Abstract
Vesicovaginal fistula (VVF) is still a major cause for concern in many developing countries. It represents a significant morbidity in female urology. Continual wetness, odor, and discomfort cause serious social problems. The diagnosis of the condition has traditionally been based on clinical methods and dye testing. A successful repair of such fistulas requires an accurate diagnostic evaluation and timely repair using procedures that exploit basic surgical principles and the application of interposition flaps. The method of closure depends on the surgeon’s training and experience. The main complication of VVF surgery is recurrent fistula formation.
Keywords: VVF, Complications, Diagnosis, Surgical principles
Introduction
Vesicovaginal fistula (VVF) is an abnormal opening between the bladder and the vagina that results in continuous and unremitting urinary incontinence. The entity is one among the most distressing complications of gynecologic and obstetric procedures. The existence of VVF is believed to have been known to the physicians of ancient Egypt, with examples present in mummies before 2,000 years bc. The literature on the subject is extensive, but is largely based on anecdote, small retrospective case series and opinion rather than on fact.
Incidence
Although the incidence of VVFs has become rare in the industrialized world, they still commonly occur in developing countries [1]. Estimates suggest that at least three million women in poor countries have unrepaired VVFs, and that 30,000–130,000 new cases develop each year in Africa alone [2]. The general public and the world medical community remain largely unaware of this problem. Ibrahim et al. [3] emphasized, as have others working in the largely Muslim culture of northern Nigeria, the high prevalence of early marriage and childbearing, the low literacy rate, and the poor uptake of conventional antenatal care among the fistula patients. Probably the most important factors contributing to the high incidence and prevalence of obstetric fistulas in Africa are socioeconomic [4]. Early marriage, low social status for women, malnutrition, and inadequately developed social and economic infrastructures are all more common in the poor areas. Most importantly, lack of access to emergency obstetric services is ubiquitous in the poor regions. In parts of the world where obstructed labor is a major contributor to maternal mortality, the rate of VVF might even approach the maternal death rate [5].
Classification
VVFs can be classified in various ways. Simple fistulas are usually small in size (≤0.5cm) and are present as single non-radiated fistulas. Complex fistulas include previously failed fistula repairs or large-sized (≥2.5 cm) fistulas, more often a result of chronic diseases or radiotherapy. Most authors consider intermediate-sized fistulas (between 0.5 and 2.5 cm) as complex ones.
Etiology
The etiology of VVF varies and may broadly be categorized into congenital or acquired, the latter being divided into obstetric, surgical, radiation, malignant, and miscellaneous causes. The most common cause of VVF is obstructed labor [6]. The fistula derived by obstructed labor is the product of a massive field injury caused by the impacted fetal head. In most of the third world countries, over 90 % of fistulas are of obstetric etiology [7]. Congenital VVFs are extremely rare and are associated with other urogenital malformations. In the industrialized world, the most common cause (>75 %) of VVF is injury to the bladder at the time of gynecologic, urologic, or other pelvic surgery. Surgical injury to the lower urinary tract most commonly occurs in the setting of hysterectomy, whereas most of the remainders are related to general surgery procedures in the pelvis, anterior colporrhaphy or cystocele repair, anti-incontinence surgery, or other urologic procedures [8].
Approximately 3–5 % of VVF in the industrialized world occur as a result of locally advanced malignancies with three most common forms such as cervical, vaginal, and endometrial carcinoma [9]. Radiation-induced fistulas occur frequently many years after treatment. Even though various predisposing factors in the formation of the postoperative fistula have been identified (infection, ischemia, arteriosclerosis, previous uterine surgery, uterine myomata, cancer treatments, and diabetes), the vast majority occur under “normal operative circumstances” [10].
Other causes of VVF include urologic or gynecologic instrumentation, including percutaneous procedure, retroperitoneal, vascular or pelvic surgery, infectious and inflammatory diseases, foreign bodies (including neglected pessaries), sexual trauma, vaginal laser procedures, and external violence (Table 1).
Table 1.
