Abstract
Background
Exposure of phosphatidylserine on the outside of red blood cells contributes to recognition and removal of old and damaged cells. The fraction of phosphatidylserine-exposing red blood cells varies between donors, and increases in red blood cell concentrates during storage. The susceptibility of red blood cells to stress-induced phosphatidylserine exposure increases with storage. Phosphatidylserine exposure may, therefore, constitute a link between donor variation and the quality of red blood cell concentrates.
Materials and methods
In order to examine the relationship between storage parameters and donor characteristics, the percentage of phosphatidylserine-exposing red blood cells was measured in red blood cell concentrates during storage and in fresh red blood cells from blood bank donors. The percentage of phosphatidylserine-exposing red blood cells was compared with red blood cell susceptibility to osmotic stress-induced phosphatidylserine exposure in vitro, with the regular red blood cell concentrate quality parameters, and with the donor characteristics age, body mass index, haemoglobin level, gender and blood group.
Results
Phosphatidylserine exposure varies between donors, both on red blood cells freshly isolated from the blood, and on red blood cells in red blood cell concentrates. Phosphatidylserine exposure increases with storage time, and is correlated with stress-induced phosphatidylserine exposure. Increased phosphatidylserine exposure during storage was found to be associated with haemolysis and vesicle concentration in red blood cell concentrates. The percentage of phosphatidylserine-exposing red blood cells showed a positive correlation with the plasma haemoglobin concentration of the donor.
Discussion
The fraction of phosphatidylserine-exposing red blood cells is a parameter of red blood cell integrity in red blood cell concentrates and may be an indicator of red blood cell survival after transfusion. Measurement of phosphatidylserine exposure may be useful in the selection of donors and red blood cell concentrates for specific groups of patients.
Keywords: aging, RBC, phosphatidylserine, storage, donor
Introduction
During storage in a blood bank, red blood cells (RBC) undergo a number of structural and biochemical changes, constituting the so-called storage lesion1. Some of these changes, such as a decrease in intracellular adenosine-5′-triphosphate (ATP), are parameters of the current RBC concentrate quality control system. This control system includes 24-hour survival of at least 75% of the transfused RBC2. The decrease in ATP concentration is readily reversible2, but the effects of the storage lesion on the capacity of the cells to deform -and thereby to deliver oxygen to the tissues- are hardly known3,4. Furthermore, the relationship between the storage lesion and the development of side effects, such as inflammatory immune reactions and iron accumulation, is not clear.
Externalised phosphatidylserine (PS) is a sensitive marker for fast recognition and removal of RBC by the reticulo-endothelial system, as suggested by the currently available data on RBC aging in vivo and in vitro, on the response of RBC to various stress treatments in vitro, and on RBC in pathological conditions5–8. The number of PS-exposing RBC increases with storage in the blood bank6,7. We have shown that storage is also accompanied by an increase in PS exposure upon hyperosmotic stress7. These data suggest a storage-associated lowering of the threshold for activation of the pathways that induce PS exposure. Various pathways have been proposed6–8, but their activity and especially their response to extracellular stimuli after transfusion in vivo remain to be elucidated. The fraction of PS-exposing RBC after near-physiological stress of stored RBC is similar to the fraction of RBC that disappears shortly after transfusion7,9. This suggests that control of PS exposure may be a determinant of RBC survival in the first 24 hours after transfusion.
We, therefore, examined whether the degree of initial PS exposure in donor blood is associated with PS exposure in the RBC concentrate and/or predictive of its susceptibility to stress-induced PS exposure. Our findings indicate that RBC concentrate production itself affects PS exposure, that initial PS exposure is correlated with RBC susceptibility to osmotic stress-induced PS exposure, and that PS exposure is associated with some, but not all, quality control parameters of RBC concentrates.
Materials and methods
Donor characteristics
A population of 97 frequent blood bank whole blood donors participated in this study (Table I). RBC from an initial group of 37 donors were used to examine PS exposure before processing and after 6 days of storage. Fresh RBC from an additional 37 donors were included to examine PS exposure with and without the application of osmotic stress (n=74). Fresh RBC from an additional 23 donors were used to determine whether the donor characteristics correlated with PS exposure (n=97). Fresh RBC and RBC concentrates from 12 donors were used to examine PS exposure during blood bank storage. The study was performed following the guidelines of the local medical ethical committee and in accordance with the declaration of Helsinki. Written informed consent was obtained from all blood donors participating in this study.
