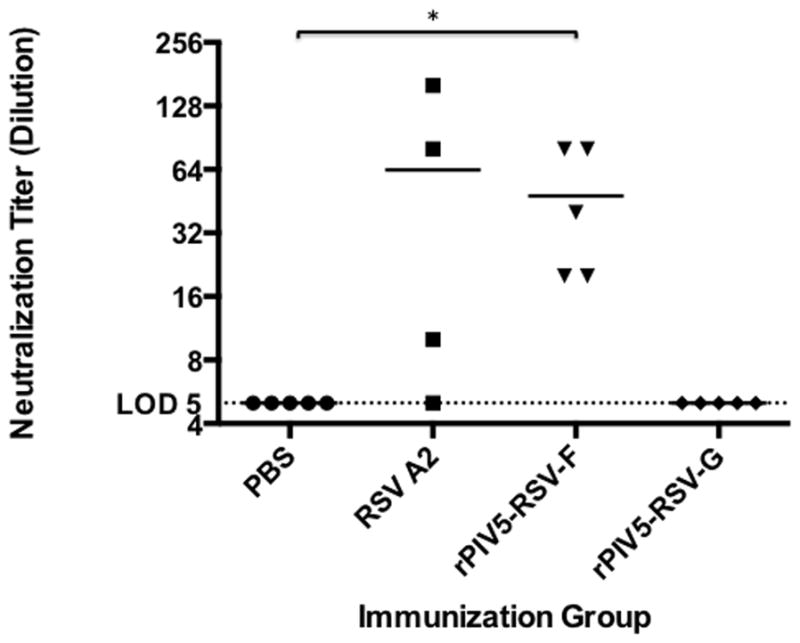
Naïve BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. At 4 weeks post-immunization, sera were collected and tested for RSV-neutralizing activity using a microneutralization assay. Neutralizing antibody titers were defined as the highest dilution of sera at which the luminescence measurement of the sample was less than that of 50 TCID50 of rA2-Rluc using a standard curve. Each group consisted of 4 to 5 mice. All samples were assayed in duplicate. Horizontal lines represent the mean antibody titer of each group. Statistical significance was determined by unpaired, two-tailed t-test. *, P < 0.05. LOD, limit of detection.
Recombinant PIV5-based RSV vaccine viruses induce protective immunity in the mouse lung
Four days post-challenge, RSV A2 titers were measured in the lungs to assess the efficacy of the recombinant vaccine viruses in reducing viral burden. Mice vaccinated with either rPIV5-RSV-F or rPIV5-RSV-G had no detectable challenge virus in the lungs. In the RSV A2-immunized group, one mouse had a viral titer of 90 PFU/lung, while all other mice in the group had no detectable virus. Mice with PBS had an average viral titer of 4.5×103 PFU/lung (Fig. 5). Therefore, immunization with the vaccine candidates induced potent immunity against RSV A2 challenge.
Figure 5. Immunization with rPIV5-RSV-F and rPIV5-RSV-G generates protective immunity against RSV A2 challenge.
