Abstract
Background
Receipt of blood transfusions is associated with the major consequences of prematurity such as bronchopulmonary dysplasia. Transfusion-mediated (iron-induced) oxidative damage, coupled with the limited ability of the premature baby to deal with enhanced iron and oxidative load may contribute to this. Adverse effects of transfusion may be related to duration of storage. This study examined the influence of storage on iron and oxidative status in paediatric packed red blood cell units.
Materials and methods
Paediatric packed red blood cell units were sampled 3 days post-donation, then at 7 days and weekly until day 35. The extracellular medium was separated and the following measured: total iron concentration, total iron binding capacity, non-transferrin-bound iron, haemoglobin, total and reduced ascorbate, glutathione and malondialdehyde.
Results
Measurable total and non-transferrin bound iron were present in the extracellular fluid of paediatric packs on day 3. Both parameters rose almost linearly to maximal values at 35 days. Haemoglobin and malondialdehyde levels rose gradually from day 3 to day 21, then more steeply to day 35. Ascorbate existed mainly in the oxidised form and fell rapidly towards the end of storage. Intracellular GSH fell throughout the period of storage. Strong correlations existed between biomarkers of oxidative damage and iron parameters.
Discussion
These data suggest that iron released following the initial preparation of packed red blood cell units may derive from free radical-mediated oxidative damage to the red blood cells and haemoglobin, rather than from extracellular haemoglobin. Iron continues to be released during storage as antioxidant protection declines. A cycle of free radical-mediated damage may initiate and then further exacerbate iron release during storage which, in turn, may mediate further free radical-mediated cellular damage. The potential consequences to recipients of older stored blood may be significant.
Keywords: iron, oxidative stress, packed cells, storage
Introduction
Many premature babies are given multiple transfusions during their first few weeks of life. A number of studies have shown correlations between the receipt of blood transfusions and the development of the serious consequences of prematurity such as chronic lung disease (CLD), retinopathy (ROP), necrotising enterocolitis and intraventricular haemorrhage (IVH)1–4. The exact mechanism of this relationship is unclear, but a number of studies have suggested that transfusion-mediated iron overload and associated oxidative stress may be major players in ROP4,5, CLD6, and IVH7. The clear benefits of restricting blood used in individual babies to that derived from a single donor8 requires blood to be stored throughout the period that individual babies require transfusions. This adds the further complication of the influence of storage age of the blood used on clinical outcome9,10. The potential adverse effects of storage on the biochemistry and validity of stored erythrocytes has been given the term “the storage lesion”11,12. The involvement of iron and oxidative status in the storage lesion has received little attention, despite the potential adverse influence of the procedures involved in the preparation of packed cell units on the iron and oxidative status of the units. The preparation of packed cell units involves the removal of most of the plasma proteins capable of binding and sequestering iron such as transferrin and albumin, and also the naturally occurring extracellular anti-oxidants such as urate and ascorbate. Thus the potential for free unbound (potentially toxic) iron to build up in the additive solution and participate in poorly protected oxidative reactions clearly exists. This can have consequences on the viability of erythrocytes, and the behaviour of haemoglobin within the erythrocytes and on iron bioavailability13–15. In addition, the premature baby is poorly equipped to deal with any additional iron or oxidative load, being deficient in iron-binding capabilities16–20 and anti-oxidant defences1,20–22. Consequently the premature baby is likely to be at risk of transfusion-related iron and oxidative overload.
A preliminary study in our laboratory confirmed that the extracellular fluid of immediately time-expired (36 days storage) paediatric packed cell units was rich in iron, a high percentage of which was in the form of the potentially toxic non-transferrin bound iron (NTBI)23. In addition, the fluid was highly redox active with limited anti-oxidant protection and iron-binding capacity23. The purpose of the current study was to further develop these findings by examining the changes in iron and oxidative status of paediatric packed cell units stored from the point of arrival at the Blood Bank (3 days after donation) up to the maximum recommended time (35 days). The study also builds on previous findings from related studies in other laboratories. For example, increases in NTBI in the extracellular medium during storage of packed cell units have been reported, along with the finding that iron-binding capacity was very low and the saturation of residual transferrin was very high24. Other studies demonstrated that membrane phospholipids in erythrocytes underwent oxidative modification with the release of malondialdehyde (MDA) during storage25, and that the addition of iron chelators or some anti-oxidants reduced the degree of lipid peroxidation in stored erythrocytes26–28. To date, no study has examined the relationship between erythrocyte viability, total iron levels, iron-binding capacity, NTBI, oxidative stress and the levels of anti-oxidants in the supernatant of stored paediatric packed cell preparations from arrival at the Blood Bank until time expiry (35 days). This study has done this by examining, in the supernatant, the following parameters: total iron concentration, iron-binding capacity, haemoglobin (Hb) concentration (as a marker of the degree of erythrocyte membrane damage), NTBI concentration, total and reduced ascorbate concentrations, total red blood cell and extracellular glutathione, and a marker of lipid peroxidation, MDA. The measurement of these related parameters in the same packs has allowed a reassessment of the potential mechanism by which adverse effects may occur during storage and how they may affect the recipients.