Table I.
Summary of donor characteristics.
| Parameter | Characteristics |
|---|---|
| Gender | 66% male, 34% female |
| Rhesus D | 80% positive, 20% negative |
| ABO group | 46% O, 41% A, 13% B |
| Age (years) | 48.9±12.9 |
| Height (cm) | 176.8±7.9 |
| Weight (kg) | 82.2±15.1 |
| Body mass index | 26.2± 4.1 |
| Haemoglobin (mmol/L) | 9.2±0.8 |
Mean and standard deviation are given for age, height, weight, body mass index and haemoglobin (normal haemoglobin ranges are 8.6–11.2 for men, and 7.5–9.4 mmol/L for women).
Red blood cell isolation
Fresh RBC were isolated from 5 mL whole blood (EDTA) as described elsewhere7. RBC concentrates were collected and processed according to standard Dutch blood bank protocols in the regional blood bank Sanquin Blood Bank South East Region, Nijmegen, The Netherlands7,9,10. Samples of 2 to 5 mL were taken aseptically from the concentrates, and RBC were washed to remove medium, plasma and vesicles using Ringer’s solution (NaCl 125 mM, KCl 5 mM, MgSO4 1 mM, CaCl2 2.5 mM, glucose 5 mM, HEPES/NaOH 32 mM, pH 7.4) by repeated centrifugation (for 5 minutes at 1,500 g, 4 °C). All experiments were performed in Ringer’s solution. Where indicated, osmotic stress was induced by adding 400 mM sucrose aseptically and incubating overnight at 37 °C in 5% CO27.
Phosphatidylserine measurement
The percentage of PS-exposing RBC was determined as described previously7. RBC were incubated for 1 hour at room temperature in the dark with Ringer’s solution containing 0.2% bovine serum albumin and annexin V FLUOS (1:25, Roche, Basel, Switzerland) to detect PS, and with PE-conjugated anti-CD235a antibody (1:100, mouse IgG1, clone KC16, Beckman Coulter, Brea, CA, USA). Flow cytometry was performed using an Epics XL-CML flow cytometer (Beckman Coulter, Brea, CA, USA), and the data were analysed with CXP Analysis software version 2.2 (Beckman Coulter, Brea, CA, USA). Only CD235a-positive events (100,000) in the RBC size range were gated for further analysis. The intra-assay variation in the number of annexin V-positive RBC was ≤0.05% before and ≤0.1% after stress treatment. Combined anti-CD41-PC5 (1:10, BioLegend, San Diego, CA, USA) and anti-CD235a-PE (1:100, Beckman Coulter, Fullerton, CA, USA) antibody staining revealed the virtual absence of platelets and the high RBC purity (>99.9%) in the samples that were tested.
Vesicle isolation and quantification
All buffers used for vesicle isolation and analysis were complemented with 0.2% bovine serum albumin and filtered (0.22 μm) before use. Samples from RBC concentrates were centrifuged (20 minutes at 1,500 g) twice to remove cells and cell debris. Vesicles were pelleted from the supernatant (1 mL) by centrifugation at 21,000 g for 20 minutes. The vesicle pellet was resuspended in 10 μL supernatant and stored at −80 °C until analysis. Frozen vesicles were thawed on ice and washed with calcium-free Ringer’s solution. Vesicles were labelled for flow cytometry analysis by incubation in Ringer’s solution with annexin V FLUOS (1:50, Roche, Basel, Switzerland) to detect PS, and with PE-conjugated anti-CD235a antibody (1:200, mouse IgG1, clone KC16, Beckman Coulter, Brea, CA, USA) for 30 minutes at 4 °C in the dark. After this incubation, vesicles were washed once by centrifugation, resuspended in Ringer’s solution, and washed Flow-Count Fluorospheres (Beckman Coulter, Brea, CA, USA) were added (1×104) for quantification. Annexin V/CD235a-double-positive vesicles were quantitated using a FACsCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) in combination with CXP Analysis software version 2.2 (Beckman Coulter, Brea, CA, USA). Sulphate latex microspheres (Invitrogen, Carlsbad, CA, USA; 0.9 μm) were used to determine the maximum upper boundary allowed for forward and sideward scatter gating. All staining solutions were centrifuged at 21,000 g and 4 °C for 20 minutes prior to use to remove fluorescent aggregates. A PE-conjugated IgG1 isotype control (Dako, Glostrup, Denmark) did not show any aspecific binding.