Etiology of vesicovaginal fistula
| • Traumatic |
| • Postsurgical |
| • Abdominal hysterectomy |
| • Vaginal hysterectomy |
| • Anti-incontinence surgery |
| • Anterior vaginal wall prolapse surgery (e.g., colporrhaphy) |
| • Vaginal biopsy |
| • Bladder biopsy/endoscopic resection/laser |
| • Other pelvic surgery (e.g., vascular, rectal) |
| • External trauma (e.g., penetrating, pelvic fracture, sexual) |
| • Radiation therapy |
| • Advanced pelvic malignancy |
| •Infectious/inflammatory |
| • Foreign body |
| • Obstetric |
| • Obstructed labor |
| • Forceps laceration |
| • Uterine rupture |
| • Caesarean section injury to bladder |
| • Congenital |
Clinical Presentation
The classical presentation sign is continuous (day and night) incontinence after a recent pelvic operation. If the fistula is small, then watery discharge from the vagina accompanied by normal voiding may be the only symptom. However, a radiation therapy-induced fistula may present even up to 20 years after the original insult [11].
Diagnosis
The evaluation of size, number, and exact location of fistula is important before curative surgery is undertaken. Better preoperative diagnosis allows better surgical planning.
Postoperative patients with a VVF usually are easily diagnosed with urine leaking through the vagina. A significant leukocytosis may be evident. Usually, fistulas occur between the 7th and 12th day after obstetric or gynecologic surgery. The diagnosis can be established by filling the bladder with a dilute solution of methylene blue. In a patient with urinary incontinence, the tampon test, where a tampon is inserted into the vagina after filling the bladder with the solution and the patient is ambulated, can lead to the confirmation of diagnosis. Cystoscopy is also of particular help and can clarify the exact anatomic origin. For small fistulas, it may be helpful to attempt to pass a small ureteric catheter through the suspected fistula tract to determine if it enters the vagina.
Physical examination is of vital importance. The site of the fistula and its surroundings must be thoroughly observed. If there are signs of associated acute inflammation, edema, necrosis, or other bladder pathologies coexist, then surgery should be postponed until these problems are resolved. In the preoperative planning, any scar at the site of the fistula, fixation to adjacent organs, rigidity of the vagina, or post-irradiation involvement of the rectum may change the surgical approach.
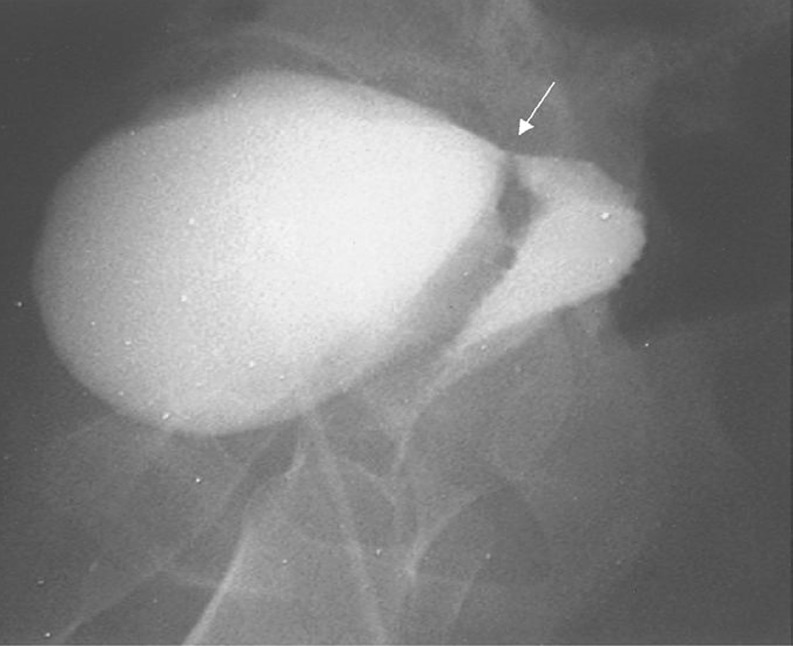
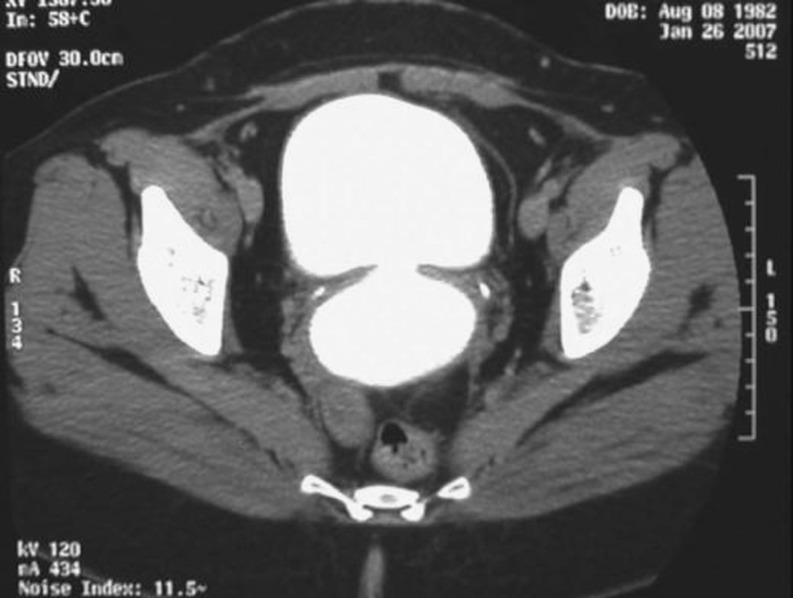