Materials and methods
Preparation and storage of packed cell units
Paediatric packed cell units were prepared at the NHS Blood and Transplant Centre (Bristol, UK) as described previously23. The adult blood is anticoagulated with citrate, phosphate, dextrose (sodium citrate 89 mmol/L; citric acid 16 mmol/L; glucose 128 mmol/L; sodium phosphate 16 mmol/L) at a ratio of 63 mL anticoagulant to 450 mL blood. The blood is filtered to remove leucocytes and centrifuged to yield the red blood cells. Most of the plasma is removed (leaving about 20 mL) and replaced with 100 mL of SAGM additive (NaCl 150 mmol/L; adenine 1.25 mmol/L; glucose 45.4 mmol/L; mannitol 28.8 mmol/L). This provides a unit containing approximately 200 mL of red blood cells and 100 mL of additive. The adult pack is then split into six paediatric units of 45–50 mL. In this study ten adult packs were used providing ten sets of paediatric packs for study. Each batch of six paediatric units was transported using the regular NHS blood transport carriers to the blood centre at Derriford Hospital (Plymouth, UK), and stored in the hospital blood bank in a quarantined section in the same refrigerator that houses units for clinical use. One pack was removed from each set on arrival at the blood bank (3 days after donation), one at 7 days after donation, and then one every 7 days up to 35 days storage.
Preparation of extracellular phase for biochemical measurements
The packed cell units were gently inverted a few times to mix the contents. The blood was removed and centrifuged in plain vacutainers. The tubes were centrifuged at 1,500×g for 10 minutes to separate the packed cells from the extracellular phase24. The extracellular phase was removed, 400 μL of this were removed and added to 400 μL of 10% metaphosphoric acid (MPA) containing 2 mM ethylene diamine tetra-acetic acid (EDTA) A for measurement of ascorbic acid. The MPA extract was centrifuged at 10,000×g at 4 °C for 10 minutes and the supernatant stored at −80 °C for later analysis. The remainder of the extracellular phase from the original centrifugation was stored at −80 °C prior to the other biochemical analyses.
Total extracellular iron and total iron-binding capacity
These two parameters were assessed by a scaled down version of the methods recommended by the International Committee for Standardisation in Haematology as described previously23. Briefly, 400 μL of extracellular phase, blanks and standards in SAGM were added to 400 μL of protein precipitation solution (0.6M trichloroacetic acid and 0.4M thioglycolic acid in 1M HCl). This was mixed thoroughly for 1 minute and incubated at 56 °C for 15 minutes in a water bath. The samples were cooled and centrifuged for 5 minutes at 1,000×g to provide an optically clear supernatant. Five hundred microlitres of this were added to 500 μL of ferene {0.5 mM 3-(2-pyridyl-5,6-bis-[2–5-furyl sulphonic acid]-1,2,4 triazine} in 1.5M sodium acetate. This was incubated for 5 minutes before absorbance was measured at 593 nm in a spectrophotometer. The iron concentration was computed from the absorbance of standards included in each batch of samples.
To determine iron-binding capacity, 350 μL of extracellular phase were added to 350 μL of iron-saturating solution (100 μM FeCl in 5 mM HCl). This was mixed and allowed to stand at room temperature for 5 minutes. Next, 35 mg of light magnesium carbonate was added and the mixture agitated for 30 minutes. The magnesium carbonate was then removed by two sequential centrifugation steps of 1,000×g for 5 minutes. Finally, 400 μL of the resultant supernatant were removed for the measurement of iron as described above.
Non transferrin-bound iron
NTBI was measured using a slight modification of the high performance liquid chromatography (HPLC) methods of Kime et al.29 and Pafetti et al.30 as described in detail previously23. Briefly, 400 μL of extracellular phase were incubated with 40 μL of 0.8M nitrilotriacetic acid (NTA) for 20 minutes at room temperature to chelate loosely bound iron, such as that chelated with residual citrate or albumin. The samples were then placed in 30 kDa Amicon Ultra 0.5 mL filters (Millipore, Watford, UK) and centrifuged at 13,000×g at 4 °C for 30 minutes. Two hundred and fifty microlitres of the ultrafiltrate were removed and incubated with 25 μL of 35 mM 3-hydroxy-1-propyl-2-methyl-pyridon-4-one for 5 minutes before being injected into the HPLC system (sample loop 20 μL). The mobile phase consisted of 5 mM PIPES buffer pH 7.0 containing 3.5 mM 3-hydroxy-1-propyl-2-methyl-pyridon-4-one and 5% acetonitrile. The column was a PEEK lined 100 mm × 5 mm C18 column (Hichrom). All tubing was PEEK. The mobile phase was pumped at a flow rate of 1 mL/minute using a Dionex pump. The absorbance of the iron-chromophore complex was determined using a Dionex UV/VIS detector at a wavelength of 450 nm and the chromatography conducted using Chromeleon software (Thermo Fisher Scientific, Loughborough, UK). The concentration of NTBI was computed from blanks and standards taken through the whole procedure with each batch of samples.
Measurement of ascorbate
Total and oxidised ascorbate were measured by the method described by Sato et al31. Two aliquots of 90 μL were removed from the initial MPA extract. To one of these 10 μl of 5% MPA were added. A further 200 μL of 5% MPA were added, the sample mixed and injected into the HPLC system (20 μL sample loop). This provided the value for reduced ascorbate. To the other aliquot, 10 μL of tris (2-carboxyethyl) phosphine hydrochloride (TCEP) (350 mM) were added and the mixture incubated at room temperature for 20 minutes to convert all the oxidised ascorbate to the reduced form31. Two hundred microlitres of 5% MPA were then added, the sample mixed and injected into the HPLC system. This provided a measure of total ascorbate. The degree of ascorbate oxidation was computed by subtracting the value for reduced ascorbate from that of the total ascorbate.
The HPLC system consisted of a 150 mm × 4.6 mm ACE 5 AQ C18 column (Hichrom, Reading, UK), a Milton Roy Constametric pump (Milton Roy, Sunderland, UK) coupled to a BAS LCD40 electrochemical detector (BASi, West Lafayette IN, USA) set at a voltage of +0.6 V. The mobile phase consisted of 50 mM phosphate buffer containing 0.54 mM EDTA and 2% methanol at pH 2.8. This was pumped at a rate of 1.0 mL/minute.
