Abstract
Background
The Haemonetics MCS®+ cell separator is a device dedicated to the collection of leucoreduced single-donor platelets. The new Universal Platelet protocol has been introduced to improve the efficiency of apheresis and increase flexibility in the collection of leucoreduced platelets in combination with red blood cells and plasma. In this study we compared its performance with that of the previous Concentrated Single Donor Platelet protocol.
Materials and methods
This observational study had a within-subject design and involved 135 donors who underwent plateletapheresis with both protocols. The primary end-point was collection efficiency; secondary end-points were other performance indices, such as procedure time and collection rate. A satisfaction questionnaire was also administered to the 135 donors to evaluate opinions on duration, comfort and side-effects of donations with the two protocols. For each parameter of interest, we tested the difference between the two protocols within donors, using a one-sample t-test or exact McNemar’s test as appropriate.
Results
The collection efficiency of the Universal Platelet protocol was significantly higher than that of the Concentrated Single Donor Platelet protocol (58% vs 47%; p<0.0001). The Universal Platelet Protocol collected more platelets in less time, leading to a higher collection rate (6.5 vs 5.0×109/min; p<0.0001). In general, donors found apheresis with the Universal Platelet protocol of equal duration or faster, of similar or greater comfort and with an equal number or fewer side effects, compared with the Concentrated Single Donor Platelet protocol.
Discussion
Our study endorses the use of the new Universal Platelet protocol in daily transfusion practice since it substantially improves collection efficiency in leucoreduced platelet procedures compared with the Concentrated Single Donor Platelet protocol. This technical improvement seems to be accompanied by equal or greater comfort for the donor.
Keywords: plateletapheresis, blood donors, blood component removal
Introduction
Compared to whole blood donation, apheresis has some advantages for the donor, including a lesser loss of red blood cells, which means that even women with low haemoglobin values could undergo apheresis. However, apheresis can also lead to specific adverse events such as citrate toxicity or, for single needle devices, extracorporeal circulation reactions1–3. Since its introduction in the late 1970s the apheresis technique has been technically improved, and yet issues such as duration of the procedure and donor’s discomfort remain to be addressed.
The Haemonetics MCS®+ cell separator (Braintree, MA, USA) has been used for therapeutic apheresis and for the collection of different cell components since 19964, and its use for the collection of leucoreduced single-donor platelets (SDP) has been significantly improved in the last 20 years. Recently, Haemonetics introduced a new protocol, the Universal Platelet protocol version A-IT (UPP), to improve efficiency and increase flexibility in the collection of SDP in combination with red blood cells and plasma (www.haemonetics.com).
The UPP introduces Super Surge® technology, which consists in reprocessing platelets regularly collected by surge at previous cycles during the last cycle, with concentrated platelets in plasma further suspended in the platelet additive solution. This type of product is better tolerated by recipients and meets requirements of current pathogen inactivation methods. Prior to reprocessing, regular platelets are collected in a plastic bag that is primed with a small volume of anticoagulant citrate dextrose A (ACD-A). This priming method was successfully tested in a donor feasibility study on 95 subjects, performed at the Establissement Francais du Sang of Rhone-Alpes in 2009 (Haemonetics Corporation, unpublished internal data). A subsequent UPP CE-marking study was performed to validate the protocol, which returns plasma to the donor in three small batches at each cycle: during a pause in the collection draw to balance donor extracorporeal volume; during dead times when extracting platelets from the bowl; and at the end of the return, while rinsing the bowl and donor line (Haemonetics Corporation; unpublished internal data). This new plasma allocation during reinfusion allows reductions in both extracorporeal volume and mean plasma return flow rate, thereby reducing potential citrate reactions in the donor.
When evaluating a new device for use in the routine production system, it is important to assess its performance with regards to cell collection efficiency, collection rate and processing time. An additional important aspect is the ability to improve donors’ experience in terms of donation time and side effects. In this observational study we compared the performance of UPP with that of the previous Concentrated Single Donor Platelet (CSDP) protocol, using collection efficiency as the primary end-point and other performance indexes, such as procedure time and collection rate, as secondary end-points. We also evaluated donors’ opinions on the two protocols, collected using a simple satisfaction questionnaire.