Further diagnostic procedures should include retrograde and voiding the cysto-urethrography (Fig. 1). A high creatinine level of the discharge can confirm the urinary leakage. However, intravenous pyelography and cystogram may not demonstrate the genital abnormalities [12]. The advanced but more invasive and/or costlier techniques include combined vaginoscopy–cystoscopy [13], subtraction magnetic resonance fistulography [14] (Fig. 2), and endocavitary ultrasound through transrectal or more properly through transvaginal route with or without Doppler or contrast agents [15]. Transvaginal sonographic evaluation can clearly visualize the exact site, size, and course of the fistula. There are reports that mention it as well-tolerated, less hazardous, and more informative than other conventional investigations [16]. However, it constitutes an operator-dependent procedure and the less experienced may not find it an easy alternative to the conventional cystogram. Finally, if there is a suspicion of malignancy, a biopsy must be taken for histologic examination. An intravenous pyeloureterogram is also recommended to rule out concomitant ureteral fistulas before proceeding with the surgical repair.
Fig. 1.
Cystography (lateral image). The arrow demonstrates the vesicovaginal fistula tract
Fig. 2.
CT scan of vesicovaginal fistula. After the intravenous administration of the contrast agent, there is high-density material in both the bladder and the vagina, consistent with a VVF
Treatment
Treatment of patients with VVF must embrace their immediate and, in most cases, subsequent surgical management. It is vital to consider the nutritional and rehabilitative needs of patients. When a delayed approach to surgery is intended, it is essential to take care of the sanitary protection and the skin. In 10 % of the cases, the fistula closes spontaneously after 0.5–2 months of urethral catheterization and anticholinergic medication, especially if the fistula is of small diameter, is detected early, or there is no epithelization of the fistula. If the diagnosis is established late and the fistula has epithelized, electrocoagulation of the mucosal layer and 2–4 weeks of catheterization may lead to closure [17]. However, in patients with a thin vesicovaginal septum, large VVF, or those with significant inflammation around the fistula tract, fulguration risks failure and the possibility of enlarging the size of the fistula and devitalizing adjacent tissues. Fibrin sealant has been used as an adjunctive measure to treat VVF. This material may be injected directly into the fistula tract following electrocoagulation. The bladder is then drained for several weeks. The therapeutic result of this approach is a result of the gel-like nature of the fibrin sealant that plugs the hole until tissue ingrowth occurs from the edges of the fistula. Fibrin sealant has also been successfully used in combination with collagen as an additional “plug” [18]. Unfortunately, in most cases, these conservative methods fail and the performance of surgery is needed.