Measurement of glutathione
Glutathione was measured by a procedure based on those described by Kand’ar et al.32 and Michaelsen et al33. For extracellular glutathione (GSH), 100 μL of sample, or standard or blank were added to either 100 μL of H2O or 100 μL of 100 mM n-ethylmaleimide [NEM] in H2O. The tubes were incubated at room temperature for 30 minutes, after which time 100 μL of trichloroacetic acid (0.1 g/mL in 2 mM EDTA) were added to each tube. The tubes were mixed well and centrifuged at 15,000×g for 5 minutes at 4 °C. Fifty microliters of supernatant were removed and added to 200 μL of o-phthaldialdehyde (OPA) derivatising solution (75 mM OPA in 0.125M borate buffer pH 9.5 containing 10% vol ethanol and 2 mM EDTA33). Samples were incubated for 2.5 minutes at room temperature before injection into the HPLC system. For the measurement of GSH in whole blood, 200 μL of thawed stored whole blood (−80 °C) were added to 200 μL of H2O. The mixture was placed in 30 kDa Amicon Ultra filters (0.5 mL) (Millipore) and centrifuged at 13,000×g for 30 minutes to separate the particulate matter from the ultrafiltrate. Two 100 μL aliquots of ultrafiltrate were removed. One was added to 100 μL of H2O, while the other was added to 100 μL of 100 mM NEM. The samples were incubated for 30 minutes at room temperature, after which time 100 μL of 0.1 g/mL trichloroacetic acid in 2 mM EDTA were added. The tubes were centrifuged at 15,000×g for 10 minutes at 4 °C. One hundred microlitres of supernatant were removed and diluted 1:10 with H2O containing 2 mM EDTA. One hundred microlitres were removed and processed as described above.
The sample was analysed on a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific) comprising an ultimate 3000 pump and fluorescence detector. Chromatography was controlled using Chromeleon software (version 6.8) (Thermo Fisher Scientific). Chromatography was conducted using a 100 mm × 4.6 mm Hichrom C18 column, protected by a guard column of the same resin and a 2 μm inline filter (Hichrom, Theale, UK). The mobile phase was pumped at a rate of 1.0 mL/min. Glutathione was detected using an excitation wavelength of 338 nm and an emission wavelength of 458 nm.
Measurement of oxidative stress
MDA is the most robust marker of oxidative damage to phospholipids and is the most abundant product of lipid peroxidation. Thus MDA is an excellent marker of oxidative stress in stored erythrocytes and was measured in this study by the method of Agarwal and Chase34, which we have used previously1,22. In a 2.0 mL screw top centrifuge tube, 50 μL of plasma, standard or blank were added to 50 μL of butylated hydroxytoluene (BHT) (0.05% in 95% ethanol), followed by 400 μL of 0.44M phosphoric acid and 100 μL of 42.0 mM thiobarbituric acid. The tubes were capped, mixed well and heated for 60 minutes at 100 °C in a dry block. After cooling, 300 μL of n-1-butanol were added to the tubes and the tubes mixed well to permit the extraction of MDA into the butanol. The tubes were centrifuged at 13,000×g at 4 °C to separate the aqueous and butanol phases. Portions (20 μL) of the butanol extract were injected directly into the HPLC system, which was a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific) comprising an ultimate 3000 pump and fluorescence detector. Chromatography was conducted using a 100 mm × 4.6 mm Hichrom C18 column, protected by a guard column of the same resin and a 2 μm inline filter (Hichrom). Chromatography was controlled using Chromeleon software (version 6.8) (Thermo Fisher Scientific). The mobile phase (50 mM potassium phosphate/methanol 80;20 v/v, pH 6.8) was pumped at a rate of 1.0 mL/min. MDA was detected using an excitation wavelength of 515 nm and an emission wavelength of 553 nm.
Measurement of extracellular haemoglobin
The viability of the erythrocyte membranes during storage was assessed by measuring the level of Hb in the extracellular phase35. A very small modification was made to the standard cyanohaemoglobin method of Hb measurement36. A 100 μL portion of extracellular phase was added to 2.0 mL of Drabkin’s reagent. This was incubated for 15 minutes at room temperature and the absorbance at 540 nm measured in a spectrophotometer. The level of Hb was computed from standards and blanks made up in SAGM fluid.
Statistics
Descriptive and correlational analysis was completed using Stat 200 (Biosoft, Cambridge, UK).
Results
Total iron, non-transferrin bound iron and iron-binding capacity
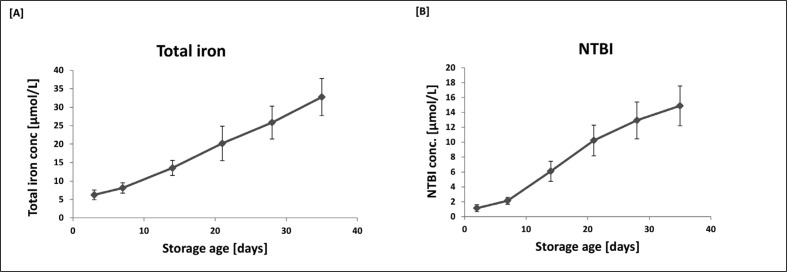
Changes in the concentration of total iron and NTBI during storage are shown in Figure 1. Even on arrival at the Blood Bank (3 days after donation) there was already measurable iron and NTBI in the extracellular fluid surrounding the packed cells. From that point on the level of total iron rose almost linearly to a level of 32.75 μmol/L at maximal storage age. NTBI levels also rose throughout the storage period reaching a maximum level of 14.88 μmol/L on day 35. Thus 45.4% of the total iron in the extracellular medium on day 35 was in the potentially highly toxic NTBI form. From day 14 onwards NTBI represented 45–50% of the total iron in the extracellular fluid. It is no surprise therefore, that the high level of NTBI was associated with a predicted very low iron-binding capacity throughout the storage period (6.014±1.813 – 6.857±2.006 nmol/mL] and that it changed very little throughout the storage period.