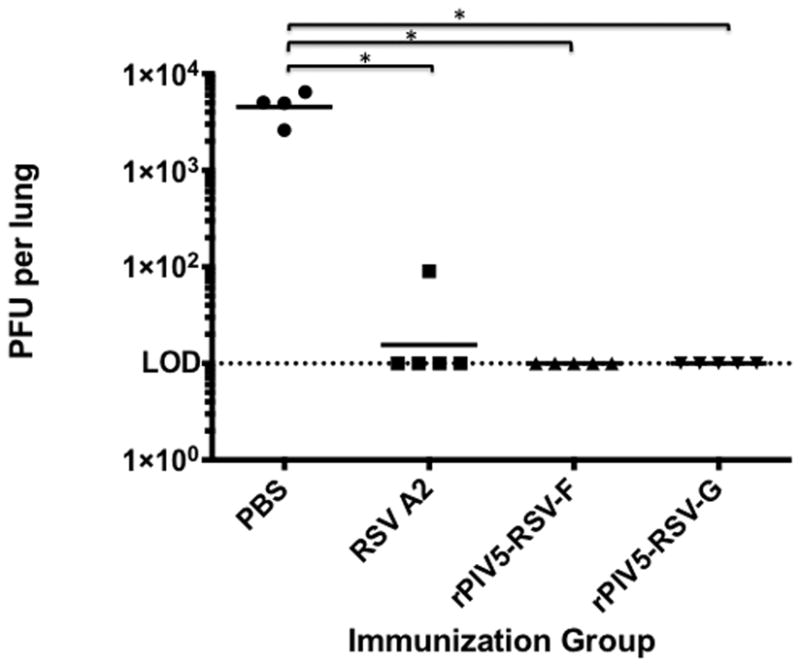
Naïve BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. At 4 weeks post-immunization, mice were challenged with 106 PFU of RSV A2. At 4 days post-challenge, lungs were harvested and viral load was determined by plaque assay in Vero cells. Each group consisted of 5 mice. Solid horizontal lines represent the geometric mean of each group. Statistical significance was determined by ANOVA and Tukey multiple comparison tests. *, P < 0.05. LOD, limit of detection.
Mice immunized with recombinant PIV5-based RSV vaccine viruses or RSV A2 display similar lung lesions
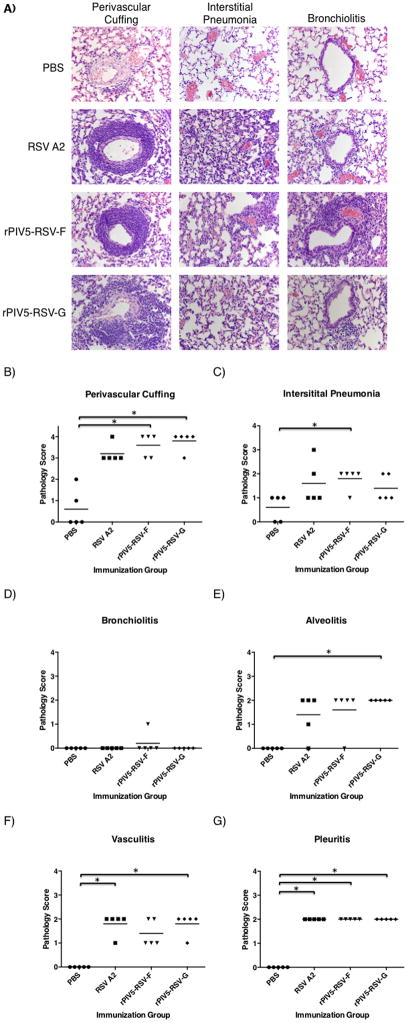
Lung histology was performed to determine if immunization with the recombinant vaccine viruses affected RSV-induced lung pathology. At low magnification, tissue from mice vaccinated with RSV A2 or the rPIV5 viruses showed similar levels of inflammatory infiltrates 4 days post-challenge. Lung tissue from the mock-vaccinated mice was the least inflamed (Fig. 6A–D), suggesting that vaccinated animals had likely generated immune responses to RSV challenge.
Figure 6. Low-magnification images of post-challenge lung lesions in mice treated with PBS or immunized with RSV A2, rPIV5-RSV-F or rPIV5-RSV-G.