The timing of intervention should aim to find the compromise between the wish to free the patient from urinary loss and to wait for the optimal conditions for closure. Surgery should be postponed if devitalized tissues, cystitis, or encrustation is present. The classical strategy is a delayed repair, undertaken after 3–6 months to allow healing of any inflammation and edema. Even a delay of 1–2 years is reasonable after radiation damage. The regular examination is fundamental to selecting the earliest date for surgery. The first step before repair is to treat any acute infection with antibiotics, while encrusted deposits must be removed both from the bladder and the vagina.
The arguments continue as to whether the abdominal or vaginal route is the most appropriate for fistula repair. In general, simple fistulas are treated using simple vaginal approaches, while complex fistulas are commonly treated either vaginally using a myocutaneous flap or through an abdominal approach. Most gynecologic surgeons favor the vaginal approach. This approach minimizes the operative complications, the hospital stay, the blood loss, and the pain following the procedure and still achieves success rates when compared with the abdominal approach [19]. At the same time, however, it can be associated with vaginal shortening and the formation of a dead space, where infection and inflammation may develop [20]. Contraindications to vaginal approach include: severely indurated vaginal epithelium around the fistula, small capacity or poorly compliant bladder, repair requiring ureteral reimplantation, involvement of other pelvic structures, vaginal stenosis, or inability to obtain proper exposure [21, 22]. The transabdominal O’Connor’s operation has been the most accepted method of repair of supratrigonal fistula to date. The traditional O’Connor operation utilizes suprapubic access for extraperitoneal dissection of the retropubic space to dissect the bladder, followed by long sagittal cystotomy (bivalving the bladder) until the fistula is reached. The fistulous tract is excised, followed by two-layer closure after tissue transposition between the bladder and vaginal walls. The abdominal approach has been recommended for: (1) high retracted fistulas in a narrow vagina, (2) fistulas which are proximal to the ureters, (3) cases with associated pelvic pathology, and (4) multiple fistulas [8]. In addition, the abdominal approach has good results with durable success (85–100 %) [23]. Transperitoneal approach offers an opportunity for wide exploration and the use of a peritoneal or omental graft in managing larger fistulas. If there is associated intra-abdominal pathology, the abdominal approach allows concomitant procedures. The transperitoneal approach is necessary if other intraperitoneal pathology is present or if there have been previous unsuccessful attempts, a rigid vaginal wall, or the need for an abdominal interposed graft. In each case, the interposed tissue serves to create an additional layer in the repair, to fill dead space, and to bring new blood supply into the area. As such, they have been most commonly used in the repair of radiation fistulas or to limit scarring and reduce post-fistula repair stress incontinence in patients with urethral and bladder neck fistulas.
VVF developed in radiated tissue should always be repaired using fresh blood supply such as flaps. In most of the cases due to the anatomic changes, the procedure is both vaginal and abdominal, but if anatomy is preserved, a vaginal approach with a flap should always be considered first. In some cases, surgical repair of a VVF will fail repeatedly, probably due to existing pelvic malignancy, severe radiation damage, and/or large soft tissue loss, especially in the setting of obstetric fistula. Furthermore, some patients may simply not be candidates for repair due to coexistent medical morbidities. For the above groups, urinary diversion, either in the form of a urinary conduit or a continent reservoir, can be considered. Fistulas in patients who are not candidates for surgical intervention may be managed by percutaneous ureteral occlusion and permanent nephrostomy [24]. In the developing world, where catheters and ostomy appliances are either too expensive or completely unavailable, continent urinary diversion or incontinent urostomies are often not practical, which present ethical issues with the alternative treatments [25]. In these situations, internal urinary diversion with ureterosigmoidostomy has some application in patients with unreconstructable lower urinary tracts. It should be recognized as a last resort operation due to its significant metabolic and neoplastic potential.
Very high or large VVFs either in close proximity to ureteric orifices or when associated with hydronephrosis, hydroureter, or urinary ascites or absent vaginal cuff are considered to be complex fistulas and require a transabdominal transvesical approach. The successful management of such fistulas is largely dependent on judicious use of interposition flaps. The omental flap is undoubtedly the most versatile; it can be used in abdominal and combined abdominal–vaginal procedures. Surgery needs to be performed in a center of excellence and questions regarding adequacy of surgical experience, technical expertise, nursing care, and facilities for blood transfusion need to be addressed before attempting complex vesicovaginal repairs.