Figure 1.
Levels of total iron [A] and NTBI [B] as a function of storage age.
Data are presented as the mean ± SD of ten sets of paediatric packs. ANOVA with post-ANOVA Duncan’s multiple comparison test confirmed significant differences (p<0.05) between all points analysed with the exception of days 3 and 7.
Extracellular haemoglobin and malondialdehyde
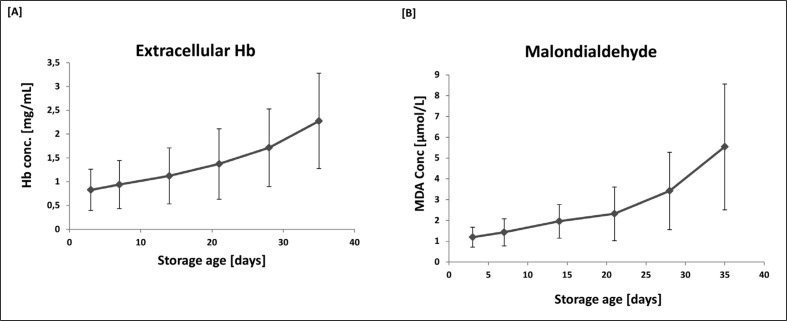
In many studies of the storage of packed red blood cell units, the appearance of Hb in the extracellular medium is used as a marker of the degree of haemolysis. MDA is a robust marker of lipid peroxidation1,22. The changes in these parameters during storage is shown in Figure 2. Unlike the pattern of changes in iron and NTBI, both Hb and MDA rose more slowly over days 3 to 21 and then more steeply towards the end of the period of storage. The mean (± SD) total Hb level in the packs was 27.31±3.26 mg/dL. Assuming that extracellular Hb is a reasonable marker of haemolysis, the maximal level of Hb in the extracellular phase was 0.83% of the total.
Figure 2.
Levels of extracellular Hb [A] and MDA [B] as a function of storage age.
Data are presented as the mean ± SD of ten sets of paediatric packs. ANOVA with post-ANOVA Duncan’s multiple comparison test confirmed significant differences (p<0.05) between all points analysed in plot A (Hb) with the exception of days 28 and 35, and days 28 and 21. Similarly, in plot B (MDA) significant differences were observed between all points with the exception of days 28 and 14.
Not surprisingly there were strong positive correlations between the level of oxidative damage (MDA) and Hb (r=0.736; p<0.001; n=60). In addition there were strong positive correlations between MDA and total iron (r=0.785; p<0.001; n=60) and MDA and NTBI (r=0.602; p<0.001; n=60).
Ascorbate and glutathione
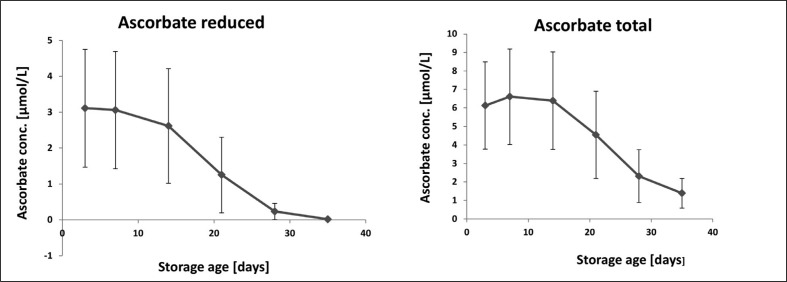
Figure 3 shows how the level of total and reduced ascorbate varied according to the storage age of the units. Levels of both parameters remained relatively constant until around day 14 when they fell significantly to minimum levels on day 35. Comparing the data from the two graphs indicates that a high proportion of total ascorbate was in the oxidised form, with this amount reaching nearly 100% on day 35. The data also indicate that the ability to convert oxidised ascorbate back to the reduced form appears to be limited in stored packed red cells. The oxidised form of ascorbate, dehydroascorbate, is itself susceptible to degradation during storage and can be rapidly and irreversibly hydrolysed to 2,3-diketogulonic acid at neutral pH, but not in acid conditions37. It is possible that hydrolysis of dehydroascorbate during storage accounted for the loss of reducible ascorbate. The relationship between MDA and reduced and oxidised ascorbate showed a positive correlation between oxidised ascorbate and MDA (r=0.465; p<0.01; n=60), and a negative correlation between reduced ascorbate and MDA (r=0.426; p<0.01; n=60). Total iron and NTBI were positively correlated with oxidised ascorbate (r=0.716; p<0.001 [total iron]; r=0.659 p<0.001 [NTBI] n=60), and negatively with reduced ascorbate (r=0.694; p<0.001 [total]; r=0.667; p<0.001 [NTBI]; n=60).
Figure 3.
Levels of extracellular total and reduced ascorbate as a function of storage age.
Data is presented as the mean ± SD of 10 sets of paediatric packs. Anova with post-anova Duncan’s multiple comparison test confirmed significant differences (P<0.05) between levels on day 03 and days 21, 28 and 35, but not between days 3, 7 and 14 in both plots.