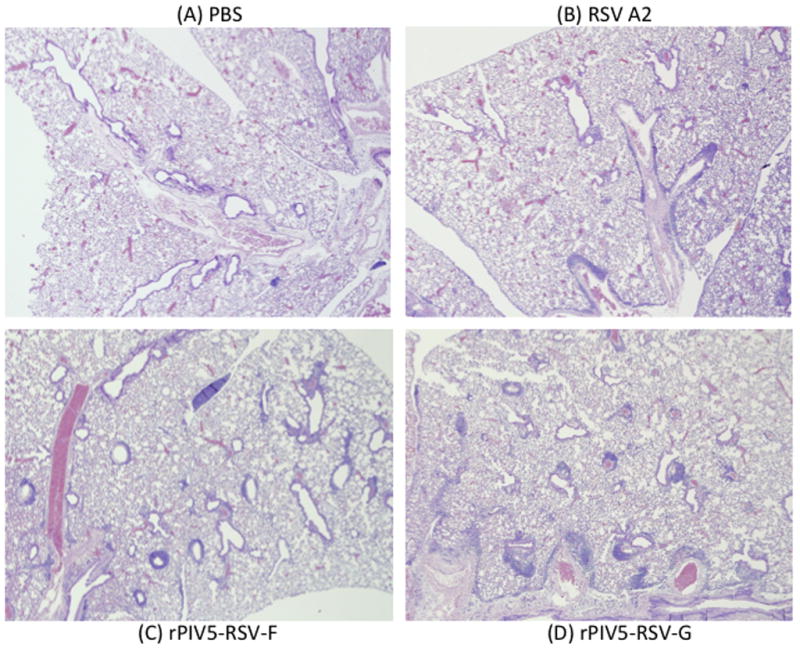
BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. Four weeks after immunization, mice were challenged with 106 PFU of RSV A2. At 4 days post-challenge, lungs were harvested and fixed with 10% formalin. Lung sections were stained with hemotoxylin and eosin. Images were taken at 4x magnification. (A) Lung section from a mouse treated with PBS. (B) Lung section from a RSV A2-immunized mouse. (C) Lung section from a rPIV5-RSV-F-immunized mouse. (D) Lung section from a rPIV5-RSV-G-immunized mouse.
At high magnification, the inflammation in the mice vaccinated with RSV A2 or the recombinant vaccine viruses was characterized most prominently by perivascular cuffing (Fig. 7A and 7B). The leukocytes surrounding the pulmonary blood vessels consisted of mostly lymphocytes and macrophages, with few neutrophils and eosinophils. Mild-to-moderate interstitial pneumonia (Fig. 7A and 9C) and little-to-no bronchiolitis (Fig. 7A and 7D) was observed in all groups. Tissue sections were also scored for alveolitis, pleuritis, and vasculitis (Fig. 7E–G). There were no significant differences in the histopathology scores of mice vaccinated with the recombinant vaccine viruses relative to the RSV A2-vaccinated controls.
Figure 7. High-magnification images of post-challenge lung lesions in mice treated with PBS or immunized RSV A2, rPIV5-RSV-F or rPIV5-RSV-G.
BALB/c mice were treated with PBS or immunized intranasally with 106 PFU of rPIV5-RSV-F, rPIV5-RSV-G, or RSV A2. Four weeks after immunization, mice were challenged with 106 PFU of RSV A2. Four days after challenge, lungs were harvested and fixed with 10% formalin. Lung sections were stained with hemotoxylin and eosin. (A) Images in the far-left column show perivascular cuffing. Images in the middle column show interstitial pneumonia. Images in the far-right column show bronchiolitis. Each image is from a representative mouse in each group. Images of lung lesions were taken at 40x magnification. (B–G) Lung sections were scored in a group-blind fashion on a scale of 0 to 4 for perivascular cuffing (B), intersititial pneumonia (C), bronchiolitis (D), alveolitis (E), vasculitis (F), and pleuritis (G). Each group consisted of 5 mice. Horizontal lines represent the mean of each group. Statistical significance was determined using the Kruskal-Wallis test. *, P < 0.05.