Quality parameters
The blood bank quality parameters extracellular haemoglobin, pH, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, ATP and 2,3-diphosphoglycerate (2,3-DPG) were measured as described previously7,11,12.
Statistical analysis
Differences before and after whole blood processing and stress were tested with a Mann-Whitney U test. Differences between multiple groups were assessed with a one way-ANOVA in combination with Tukey’s post-test. Pearson’s correlation coefficients were computed to measure bivariate correlations. The reported P values are two-sided, and a P value of <0.05 was considered to be statistically significant.
Results
Donor variation in phosphatidylserine exposure before and after the production of red blood cell concentrates
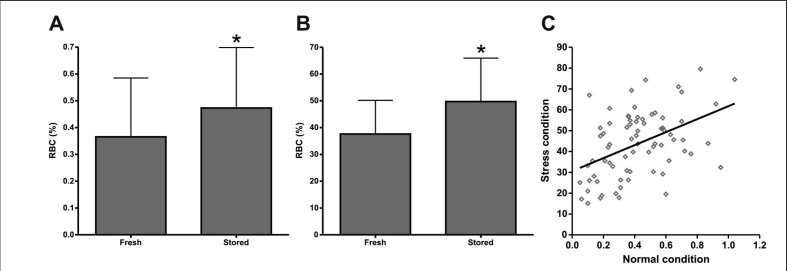
As a first step to examine a putative correlation between donor characteristics and RBC concentrate quality, we determined whether there is donor variation in the percentage of PS-exposing RBC, both in freshly drawn blood before RBC concentrate production and during the first week of storage. A large donor variation in the amount of PS-exposing RBC was observed in both fresh and briefly stored RBC concentrates (Figure 1A). There was a small, but statistically significant, increase in the number of PS-exposing RBC during blood bank processing and/or within the first week of storage (Figure 1A). Osmotic stress induced a strong increase in the number of PS-exposing RBC, with similar donor variation (Figure 1B) in the fresh RBC and in RBC that had been stored for 1 week under blood bank conditions. After a short period of storage, RBC were more susceptible than fresh RBC to osmotic stress (Figure 1B). The latter data are in accordance with previous observations showing an increase in susceptibility with storage time7.
Figure 1.
Exposure of PS by RBC before and after the first week of storage in the presence or absence of osmotic stress.
RBC were collected from freshly drawn blood [fresh] and from 6-day old RBC concentrates [stored] obtained from the same donor. Using flow cytometry, the percentage of PS-exposing RBC [RBC (%)] was determined before (A, *P=0.04) and after (B, *P=0.001) osmotic stress. Results are expressed as the mean ± SD (n=37). The correlation between PS exposure of fresh RBC before and after osmotic stress (C) had a Pearson’s coefficient of 0.64 (n=74).
Phosphatidylserine exposure in red blood cell concentrates predicts susceptibility to stress
Since PS exposure increases with RBC storage6,7, and is involved in the recognition and removal of damaged cells5,13, stress-induced PS exposure might be involved in the removal of up to 30% of the transfused RBC that is known to occur within the first hours after transfusion9. We, therefore, examined whether there is a relationship between initial PS exposure of RBC and their susceptibility to osmotic stress-induced PS exposure. We observed a strong positive correlation between PS exposure before and after stress (Figure 1C), showing that the percentage of PS-exposing RBC may serve as a marker for the susceptibility of the entire RBC population.