The level of total glutathione was very low in the extracellular medium of the packed cell units and changed very little during storage (Table I). Furthermore, the addition of NEM to the samples during analysis effectively abolished the glutathione fluorescence indicating that, unlike the situation with regard to ascorbate, glutathione remains almost exclusively in the reduced form. In contrast, the level of GSH in whole blood was predictably high and decreased gradually from a maximum level on day 3 to a lowest value on day 35. These findings are similar to those reported previously38,39. In contrast to the previous studies, in this study a rise was not seen in GSSG in the later stages of storage. Over 90% of the GSH was in the reduced form. Oxidised GSSG changed little throughout the storage period. The magnitude of the loss of GSH over time in comparison to the level of GSSG indicates that in this study, the loss of GSH is more likely to be due to metabolism via a route other than oxidation to GSSG40, coupled with reduced de novo synthesis in the latter stages of storage38. There was a strong negative correlation between total blood GSH and total extracellular iron (r=0.723 p<0.001; n=60) and NTBI (r=0.691 p<0.001; n=60).
Table 1.
GSH and GSSG levels as a function of storage age.
Data is presented as mean level ± standard deviation of 10 observations. Anova with post-Anova Duncan’s multiple comparison test confirmed significant differences in total blood glutathione (p<0.05) between the level at day 3 and all other days with the exception of day 7.
| Storage age [days] | Total blood glutathione [μmol/L] | Total blood oxidised glutathione [μmol/L] | Extracellular glutathione [μmol/L] |
|---|---|---|---|
| 03 | 621.10±64.56 | 5.700±1.318 | 0.709±0.467 |
| 07 | 581.11±48.60 | 5.253±0.992 | 0.713±0.376 |
| 14 | 570.09±56.05 | 4.995±2.182 | 0.532±0.176 |
| 21 | 520.28±39.21 | 4.615±1.516 | 0.521±0.196 |
| 28 | 480.39±39.38 | 4.799±1.671 | 0.525±0.178 |
| 35 | 442.79±37.78 | 3.494±0.952 | 0.574±0.231 |
Discussion
The results of this study have confirmed and extended a number of findings from previous work24–28,41. In addition, the combination of parameters measured in this study have provided data from which a potential mechanism may be proposed to account for the changes observed in paediatric packed red blood cells during storage. The presence of iron and NTBI in the extracellular medium surrounding the packed red blood cells on arrival at the blood bank, coupled with the presence of MDA and Hb indicates that initial changes occurring during blood collection and processing lead to oxidative damage to components of the packed cell units. This provides an initial pro-oxidant environment around and, probably, within the red blood cells. Following on from the initial damage to the erythrocytes, oxidative damage is on-going and progressive throughout the period of storage. The very high level of iron and NTBI in the extracellular phase indicate that it could not all have originated from the relatively low level of Hb present in the extracellular environment surrounding the red blood cells. It must have derived as a result of damage to iron-binding proteins within the red blood cell and leaked out into the extracellular fluid. Hb within the red blood cell is usually well protected by an array of antioxidants and other protective molecules42. When red blood cells are stored under refrigerated conditions the mechanisms protecting the cells lose their effectiveness and Hb becomes vulnerable to oxidation43. This has been confirmed in many previous studies44. In this study and others38,39,45, the intracellular GSH level fell consistently throughout the period of storage and the extracellular level of MDA rose, indicating that oxidative damage to membrane phospholipids was ongoing. GSH is also an important anti-oxidant which protects haem and Hb against excessive oxidation46, and the loss of intracellular GSH during storage would limit this degree of protection. Oxidative modification of Hb may include damage to the haem moiety at any of the double bonds which would lead to the release of free iron which may then participate in further generation of reactive oxygen species47. The free iron, and iron bound to haem may then be able to leave the red blood cell and accumulate in the extracellular medium of the stored packs. This scenario is likely as a variety of oxidising agents have been shown to cause the release of iron from erythrocytes13,14, and this appears to be associated with the formation of methaemoglobin, a relatively unstable Hb derivative which can readily release the haem moiety from the haem pocket11,15. The iron that is released from Hb under these conditions appears to be redox active13, and thus potentially damaging. The nature of NTBI in plasma is not fully understood48, and even less is known about the nature of the NTBI in the extracellular fluid of stored packed cells. It is possible that a portion of the NTBI measured is iron bound to or removed from haem during the assay procedure. Previous studies in stored adult packs49 showed that sufficient haem could be present in the extracellular fluid to contribute significantly to the measured total iron and NTBI levels. Further studies are required to find out more about the nature of the iron in these packs.
Previous studies also proposed that oxidative stress during storage drives many of the changes in protein and lipid structure that occur during storage39,45. Previous studies indicated that iron-induced free radical generation is capable of initiating lipid peroxidation and causing haemolysis in stored and circulating red blood cells50–52. Studies have shown positive effects of some anti-oxidants and iron chelators in defending against lipid peroxidation in stored erythrocytes27,28, and fresh erythrocytes challenged with free radicals in vitro53. Ascorbate is particularly adept at defending against oxidative damage to the haemoglobin molecule54. Ascorbate is able to reduce the products of Hb oxidation and protect against the consequences of Hb oxidation such as the release of redox active iron54. The ability of ascorbate to reduce methaemoglobin is particularly important55, as is its ability to reduce ferryl Hb54 which is another derivative of Hb formed when Hb is oxidised15. The relative paucity of ascorbate, and the high degree of ascorbate oxidation would limit the protective action of ascorbate in packed cell preparations. Interestingly, a recent study demonstrated that the addition of ascorbate to stored murine red blood cells improved post-transfusion recovery of the red blood cells45.