DISCUSSION
The most advanced area of investigation for RSV vaccine candidates is live attenuated viruses. These viruses have several benefits: 1) enhanced RSV disease has not been observed either after natural infection or vaccination with live attenuated viruses [33–35]; 2) live attenuated RSV vaccines induce balanced immune responses that more closely match natural immunity compared with subunit or inactivated vaccines [36, 37]; 3) intranasal vaccination with live attenuated viruses should induce better local immunity compared with intramuscular injection of subunit vaccines. Live attenuated RSV vaccines have been in development for several decades, using a combination of cold passage (cp) and chemical mutagenesis to induce temperature sensitivity (ts). A number of cpts RSV vaccine candidates have been tested clinically. The cpts 248/404 candidate was sufficiently attenuated in adults and sero-negative children and tested in 1 to 3-month-old infants. However, cpts 248/404 caused nasal congestion in these infants, an unacceptable adverse effect [33]. More recently, the advent of reverse genetics systems for RSV, whereby infectious virus is derived from cDNAs, has allowed the development of live attenuated RSV vaccine candidates encoding specific combinations of attenuating mutations and deletions of nonessential viral genes. RSV lacking NS2 (rA2ΔNS2) was tested in clinical trials as a vaccine for the elderly since it was less attenuated in chimpanzees than cpts 248/404. It was shown to be over-attenuated in adults but under-attenuated in children, a contraindication for testing in infants [38]. Subunit and other synthetic vaccines have shown only moderate immunogenicity in clinical trials, even with the development of newer adjuvant regimens. Vectored vaccines expressing RSV F and/or G have been generated based on paramyxoviruses such as Sendai virus (SeV), Newcastle disease virus (NDV), and a chimeric recombinant bovine parainfluenza virus 3 (PIV3) expressing human PIV3 F/HN and RSV-F (MEDI-534). Sendai virus expressing RSV-F or G protected the lower respiratory tract (LRT) of cotton rats against RSV infection [39, 40]. SeV-RSV-F also conferred LRT protection in African green monkeys [41]. Immunization of mice with NDV expressing RSV-F was only modestly effective, reducing RSV burden in lungs by approximately 1 log10 [42]. MEDI-534 was attenuated and safe in clinical trials, but it was only minimally immunogenic in adults and children [43]. Furthermore, the vaccine candidate genome was unstable, with mutations observed in vivo and in vitro [44, 45]. Thus, while many RSV vaccine candidates have been researched extensively, an important public health gap remains for RSV disease prevention.
This work demonstrated that PIV5-based RSV vaccine candidates provide a promising alternative for RSV vaccine development. Single-dose immunization with rPIV5-RSV-F or rPIV5-RSV-G induced potent immunity against RSV challenge in mice. Importantly, the recombinant vaccine viruses did not exacerbate lung lesions relative to the RSV A2-immunized controls. Natural infection with RSV does not lead to enhanced disease upon reinfection, in contrast to immunization with formalin-inactivated RSV [46]. Inflammation in the lung tissue of mice immunized with the vaccine candidates was likely due to the induction of host immunity in response to RSV challenge.
Serum neutralizing antibodies were generated in rPIV5-RSV-F-immunized mice, suggesting that the vaccine candidate induces a functional, systemic humoral response against RSV. Immunization with rPIV5-RSV-G did not generate neutralizing antibodies, but reduced viral burden in the lungs. The mechanism is unclear, but rPIV5-RSV-G immunization may generate protective antibodies that are non-neutralizing in vitro. In the case of the RSV-G subunit vaccine candidate, BBG2Na, passively transferred serum from immunized mice reduced lung viral burden in recipient mice at dilutions negative for neutralizing activity [47]. Other groups have also shown that neutralizing activities of RSV-G or RSV-F-specific antibodies in vitro do not necessarily correlate with lung protection in vivo [48–50].
In summary, PIV5 is safe, stable, efficacious, cost-effective to produce, and overcomes pre-existing anti-vector immunity. In this work, we have shown that PIV5-based RSV vaccine candidates have the potential to be an effective RSV vaccine, providing an additional option for RSV vaccine development.
Research Highlights.
We generated a recombinant PIV5 expressing RSV F and G (rPIV5-RSV-F and rPIV5-RSV-G).
rPIV5-RSV-F and rPIV5-RSV-G generated balanced immune responses in mice.
rPIV5-RSV-F and rPIV5-RSV-G protected mice against RSV challenge.