Relation of phosphatidylserine exposure with red blood cell concentrate quality parameters and vesiculation
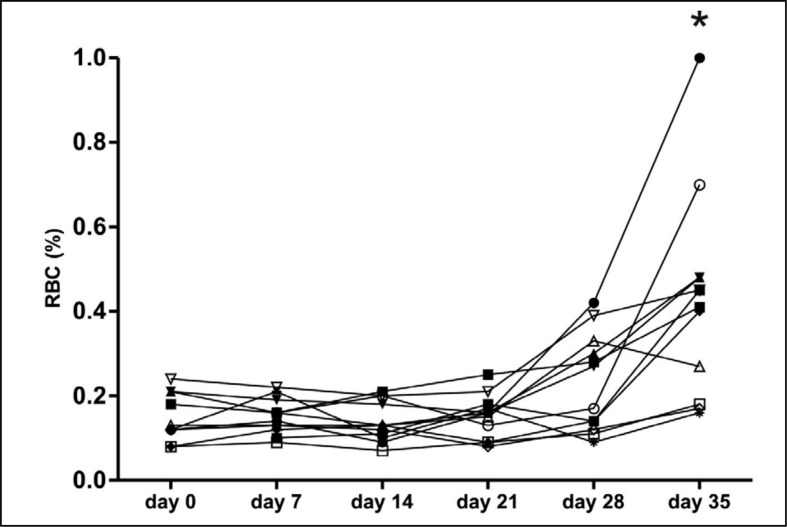
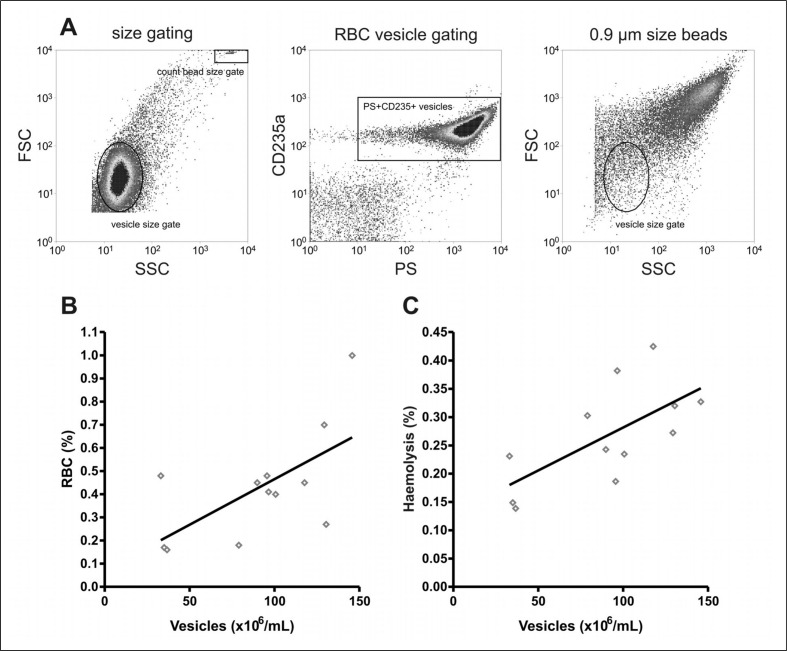
The variation in PS exposure between donors, together with the correlation with stress-induced PS exposure, raised the question of whether PS exposure is related to any of the regular quality parameters of RBC concentrates. Therefore, the percentage of PS-exposing RBC, as well as extracellular haemoglobin, pH, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, ATP and 2,3-DPG of 12 RBC concentrates were followed in time. In six of the 12 units tested, the percentage of PS-exposing RBC started to increase in the fourth week of storage (Figure 2). In the fifth week, an additional three RBC concentrates showed an increased percentage of PS-exposing RBC. Variation in PS exposure between donors became more apparent from week 4 onwards (Figure 2). The rise in the percentage of PS-exposing RBC ran parallel with decreases in ATP, 2,3-DPG, and pH, and increases in MCV and haemolysis, confirming previous data11,12. However, only the degree of haemolysis showed a positive correlation with the percentage of PS-exposing RBC at multiple time points during storage (data not shown). Furthermore, both the percentage of PS-exposing RBC and the degree of haemolysis showed a positive correlation with the number of RBC vesicles in the 35 day-old concentrate (Figure 3).
Figure 2.
PS exposure during storage of RBC under blood bank conditions.
RBC from 12 donors were analysed for the percentage of PS-exposing RBC [RBC (%)] by flow cytometry, just prior to (day 0), and during RBC concentrate storage. *P≤0.0001.
Figure 3.