In this study we used extracellular Hb as a marker of haemolysis35,56. Comparing the measured total Hb and extracellular Hb, and assuming a haematocrit of around 60% (the target haematocrit during preparation of the packs), a calculated figure for the degree of haemolysis even at 35 days storage was only 0.33%. This is very close to the value reported by Gevi et al.38, and well below the maximum of 0.8% recommended by the European guidelines. This further supports the view that the high level of extracellular iron and NTBI did not originate as a result of haemolysis but through intracellular oxidative damage, as outlined above. It is likely that some of the measured Hb is present in Hb-rich microparticles which are shed from the red blood cells during storage57,58. The proportion of Hb present in microvesicles during storage can be large and even exceed free extracellular levels57. These microparticles also contain other proteins and lipids, some of which may be oxidatively modified59. The purpose of the generation of microvesicles is unclear, but the Hb contained within them could contribute to the measured Hb in this study and be a source of free iron if oxidatively modified. Whether the Hb contained within microparticles can be considered as a valid marker of haemolysis is unclear. Consequently, the calculated degree of haemolysis based on extracellular Hb as measured in this study may be an overestimate. Importantly, the large build-up of iron and NTBI in the extracellular environment during storage occurs despite limited haemolysis.
The question that needs to be addressed is whether or not the changes in iron and other factors during storage might have a detrimental effect on the recipients. To put the data in context, total iron rose to about 30 μM and NTBI to about 15 μM in this study. Studies in our laboratory in adult controls and type 2 diabetics provided figures of total iron of 19±6.6 μM (controls, n=24) and 17.9±7.6 μM (diabetics, n=54). The median NTBI was 0.34 μM (range, 0–2.08) in controls and 0.21 μM (range, 0–1.34) in diabetics. Consequently, the iron status of the stored packs, particularly NTBI, is massively in excess of the normal values. Given that premature babies receive approximately 10% of their blood volume over a 4-hour transfusion period, the potential for iron and oxidative overload is significant, particularly since they are poorly equipped to deal with additional iron and oxidative load18–20,60 being limited in the processes which operate to sequester free iron such as transferrin, caeruloplasmin and albumin. Premature babies also have limited anti-oxidant defences to protect against circulating free radicals1,20,21,61. Furthermore, the concentration of the low molecular weight anti-oxidants ascorbate, urate and possibly glutathione in serum and bronchoalveolar lavage fluid in premature babies falls during the first week of life and recovers over the next few weeks1,21,61–63. Premature babies who require blood transfusions will receive their first transfusion, and possibly the majority of their treatments within the first week of life. Thus the receipt of blood, with the possibility of generating excessive free radicals, coincides with a period when anti-oxidant protection is falling. Consequently, the premature baby may be particularly susceptible to adverse reactions of transfused iron because of their poor anti-oxidant status and inability to deal with any excess iron. In addition, any free Hb or haem present in the transfused blood, and supplemented by post-transfusion haemolysis64 would be potentially toxic in its own right65,66 unless it is rapidly sequestered by haptoglobin65,67 and hemopexin68. The removal of plasma and the replacement with additive effectively removes most of the haptoglobin and hemopexin such that free Hb and haem could be available in the transfused blood. Premature babies appear to have haptoglobin in the circulation69 and may be able to up-regulate the level as necessary70. Thus the potentially toxic influence of free Hb may be limited by the presence of haptoglobin in the recipients. In contrast, hemopexin levels are low71 in premature babies who could be at risk of haemmediated toxicity.
This study has highlighted potential mechanisms for storage-related changes in iron and oxidative load in paediatric packed red blood cell units. There is evidence and logic to suggest that these changes could be potentially dangerous if older stored blood were to be transfused to premature babies. However, these are effectively just theoretical potential risks and it is generally considered that there is no urgency to change transfusion practice in the neonatal intensive care unit.
This view is supported by the results of a recent randomised controlled study which examined the influence of blood stored for less than 7 days compared to that administered according to standard practice72. The mean storage age of the “fresh” blood was 5.1 days and that of the “older” blood 14.6 days. The study showed no changes in outcome with regard to the major consequences of prematurity viz necrotising enterocolitis, ROP, bronchopulmonary dysplasia and IVH as well as death. Our data would suggest that any major impact of iron and/or oxidative load in premature babies is likely to occur following transfusion with blood that has been stored in excess of 21 days and possibly more than 14 days.
This is supported by other studies which indicated that, from biochemical and molecular standpoints (in addition to iron and related factors), the parameters defining the integrity of SAGM-stored leucoreduced red blood cells (as studied here) may be acceptable up to 14 days of storage, but then decline39. For this reason, it may be pertinent to study the clinical impact of blood stored for longer than 14–21 days.
Acknowledgements
This study was funded by The Northcott Devon Medical Foundation. We would like to acknowledge the help of Samantha Harle-Stephens of the Derriford Hospital Blood Bank for arranging the receipt, storage and availability of the paediatric blood packs used in this study.
Footnotes
The Authors declare that they have no conflicts of interest relevant to the manuscript.