Materials and methods
Study design
In 2011 our Service collected 1,140 SDP from 393 donors, with a mean annual collection rate per donor of 2.9. Each donor contributed a single SDP unit per donation, since our departmental policy does not allow collection of multiple SDP units.
In April 2012 the UPP was introduced in our Service to replace the previous CSDP, and a satisfaction questionnaire was administered to all consecutive donors from April to October 2012. To evaluate both performance of and donors’ satisfaction with the UPP, and compare these with those of the CSDP, we performed an observational study with a within-subject design on all consecutive donors who had donated with both protocols. In particular, we compared data on SDP obtained using the UPP (from April to October 2012) with paired retrospective data from the same donor’s last donation using the CSDP (from 2009 to 2012).
All collections were taken from healthy periodic cytapheresis donors according to Italian and European guidelines5,6, which specify the requirements for the cellular content of the products: >2×1011 platelets/unit and <1×106 white blood cells/unit.
Study population
Donors in our Service are selected using the following criteria: (i) weight >55 kg; (ii) age between 18 and 65 years; (iii) negative tests for human immunodeficiency virus, hepatitis B and C viruses and syphilis; (iv) absence of active illness; and (v) no consumption of non-steroid anti-inflammatory drugs.
Adverse events requiring any intervention or treatment (e.g. citrate toxicity, vasovagal reaction, haematoma) were documented by a nurse using a standardised form provided by our internal standard operating procedure.
After each donation, donors were asked to fill in a standardised questionnaire used in our Service when evaluating new apheresis protocols. The first part of the questionnaire evaluated the duration and comfort of the current UPP donation using a scale from 1 (absolutely unsatisfactory) to 5 (very satisfactory) (Figure 1). The second part asked whether the current UPP donation was faster and more comfortable than the previous CSDP donation, using a scale from 1 (absolutely not) to 5 (absolutely yes) for both questions, and whether the donors experienced fewer or more citrate-related side effects, using a scale from 1 (much more) to 5 (much less) (Figure 1).
Figure 1.
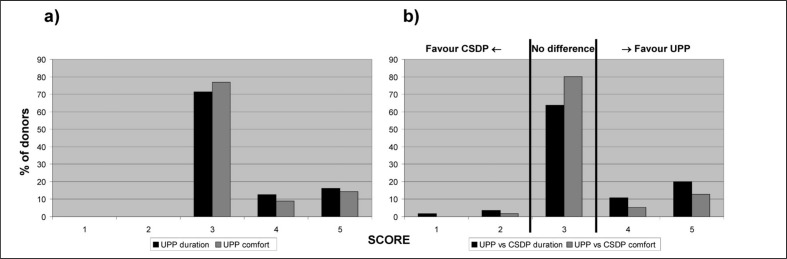
Results of the donor satisfaction questionnaire.
a) Results for UPP donation (“today’s donation”), in terms of duration [“What do you think about the duration of today’s donation?”; scale: 1=absolutely unsatisfactory, too long → 5=very satisfactory] and comfort [“How would you evaluate the comfort of today’s donation?”; scale: 1=absolutely unsatisfactory → 5=very satisfactory]; b) Results for the comparison between UPP and CSDP donation, in terms of duration [“Do you think that today’s donation (UPP) has been faster than your previous donation (CSDP)?”; scale: 1=absolutely not → 5= absolutely yes] and comfort [“Do you think that today’s donation (UPP) has been more comfortable than your previous donation (CSDP)?”; scale: 1=absolutely not → 5= absolutely yes].
Single-donor platelet equipment
We used the apheresis device Haemonetics MCS®+ cell separator, a typical discontinuation separation system that operates with the 225 mL Latham bowl7. Repeated cycles of collection and return allow the gradual accumulation of platelet-rich plasma. All procedures were performed following the manufacturer’s instructions (www.haemonetics.com) and our internal standard operating procedure based on the Italian Standard of Transfusion Medicine5.