Correlation of RBC PS exposure and haemolysis with vesicle content in RBC concentrates.
After 35 days of storage haemolysis was measured, and the percentage of PS-exposing RBC was determined by flow cytometry detection of annexin V binding. The number of PS+CD235a+ vesicles was determined per millilitre of transfusion concentrate supernatant as described in the Materials and methods section. (A) Vesicle characteristics and quantification. (B) The correlation between vesicle number and PS exposure [RBC (%); Pearson coefficient of 0.64, n=12]. (C) The correlation between vesicle number and haemolysis (Pearson coefficient of 0.67, n=12).
Relation of phosphatidylserine exposure with donor parameters
Since the percentage of PS-exposing RBC varies between RBC concentrates from different donors (Figure 1), we investigated whether it would be possible to identify donors with high or low PS exposure by readily available donor parameters (Table I). Among the 97 donors examined, the numbers of PS-exposing RBC in freshly drawn donor blood, i.e. before blood bank processing, did not differ significantly depending on gender, age, body mass index and ABO or Rhesus D blood groups. There was a weak positive correlation between PS exposure and haemoglobin concentration of the donor plasma (Pearson’s coefficient 0.21), but not with the other parameters (data not shown).
Discussion
Stored RBC are less resistant than fresh RBC to hypo-osmotic and mechanical stress-induced haemolysis14. The positive correlation we observed between PS exposure and haemolysis suggests that PS exposure is an indicator of RBC integrity during storage in blood bank conditions. Indeed, time-lapse microscopy data show that PS exposure precedes lysis when exposed to various stressors15. The small, but significant difference we observed in PS exposure between freshly isolated RBC and RBC sampled from the RBC concentrates within the first week after their processing suggests that processing of the blood before storage induces membrane restructuring. In addition, the correlation between the percentage of PS-exposing RBC and vesicle concentration in RBC concentrates is compatible with the hypothesis that the changes in membrane organisation that lead to the appearance of PS in the outer layer of the cell membrane also lead to the generation of vesicles16. Our data suggest that concentrates from donors with higher initial numbers of PS-exposing RBC may be less effective upon transfusion, due to enhanced susceptibility to physiological stress in the circulation and subsequent removal. We propose testing PS exposure as a novel quality parameter of RBC concentrates, since the conventional parameters do not accurately predict RBC survival after transfusion17.
Of the donor parameters that we examined, only the haemoglobin concentration showed a correlation with RBC PS exposure. It might, therefore, be worth investigating whether other donor parameters might be predictive of PS exposure. Recent findings indicate that donor differences in PS exposure may reflect variation in iron status and hormonal factors18,19. Accumulation of HbA1c and other modified haemoglobin species in RBC-derived vesicles20 suggests a functional relationship between haemoglobin composition and RBC structure in healthy donors. Thus, the recently described donor variation in the rate of haemoglobin glycation21 may contribute to the variation from concentrate to concentrate. This leads to our prediction that in donors, HbA1c content is positively correlated to both a decrease in RBC life span and PS exposure. Indeed, the percentage of PS-exposing RBC in individuals with type 2 diabetes mellitus is twice that of control subjects22. Similarly, the increased susceptibility to mechanical stress of RBC in post-menopausal women19 may very well correlate with the number of PS-exposing RBC.
In conclusion, the correlations between PS exposure, donor characteristics and RBC concentrate quality parameters indicate that PS exposure may be a biologically relevant parameter of RBC quality. These results emphasise the need for elucidation of the mechanisms that stimulate PS exposure on RBC in vivo. Such knowledge could lead to the development of methods to improve RBC survival during storage and after transfusion, and could enable the selection of RBC concentrates -and even donors- for specific groups of patients. It has been suggested that RBC concentrates with a high haemoglobin content may be selected for patients with the largest blood volume23. Similarly, it may be feasible to select concentrates with the lowest PS exposure for chronically transfused and/or critically ill patients, or remove the RBC that are susceptible to stress-induced PS exposure from the RBC concentrate. This would be of benefit especially for chronically transfused patients, as a reduction in the number of rapidly removed RBC would lead to enhanced transfusion efficacy and a significant reduction in the rate of iron accumulation and pathological activation of the immune system.