References
- 1.Collard KJ, Godeck S, Holley JE, Quinn MW. Pulmonary antioxidant levels and oxidative damage in ventilated premature babies. Arch Dis Child. 2004;89:F412–6. doi: 10.1136/adc.2002.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer VL, Lambert DK, Henry E, et al. Among very-low-birth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011;51:1170–8. doi: 10.1111/j.1537-2995.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Dib M, Narang S, Lee E, et al. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. 2011;31:183–7. doi: 10.1038/jp.2010.157. [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli C. Risk factors and growth factors in ROP. Early Hum Devel. 2009;85:S79–82. doi: 10.1016/j.earlhumdev.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Akkoyun I, Oto S, Yilmaz G, et al. Risk factors in the development of mild and severe retinopathy of prematurity. J Am Ass Pediatr Ophthalmol Strabismus. 2006;10:449–53. doi: 10.1016/j.jaapos.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypoth. 2006;66:355–64. doi: 10.1016/j.mehy.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Perrone S, Tataranno ML, Negro S, et al. Early Hum Devel. 2010;86:241–4. doi: 10.1016/j.earlhumdev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Bell EF. When to transfuse preterm babies. Arch Dis Child Fetal Neonatal Ed. 2008;93:F469–73. doi: 10.1136/adc.2007.128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–1913. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 10.Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care. 2010;14:R57–64. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Ferrali M, Signorini C, Ciccoli L, Comporti M. Iron release and membrane damage in erythrocytes exposed to oxidising agents, phenylhydrazine, divicine, and isouramil. Biochem J. 1992;285:295–301. doi: 10.1042/bj2850295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Rad Biol Med. 2002;32:568–76. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 15.Kanias T, Acker JP. Biopreservation of red blood cells – the struggle with haemoglobin oxidation. FEBS J. 2010;277:343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 16.Moison RMW, Hasnoot AA, Van Zoren-Grobben D, Berger HM. Plasma proteins in acute and chronic lung disease of the newborn. Free Rad Biol Med. 1998;25:321–8. doi: 10.1016/s0891-5849(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 17.Lackmann GM, Hess L, Tollner U. Reduced iron-associated antioxidants in premature newborns suffering intracerebral haemorrhage. Free Rad Biol Med. 1996;20:407–9. doi: 10.1016/0891-5849(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 18.Lindeman JHN, Lentjes EGWM, Van Zoeren-Grobben D, Berger HM. Postnatal changes in plasma caeruloplasmin and transferrin antioxidant activities in preterm babies. Biol Neonate. 2000;78:73–6. doi: 10.1159/000014252. [DOI] [PubMed] [Google Scholar]
- 19.Galinier A, Periquet B, Lambert W, et al. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum Devel. 2005;81:583–93. doi: 10.1016/j.earlhumdev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JL. Iron, plasma antioxidants, and the ‘oxygen radical disease of prematurity. Am J Dis Child. 1988;142:1341–4. doi: 10.1001/archpedi.1988.02150120095048. [DOI] [PubMed] [Google Scholar]
- 21.Vyas JR, Currie A, Dunster C, et al. Ascorbic acid concentration in airways lining fluid from infants who develop chronic lung disease of prematurity. Eur J Pediatr. 2001;160:177–84. doi: 10.1007/s004310000709. [DOI] [PubMed] [Google Scholar]
- 22.Collard KJ, Godeck S, Holley JE. Blood transfusion and pulmonary lipid peroxidation in ventilated premature babies. Pediatr Pulmonol. 2005;39:257–61. doi: 10.1002/ppul.20190. [DOI] [PubMed] [Google Scholar]
- 23.Collard K, White D, Copplestone A. The effect of maximum storage on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2012;12:1–9. doi: 10.2450/2012.0046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marwah SS, Blann A, Harrison P, et al. Increased non-transferrin bound iron in plasma depleted SAG-M red blood cell units. Vox Sang. 2002;82:122–6. doi: 10.1046/j.1423-0410.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumaswala UJ, Zhuo L, Jacobsen DW, et al. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Rad Biol Med. 1999;27:1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 26.Knight J, Voorhees RP, Martin L. The effect of metal chelators on lipid peroxidation in stored erythrocytes. Ann Clin Lab Sci. 1992;22:207–13. [PubMed] [Google Scholar]
- 27.Knight J, Searles DA, Clayton FC. The effect of desferrioxamine on stored erythrocytes: lipid peroxidation, deformity and morphology. Ann Clin Lab Sci. 1996;26:283–90. [PubMed] [Google Scholar]
- 28.Knight JA, Searles DA. The effects of various antioxidants on lipid peroxidation in stored whole blood. Ann Clin Lab Sci. 1994;24:294–301. [PubMed] [Google Scholar]
- 29.Kime R, Gibson A, Yong W, et al. Chromatographic method for the determination of non-transferrin-bound iron suitable for use on the plasma and bronchoalveolar lavage fluid of preterm babies. Clin Sci. 1996;91:633–9. doi: 10.1042/cs0910633. [DOI] [PubMed] [Google Scholar]
- 30.Paffetti P, Perrone S, Longini M, et al. Non-protein bound iron detection in small samples of biologic fluids and tissues. Biol Trace Elem Res. 2006;112:221–32. doi: 10.1385/BTER:112:3:221. [DOI] [PubMed] [Google Scholar]
- 31.Sato Y, Uchiki T, Iwama M, et al. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull. 2010;33:364–9. doi: 10.1248/bpb.33.364. [DOI] [PubMed] [Google Scholar]
- 32.Kand’ar R, Zakova P, Lotkova H, et al. Determination of reduced and oxidised glutathione in biological samples using liquid chromatography with fluorometric detection. J Pharmaceut Biomed Anal. 2007;43:1382–7. doi: 10.1016/j.jpba.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Michaelsen JT, Dehnert S, Giustorini D, et al. HPLC analysis of human eryhthrocytic glutathione forms using OPA and N-acetyl-cysteine ethyl ester: evidence for nitrite-induced GSH oxidation to GSSG. J Chromatog B. 2009;877:3405–17. doi: 10.1016/j.jchromb.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal R, Chase S. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatog B. 2002;775:121–6. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 35.Almizraq R, Tchir JDR, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–67. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 36.Moore GL, Ledford ME, Merydith A. A micromodification of the Drabkin haemoglobin assay for measuring plasma haemoglobin in the range of 5 to 2000 mg/dl. Biochem Med. 1981;26:167–73. doi: 10.1016/0006-2944(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 37.Karlsen A, Blomhoff R, Gundersen TE. Stability of whole blood and plasma ascorbic acid. Eur J Clin Nutr. 2007;61:1233–6. doi: 10.1038/sj.ejcn.1602655. [DOI] [PubMed] [Google Scholar]