Acknowledgments
We appreciate the helpful discussion and technical assistance from all members of Biao He’s laboratory. This work was partially supported by grants from the National Institute of Allergy and Infectious Disease (R01AI070847) to B.H. and (R01AI081977) to M.N.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 1443–85. [Google Scholar]
- 2.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 3.Binn LN, Eddy GA, Lazar EC, Helms J, Murnane T. Viruses recovered from laboratory dogs with respiratory disease. Proc Soc Exp Biol Med. 1967;126:140–5. doi: 10.3181/00379727-126-32386. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg FJ, Lief FS, Todd JD, Reif JS. Studies of canine respiratory viruses. I. Experimental infection of dogs with an SV5-like canine parainfluenza agent. Am J Epidemiol. 1971;94:147–65. doi: 10.1093/oxfordjournals.aje.a121307. [DOI] [PubMed] [Google Scholar]
- 5.Cornwell HJ, McCandlish IA, Thompson H, Laird HM, Wright NG. Isolation of parainfluenza virus SV5 from dogs with respiratory disease. Vet Rec. 1976;98:301–2. doi: 10.1136/vr.98.15.301. [DOI] [PubMed] [Google Scholar]
- 6.McCandlish IA, Thompson H, Cornwell HJ, Wright NG. A study of dogs with kennel cough. Vet Rec. 1978;102:293–301. doi: 10.1136/vr.102.14.293. [DOI] [PubMed] [Google Scholar]
- 7.Azetaka M, Konishi S. Kennel cough complex: confirmation and analysis of the outbreak in Japan. Nippon Juigaku Zasshi. 1988;50:851–8. doi: 10.1292/jvms1939.50.851. [DOI] [PubMed] [Google Scholar]
- 8.Goswami KKA, Lange LS, Mitchell DN, Cameron KR, Russell WC. Does simian virus 5 infect humans. J Gen Virol. 1984;65:1295–303. doi: 10.1099/0022-1317-65-8-1295. [DOI] [PubMed] [Google Scholar]
- 9.Goswami KK, Randall RE, Lange LS, Russell WC. Antibodies against the paramyxovirus SV5 in the cerebrospinal fluids of some multiple sclerosis patients. Nature. 1987;327:244–7. doi: 10.1038/327244a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Xu P, Salyards GW, Harvey SB, Rada B, Fu ZF, et al. Evaluating a Parainfluenza Virus 5-Based Vaccine in a Host with Pre-Existing Immunity against Parainfluenza Virus 5. PLoS One. 2012;7:e50144. doi: 10.1371/journal.pone.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chladek DW, Williams JM, Gerber DL, Harris LL, Murdock FM. Canine parainfluenza-Bordetella bronchiseptica vaccine immunogenicity. Am J Vet Res. 1981;42:266–70. [PubMed] [Google Scholar]
- 12.Chen Z, Xu P, Salyards GW, Harvey SB, Rada B, Fu ZF, et al. Evaluating a PIV5-based vaccine in a host with pre-existing immunity against PIV5. PLoS One. 2012 doi: 10.1371/journal.pone.0050144. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young DF, Randall RE, Hoyle JA, Souberbielle BE. Clearance of a persistent paramyxovirus infection is mediated by cellular immune responses but not by serum-neutralizing antibody. J Virol. 1990;64:5403–11. doi: 10.1128/jvi.64.11.5403-5411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Zhou M, Gao X, Zhang G, Ren G, Gnanadurai CW, et al. A novel rabies vaccine based on a recombinant parainfluenza virus 5 expressing rabies virus glycoprotein. Journal of virology. 2012 doi: 10.1128/JVI.02886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Mooney AJ, Gabbard JD, Gao X, Xu P, Place RJ, et al. Recombinant Parainfluenza Virus 5 Expressing Hemagglutinin of Influenza A Virus H5N1 Protected Mice against Lethal Highly Pathogenic Avian Influenza Virus H5N1 Challenge. Journal of virology. 2013;87:354–62. doi: 10.1128/JVI.02321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Gabbard JD, Mooney A, Gao X, Chen Z, Place RJ, et al. SIngle Dose Vaccination of A Recombinant Parainfluenza Virus 5 Expressing NP from H5N1 Provides Broad Immunity Against Influenza A VIruses. Journal of Virology. 2013:87. doi: 10.1128/JVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooney A, Li Z, Gabbard JD, He B, Tompkins SM. Recombinant PIV5 vaccine encoding the influenza hemagglutinin protects against H5N1 highly pathogenic avian influenza virus infection when delivered intranasally or intramuscularly. J Virol. 2012 doi: 10.1128/JVI.02330-12. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus research. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunologic research. 2007;39:225–39. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz JL. Respiratory syncytial virus vaccine development. Expert review of vaccines. 2011;10:1415–33. doi: 10.1586/erv.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nature immunology. 2001;2:732–8. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, Tripp RA. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral immunology. 2012;25:193–203. doi: 10.1089/vim.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, et al. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. Journal of virology. 2010;84:1148–57. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harcourt JL, Karron RA, Tripp RA. Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C-CX3CR1 binding and leukocyte chemotaxis. The Journal of infectious diseases. 2004;190:1936–40. doi: 10.1086/425516. [DOI] [PubMed] [Google Scholar]