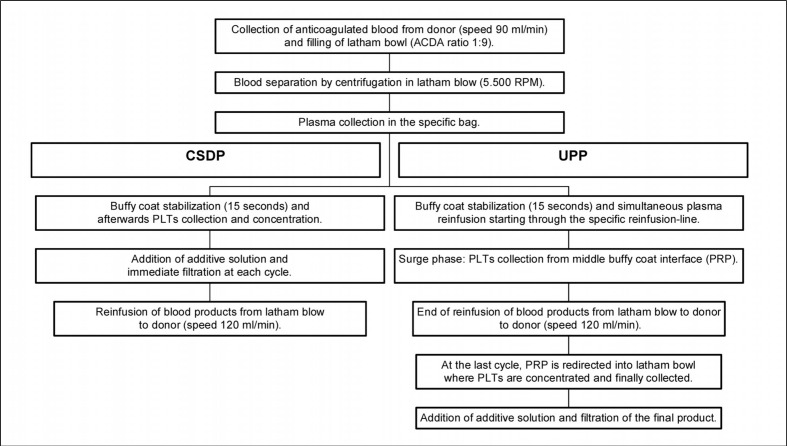
We programmed the machines to collect one SDP unit containing 3.5×1011 platelets in a volume of 250–300 mL, and an additional unit of fresh-frozen plasma of 500 mL. Details on the procedure for SDP collection using the two protocols are reported in Figure 2. The UPP limits the maximum extracorporeal volume during a draw cycle to 15%, and the maximum at the end of the procedure to 13%, net of ACD-A.
Figure 2.
SDP collection using the UPP and the CSDP protocols.
Laboratory analysis
Peripheral blood samples from donors were drawn before each SDP collection. SDP were conserved at 20–22 °C on a platelet shaker. The SDP samples for determining the platelet count were obtained after approximately 24 hours, using EDTA tubes (Venosafe, Terumo Europe, Leuven, Belgium). We used an automated blood cell counter (Advia 120 Hematology System, Siemens, Erlangen, Germany) to determine the donor and SDP values of haemoglobin, haematocrit, red blood cells, white blood cells and platelets.
Statistical analysis
The performance of the two protocols for platelet collection was evaluated in terms of collection efficiency, which represented our primary end-point, as well as collection rate, platelet yield, procedure time, and processed and collected volumes as secondary end-points.
The collection efficiency was calculated by dividing the platelet yield by the number of processed platelets, with processed platelets defined as the product of the pre-donation platelet counts times the volume of blood processed. The collection rate was calculated by dividing the platelet yield by the procedure time. The procedure time was defined as the time from the start of blood withdrawal to the end of blood reinfusion.
For all parameters of interest, we calculated the difference between UPP and CSDP within each donor. We tested the statistical significance of the difference using a one-sample, two-tailed t-test (null hypothesis of zero difference), with a P-value threshold of 0.05. Variables were log transformed to achieve normality whenever necessary. Differences in proportions were tested using the exact McNemar’s test.
Statistical analyses were performed using STATA software version 10 (StataCorp LP, College Station, TX, USA).
Results
From April to October 2012, 154 donors underwent SDP collection in our Service. Nineteen donors, of whom eight at their first apheresis donation, were excluded from the analyses because previous data using CSDP were not available. This left 135 donors, with a total of 270 donations (one with UPP and one with CSDP), available for analysis. The median time between the UPP and previous CSDP donation was 7 months (interquartile range: 4–12 months).
Clinical and laboratory findings are summarised in Table I, which shows that pre-donation values of white blood cells, haemoglobin, haematocrit and platelets were similar for UPP and CSDP donations.
Table I.