- 38.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 39.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red blood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu G, Fang Y-Z, Yang S, et al. Glutathione metabolism and its implications for health. J Nutrition. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 41.Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child Fetal Neonatal Ed. 2013;98:F424–9. doi: 10.1136/archdischild-2012-303353. [DOI] [PubMed] [Google Scholar]
- 42.Gladwin MT, Kanias T, Kim-Shapiro DM. Hemolysis and cell free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122:1205–8. doi: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang W, Weisbach V, Sticht H, et al. Oxidative stress-induced postranslational modifications of human hemoglobin in erythrocytes. Arch Biochem Biophys. 2013;529:34–44. doi: 10.1016/j.abb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Stowell SR, Smith NH, Zimring JC, et al. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;54:2248–57. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- 46.Simoni J, Villanueva-Mayer J, Simoni G, et al. Artif Organs. 2009;33:115–26. doi: 10.1111/j.1525-1594.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 47.Maitra D, Byun J, Andreana PR, et al. Reaction of haemoglobin with HOCl: Mechanism of heme destruction and free iron release. Free Rad Biol Med. 2011;51:374–86. doi: 10.1016/j.freeradbiomed.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans RW, Rafique R, Zarea A, et al. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem. 2008;13:57–74. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 49.Ozment CP, Mamo LB, Campbell ML, et al. Transfusion-related biologic effects and free haemoglobin, heme, and iron. Transfusion. 2013;54:732–40. doi: 10.1111/j.1537-2995.2012.03837.x. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhary R, Katharia R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfus. 2012;10:59–62. doi: 10.2450/2011.0107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 52.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Membrane protein carbonylation in non-leukodepleted CPDA-preserved red blood cells. Blood Cell Mol Dis. 2006;36:279–82. doi: 10.1016/j.bcmd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Ertabak A, Kutluay T, Unlu A, et al. The effect of deferrioxamine on peroxynitrite-induced oxidative damage in erythrocytes. Cell Biochem Funct. 2004;22:149–52. doi: 10.1002/cbf.1056. [DOI] [PubMed] [Google Scholar]
- 54.Dunne J, Caron A, Menu P, et al. Ascorbate removes key precursors to oxidative damage by cell free haemoglobin in vitro and in vivo. Biochem J. 2006;399:513–24. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free haemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gkoumassi E, Dijkstra-Tiekstra MJ, Hoentjen D, de Wildt-Eggen J. Hemolysis of red blood cells during processing and storage. Transfusion. 2012;52:489–92. doi: 10.1111/j.1537-2995.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- 57.Greenwalt TJ, McGuiness CG, Dumasawala UJ. Studies in red blood cell preservation, 4: plasma vesicle haemoglobin exceeds free haemoglobin. Vox Sang. 1991;61:14–7. doi: 10.1111/j.1423-0410.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 58.Greenwalt TJ. The how and why of exocytotic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 59.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signalling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 60.Marzocchi B, Perrone S, Pafetti P, et al. Nonprotein-bound iron and plasma protein oxidative stress at birth. Pediatr Res. 2005;58:1295–1295. doi: 10.1203/01.pdr.0000183658.17854.28. [DOI] [PubMed] [Google Scholar]
- 61.Silvers KM, Gibson AT, Russell JM, Powers HJ. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child. 1998;78:F214–9. doi: 10.1136/fn.78.3.f214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15:191–5. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Rook D, Te Braake FWJ, Schierbeek H, et al. Glutathione synthesis rates in early postnatal life. Pediatr Res. 2010;67:407–11. doi: 10.1203/PDR.0b013e3181d22cf6. [DOI] [PubMed] [Google Scholar]
- 64.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baek JH, D’Agnillo FD, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buehler PW, D’Agnillo F. Toxicological consequences of extracellular haemoglobin: biochemical and physiological perspectives. Antioxid Redox Signal. 2010;12:275–91. doi: 10.1089/ars.2009.2799. [DOI] [PubMed] [Google Scholar]
- 67.Boretti FS, Buehler PW, D’Agnillo F, et al. Sequestration of extracellular haemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–80. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hvidberg V, Manieki MB, Jacobsen C, et al. Identification of the receptor scavenging hemopexin-heme complexes. Red Cells. 2005;106:2572–9. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 69.Chavez-Bueno S, Beasley JA, Goldbeck JM, et al. Haptoglobin concentrations in preterm and term newborns. J Perinatol. 2011;31:500–3. doi: 10.1038/jp.2010.197. [DOI] [PubMed] [Google Scholar]
- 70.Buhimschi CS, Bhandari V, Dulay AT, et al. Proteomics mapping of cord blood identifies haptoglobin ‘switch-on’ pattern as biomarkers of early-onset neonatal sepsis in preterm newborns. PLoS One. 2011;6:e26111. doi: 10.1371/journal.pone.0026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanakoudi F, Drossou V, Tzmouli V, et al. Serum concentrations of 10 acute phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem. 1995;41:605–8. [PubMed] [Google Scholar]
- 72.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature very low birth-weight infants. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]