- 25.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. The Journal of infectious diseases. 2009;200:439–47. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 26.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. Journal of virology. 2010;84:9632–6. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He B, Paterson RG, Ward CD, Lamb RA. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–60. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 28.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nature immunology. 2001;2:732–8. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 29.Ulane CM, Horvath CM. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology. 2002;304:160–6. doi: 10.1006/viro.2002.1773. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes S, Crim RL, Beeler J, Teng MN, Golding H, Khurana S. Development of a simple, rapid, sensitive, high-throughput luciferase reporter based microneutralization test for measurement of virus neutralizing antibodies following Respiratory Syncytial Virus vaccination and infection. Vaccine. 2013;31:3987–94. doi: 10.1016/j.vaccine.2013.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. Journal of Virology. 1998;72:2871–80. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun D, Luthra P, Li Z, He B. PLK1 down-regulates parainfluenza virus 5 gene expression. PLoS Pathog. 2009;5:e1000525. doi: 10.1371/journal.ppat.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 34.Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–6. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright PF, Shinozaki T, Fleet W, Sell SH, Thompson J, Karzon DT. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. J Pediatr. 1976;88:931–6. doi: 10.1016/s0022-3476(76)81044-x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PR, Jr, Feldman S, Thompson JM, Mahoney JD, Wright PF. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. J Med Virol. 1985;17:325–35. doi: 10.1002/jmv.1890170405. [DOI] [PubMed] [Google Scholar]
- 37.Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986;154:121–7. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- 38.Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, et al. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis. 2006;193:573–81. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 39.Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78:6043–7. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–93. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, et al. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30:959–68. doi: 10.1016/j.vaccine.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. Journal of Virology. 2006;80:1130–9. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, et al. Development of a PIV-vectored RSV vaccine: preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine. 2008;26:6373–82. doi: 10.1016/j.vaccine.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Nelson CL, Tang RS, Stillman EA. Genetic stability of RSV-F expression and the restricted growth phenotype of a live attenuated PIV3 vectored RSV vaccine candidate (MEDI-534) following restrictive growth in human lung cells. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Yang CF, Wang CK, Malkin E, Schickli JH, Shambaugh C, Zuo F, et al. Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in Phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine. 2013;31:2822–7. doi: 10.1016/j.vaccine.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. Journal of Virology. 2008;82:2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power UF, Plotnicky-Gilquin H, Huss T, Robert A, Trudel M, Stahl S, et al. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology. 1997;230:155–66. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 48.Taylor G, Stott EJ, Bew M, Fernie BF, Cote PJ, Collins AP, et al. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52:137–42. [PMC free article] [PubMed] [Google Scholar]
- 49.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–57. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 50.Routledge EG, Willcocks MM, Samson AC, Morgan L, Scott R, Anderson JJ, et al. The purification of four respiratory syncytial virus proteins and their evaluation as protective agents against experimental infection in BALB/c mice. The Journal of general virology. 1988;69 (Pt 2):293–303. doi: 10.1099/0022-1317-69-2-293. [DOI] [PubMed] [Google Scholar]