Moreover, the laparoscopic repair of vesicovaginal fistula without opening the bladder and using intracorporeal suturing and omentum interpositioning is a feasible procedure in selected patients. It will be a useful adjunct to transvaginal repair of fistulas if the surgical morbidity of the open abdominal approach is decreased. Laparoscopic VVF repair is most useful in the same scenarios as the transabdominal repair, such as in the setting of a high VVF in which a vaginal operation would be anatomically challenging. Although the laparoscopic approach in expert hands may provide high success rate, it is not widely practiced due to the costs and considerable learning curves imposed by intracorporeal laparoscopic suturing, a requirement for VVF repair, which is an advanced skill many surgeons lack [26, 27]. Successful robotic VVF repair was first reported in 2005 [28]. A five-port technique has been described using a vaginal pack to maintain pneumoperitoneum throughout the case [29]. The successful closure was confirmed by the retention of pneumoperitoneum after the removal of the vaginal pack. Advantages to the robotic technique include three-dimensional visualization, wristed instrumentation reducing the severe angulation required for laparoscopic VVF repair, and technically simpler intracorporeal knot tying. It is doubtful that a single procedure will emerge as the optimal surgery for all patients with VVF, given the variability in the nature of the condition, the patients on whom it occurs, and the expertise of the individual surgeon.
Post-Fistula Stress Incontinence
Stress incontinence has long been recognized as a complication of VVF. It is most likely occur in obstetric fistula patients when the injury involves the sphincter mechanism, particularly if there is a tissue loss. It affects at least 10 % of obstetric fistula patients and has also been reported in patients with surgical fistulas involving the urethra or bladder neck [30]. The use of a labial musculo-fat graft in the initial repair may reduce the likelihood of this complication and a number of other techniques have been attempted [31].
Postoperative Care
Continuous bladder drainage via a urethral Foley catheter is essential. In patients with a fistula involving the bladder neck, the balloon should not be inflated, but the catheter should be sutured in place. The postoperative management is of vital importance. A high-fluid input and output should be maintained until the urine is clear of blood; continuous bladder drainage is essential. If the catheter is blocked, this is the most likely cause of failure of the repair and nurses should be instructed to ensure that the catheter is freely draining, both day and night. The bladder should remain catheterized for 2–3 weeks after repair. Cystography is undertaken before catheter removal if there is any doubt about the integrity of the repair. Anticholinergic drugs should be administered if bladder spasms occur. As far as causing discomfort to the patient, it has been suggested that these contractions may compromise healing of the repair. An antiseptic tampon is placed in the vagina for a day. Patients should avoid sexual intercourse for 3 months. Regardless of the surgical approach to VVFs, the key to postoperative management is the maintenance of a dry, uninfected suture line. For this reason, the use of antibiotics is recommended for a prolonged period after surgery, usually until all catheters are removed [32].
Recurrence
Successful closure of a VVF requires an accurate and a timely repair using procedures that exploit basic surgical principles. The rate of successful fistula repair reported in the literature varies between 70 and 100 % in non-radiated patients, with similar results when a vaginal or abdominal approach is performed; the mean success rates being 91 and 97 %, respectively. Fistulas in radiated patients are less frequently repaired and the success rate varies between 40 and 100 %. Generally, multiple fistula (two or more), size (>10 mm) and type of the fistula (complex VVF which involves the bladder cervix or the urethra), urinary tract infection, and obstetrical etiology constitute the recurrence risk factors. The interposition of flaps is considered to be a protective factor for the recurrent cases [33]. The omentum, especially, supplies excellent lymphatic drainage and maintains its suppleness following resolution of inflammation.
Conclusion
VVFs are among the most distressing complications of gynecologic and obstetric procedures. The diagnosis of the condition has traditionally been based on clinical methods and dye testing. The best chance of a successful repair is at the first attempt. The arguments about the most appropriate route for repair continue and are not clarified by the publications so far. However, the role of interposition grafts at both abdominal and vaginal repairs is viewed positively by the respective authors. Adjuvant techniques are needed for complex fistulas. Measures for prevention must include universal education, improvement in the status of women, and improved and accessible medical services.