Acknowledgements
We thank the Sanquin Blood Supply Foundation, Amsterdam, The Netherlands for performing the ATP and 2,3-DPG measurements. This study was financed by the Radboud University Medical Centre.
Footnotes
Authors’ Contributions
Sip Dinkla, Malou Peppelman, Jori van der Raadt, Giel J.C.G.M. Bosman, Vĕra M.J. Novotný and Irma Joosten designed the research; Sip Dinkla, Malou Peppelman and Jori van der Raadt performed the experiments; Sip Dinkla, Malou Peppelman, Jori van der Raadt and Femke Atsma analysed the data; Sip Dinkla, Giel J.C.G.M. Bosman, Marian G.J. van Kraaij, Femke Atsma, Vĕra M.J. Novotný and Irma Joosten wrote and edited the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Hogman CF, Meryman HT. Red blood cells intended for transfusion: quality criteria revisited. Transfusion. 2006;46:137–42. doi: 10.1111/j.1537-2995.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 3.Raat NJ, Hilarius PM, Johannes T, et al. Rejuvenation of stored human red blood cells reverses the renal microvascular oxygenation deficit in an isovolemic transfusion model in rats. Transfusion. 2009;49:427–4. doi: 10.1111/j.1537-2995.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- 4.Raat NJ, Ince C. Oxygenating the microcirculation: the perspective from blood transfusion and blood storage. Vox Sang. 2007;93:12–18. doi: 10.1111/j.1423-0410.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy -le-grand) 2004;50:147–58. [PubMed] [Google Scholar]
- 6.Verhoeven AJ, Hilarius PM, Dekkers DW, et al. Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox Sang. 2006;91:244–51. doi: 10.1111/j.1423-0410.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Bosman GJ, Cluitmans JC, Groenen YA, et al. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion. 2011;51:1072–8. doi: 10.1111/j.1537-2995.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 8.Lang F, Lang KS, Lang PA, et al. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–92. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 9.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 10.Pistorius AM, Luten M, Bosman GJ, et al. A single assay for multiple storage-sensitive red blood cell characteristics by means of infrared spectroscopy. Transfusion. 2010;50:366–75. doi: 10.1111/j.1537-2995.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- 11.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–62. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 12.Luten M, Roerdinkholder-Stoelwinder B, Bost HJ, et al. Survival of the fittest?--survival of stored red blood cells after transfusion. Cell Mol Biol (Noisy-le-grand) 2004;50:197–203. [PubMed] [Google Scholar]
- 13.Lee SJ, Park SY, Jung MY, et al. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood. 2011;117:5215–23. doi: 10.1182/blood-2010-10-313239. [DOI] [PubMed] [Google Scholar]
- 14.Gelderman MP, Vostal JG. Rejuvenation improves roller pump-induced physical stress resistance of fresh and stored red blood cells. Transfusion. 2011;51:1096–104. doi: 10.1111/j.1537-2995.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 15.Dinkla S, Wessels K, Verdurmen WP, et al. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis. 2012;3:e410. doi: 10.1038/cddis.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–62. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 17.Hogman CF, Meryman HT. Storage parameters affecting red blood cell survival and function after transfusion. Transfus Med Rev. 1999;13:275–96. doi: 10.1016/s0887-7963(99)80058-3. [DOI] [PubMed] [Google Scholar]
- 18.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–22. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–21. doi: 10.1111/j.1423-0410.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 20.Bosman GJ, Lasonder E, Groenen-Dopp YA, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Wenk RE, McGann H, Gibble J. Haemoglobin A1c in donor erythrocytes. Transfus Med. 2011;21:349–50. doi: 10.1111/j.1365-3148.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 22.Calderon-Salinas JV, Munoz-Reyes EG, Guerrero-Romero JF, et al. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol Cell Biochem. 2011;357:171–9. doi: 10.1007/s11010-011-0887-1. [DOI] [PubMed] [Google Scholar]
- 23.Reikvam H, van de Watering L, Prowse C, et al. Evaluation of noninvasive methods for the estimation of haemoglobin content in red blood cell concentrates. Transfus Med. 2011;21:145–9. doi: 10.1111/j.1365-3148.2010.01059.x. [DOI] [PubMed] [Google Scholar]