References
- 1.Waaldijk K. Surgical classification of obstetric fistulas. Int J Gynecol Obstet. 1995;49:161–163. doi: 10.1016/0020-7292(95)02350-L. [DOI] [PubMed] [Google Scholar]
- 2.Wall LL. Obstetric vesicovaginal fistula as an international public-health problem. Lancet. 2006;368:1201–1209. doi: 10.1016/S0140-6736(06)69476-2. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim T, Sadiq A, Daniel S. Characteristics of VVF patients as seen at the specialist hospital Sokoto, Nigeria. West Afr Med J. 2000;19:59–63. [PubMed] [Google Scholar]
- 4.Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med. 1944;38:1091–1110. doi: 10.1016/0277-9536(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 5.Cron J (2003) Lessons from the developing world: obstructed labor and the vesico-vaginal fistula. Medscape Gen Med 5(3) [PubMed]
- 6.Tahzib F. Epidemiological determinants of vesicovaginal fistulas. Br J Obstet Gynaecol. 1983;90:387–391. doi: 10.1111/j.1471-0528.1983.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 7.Hilton P. Surgical fistulae and obstetric fistulae. In: Cardozo LD, Staskin D, editors. Textbook of female urology and urogynaecology. London: Isis Medical Media Ltd; 2001. pp. 691–719. [Google Scholar]
- 8.Armenakas NA, Pareek G, Fracchia JA. Iatrogenic bladder perforations: long-term follow-up of 65 patients. J Am Coll Surg. 2004;198:78–82. doi: 10.1016/j.jamcollsurg.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Penalver M, Angioli R. Urinary diversion. Chapter 11. In: Glenn HW, editor. Urogynecologic surgery. 2. New York: Lippincott-Raven; 2000. pp. 193–206. [Google Scholar]
- 10.Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surg. 1994;49(12):840–847. doi: 10.1097/00006254-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Zoubek J, McGuire EJ, Noll F, DeLancey JOL. The late occurrence of urinary tract damage in patients successfully treated by radiotherapy for cervical cancer. J Urol. 1984;141:1347–1349. doi: 10.1016/s0022-5347(17)41303-6. [DOI] [PubMed] [Google Scholar]
- 12.Adetiloye VA, Dare F. Obstetric fistula: evaluation with ultrasonography. J Ultrasound Med. 2000;19:243–249. doi: 10.7863/jum.2000.19.4.243. [DOI] [PubMed] [Google Scholar]
- 13.Andreoni C, Bruschini H, Trazzi JC, Simmetti R, Srougi M. Combined vaginopscopy-cystoscopy: a novel simultaneous approach improving VVF evaluation. J Urol. 2003;170:2330–2332. doi: 10.1097/01.ju.0000096343.03276.75. [DOI] [PubMed] [Google Scholar]
- 14.Dwarkasing S, Hussain SM, Hop WC, Krestin GP. Anovaginal fistulas: evaluation with endoanal MR imaging. Radiol. 2004;231:123–128. doi: 10.1148/radiol.2311021190. [DOI] [PubMed] [Google Scholar]
- 15.Volkmer BG, Keufer R, Nesslaner T, Loeffler M, Gottfried HW. Color Doppler ultrasound in vesicovaginal fistulae. Ultrasound Med Biol. 2000;26:771–775. doi: 10.1016/S0301-5629(00)00210-6. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi IA, Hidayaatullah AAH, Ashfag S, Nayyar S. Transvaginal versus transabdominal sonography in the evaluation of pelvic pathology. J Coll Physi Surg Pak. 2004;14:390–393. [PubMed] [Google Scholar]
- 17.Kursch ED, Stovsky M, Ignatoff JM, Nanniraga WF, O’Connor VJ. Use of fulguration in the treatment of vesicovaginal fistula. J Urol. 1993;149:292A. [Google Scholar]
- 18.Kumar U, Albala DM. Fibrin glue applications in urology. Curr Urol Rep. 2001;2(1):V79–V82. doi: 10.1007/s11934-001-0029-5. [DOI] [PubMed] [Google Scholar]
- 19.Angioli R, Penalver M, Muzii L, Mendez L, Mirhashemi R, Bellati F, Crocè C, Panici PB. Guidelines of how to manage vesicovaginal fistula. Crit Rev Oncol/Hematol J. 2003;48(3):295–304. doi: 10.1016/S1040-8428(03)00123-9. [DOI] [PubMed] [Google Scholar]
- 20.Enzelseberger H, Gitsch E. Surgical management of vesicovaginal fistulas according to Chassar Moir’s method. Surg Gynecol Obstet. 1991;173:183–186. [PubMed] [Google Scholar]
- 21.Carr LK, Webster GD. Abdominal repair of vesicovaginal fistula. Urol. 1996;48(1):10–11. doi: 10.1016/s0090-4295(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor R, Ansari MS, Singh P, Gupta P, Khurana N, Mandhani A, Dubey D, Srivastava A, Kumar A. Management of vesicovaginal fistula: an experience of 52 cases with a rationalized algorithm for choosing the transvaginal or transabdominal approach. Indian J Urol. 2007;23(4):372–376. doi: 10.4103/0970-1591.36709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalela D, Ranjan P, Sankhwar PL, Sankhwar SN, Naja V, Goel A. Supratrigonal VVF repair by modified O’Connor’s technique: an experience of 26 cases. Eur Urol. 2006;49(3):551–556. doi: 10.1016/j.eururo.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Shindel AW, Zhu H, Hovsepian DM, Brandes SB. Ureteric embolization with stainless-steel coils for managing refractory lower urinary tract fistula: a 12-year experience. BJU. 2007;99(2):364–368. doi: 10.1111/j.1464-410X.2006.06569.x. [DOI] [PubMed] [Google Scholar]
- 25.Wall LL, Wilkinson J, Arrowsmith SD, Ojengbede O, Mabeya H. A code of ethics for the fistula surgeon. Int J Gynaecol Obstet. 2008;101(1):84–87. doi: 10.1016/j.ijgo.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi SJ, Gupta R, Patel S, Trivedi A, Trivedi P, Modi P. Modified laparoscopic abdominal vesico-vaginal fistula repair—"Mini-O’Conor" vesicotomy. J Laparoendosc Adv Surg Tech A. 2010;20(1):13–15. doi: 10.1089/lap.2009.0176. [DOI] [PubMed] [Google Scholar]
- 27.Zambon J, Batezini N, Pinto E, Skaff M, Girotti M, Almeida F. Do we need new surgical techniques to repair vesico-vaginal fistulas? Int Urogynecol J. 2010;21:337–342. doi: 10.1007/s00192-009-1040-5. [DOI] [PubMed] [Google Scholar]
- 28.Melamud O, Elehel L, Turbow B, Shanberg A. Laparoscopic vesicovaginal fistula repair with robotic reconstruction. Urol J. 2005;65:163–166. doi: 10.1016/j.urology.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 29.Hemal A, Wadwa P. Robotic repair of vesico-vaginal fistula. In: Robotics in genitourinary surgery. London: Springer; 2011. pp. 611–616. [Google Scholar]
- 30.Hilton P. The urodynamic findings in patients with urogenital fitulae. Br J Urol. 1998;81:539–542. doi: 10.1046/j.1464-410x.1998.00596.x. [DOI] [PubMed] [Google Scholar]
- 31.Hilton P, Ward A, Molloy M, Umana O. Periurethral injection of autologous fat for the treatment of post-fistula repair stress incontinence: a preliminary report. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:118–121. doi: 10.1007/BF01982221. [DOI] [PubMed] [Google Scholar]
- 32.Smith GL, Williams G. Vesicovaginal fistula. BJU Int. 1999;83:564–570. doi: 10.1046/j.1464-410x.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 33.Ockrim JL, Greenwell TJ, Foley CL, Wood DN, Shah PJR. A tertiary experience of vesico-vaginal and urethro-vaginal fistula repair: factors predicting success. BJU Int. 2009;103:1122–1126. doi: 10.1111/j.1464-410X.2008.08237.x. [DOI] [PubMed] [Google Scholar]