Clinical and laboratory characteristics of the 135 donors.
| Variable | At UPP donation | At CSDP donation |
|---|---|---|
| Age, years (mean, SD) | 48.5 (8.9)* | |
| Sex (male) | 94 (69.6%) | |
| Weight, kg (mean, SD) | 75 (10.5)* | |
| WBC, 109/L (mean, SD) | 5.9 (1.4) | 5.9 (1.2) |
| Hb, g/dL (mean, SD) | 14.4 (1.1) | 14.3 (1.2) |
| Platelets, 109/L (mean, SD) | 287.2 (52.5) | 281.9 (49.5) |
SD: standard deviation; WBC: white blood cells; Hb: haemoglobin. Measured at the time of the UPP donation.
Performance indices
Results for all performance indices are summarised in Table II. European and Italian product standards were satisfied for both protocols, with all apheresis donations containing >2×1011 platelets and <1×106 white blood cells.
Table II.
Performance indices for the two protocols. The P-values for the differences between UPP and CSDP were calculated using a one-sample, two-tailed t-test.
| Parameter | UPP | CSDP | P-value |
|---|---|---|---|
| Collection efficiency, % (mean, SD) | 58.2 (5.9) | 47.3 (6.1) | <0.0001 |
| Procedure time, min (mean, SD) | 60 (9) | 70 (10) | <0.0001 |
| Collection rate, ×109/min (mean, SD) | 6.5 (1.6) | 5.0 (1.2) | <0.0001 |
| Platelets yielded, 1011 (mean, SD) | 3.9 (0.6) | 3.4 (0.6) | <0.0001 |
| Volume processed, mL (mean, SD) | 2,336 (281) | 2,583 (305) | <0.0001 |
SD: standard deviation; mL: millilitre; min: minutes.
The collection efficiency was 58.2% for the UPP and 47.3% for the CSDP, with the difference being highly statistically significant (p<0.0001). The UPP yielded more platelets per bag than did the CSDP protocol (3.9 vs 3.4×1011, respectively; p<0.0001) and the platelets were collected in a shorter time (60 vs 70 minutes, respectively; p<0.0001), which resulted into a higher collection rate for the UPP (6.5 vs 5.0×109/min; p<0.0001). As a consequence of the shorter procedure time, the processed and collected volumes were lower for the UPP (2,336 vs 2,583 mL, p<0.0001, and 312 vs 349 mL, p<0.0001, respectively).
In nine donations made with the UPP (6.7%), the volume of plasma collected was <350 mL and the product was discarded, while this never happened with the CSDP (p=0.004). Macroscopic contamination of the SDP units by red blood cells was observed in five of the units collected with the CSDP protocol (3.7%) but only one of those collected with the UPP (0.7%), although the difference was not statistically significant (p=0.21). Both protocols use the same leucodepletion filter, with leucodepletion taking place in every cycle for CSDP and at the end the procedure for UPP. There was no difference in residual, cytofluorimetrically determined white blood count per SDP unit, with both protocols satisfying European and Italian product standards of a white blood cell content <1×106 (0.07×106 for CSDP and 0.05×106 for UPP; p=0.26).
Donors’ comfort and adverse reactions
There was no difference in the number of adverse events recorded by the nursing staff between UPP and CSDP donations (p=0.754). In detail, there were five (3.7%) adverse events for the UPP (four episodes of citrate toxicity and one of nausea) and seven (5.2%) for the CSDP (six episodes of citrate toxicity and one vasovagal reaction), with none of these being severe. Four donors needed a saline infusion at the end of the donation with the CSDP, while this never happened with the UPP, although the difference did not reach statistical significance (p=0.125).
Fifty-six donors (41%) filled in the satisfaction questionnaire, and the results are presented in Figure 1. Duration and comfort of the donation with the UPP were reported as very satisfactory (scores 4 and 5) by 29% and 23% of donors, respectively, with no donor reporting poor satisfaction for either aspect. For questions comparing duration and comfort of the UPP donation with those of the previous CSDP donation, no difference was reported by 64% and 80% of donors, respectively. Among the remaining donors who did find a difference, the large majority expressed a preference for the UPP for both duration and comfort (85% and 91% of donors, respectively; binomial probability test with null hypothesis of 50%: p=0.003 and p=0.012). As for the frequency of citrate-related side effects with the two protocols, no difference was reported by 63% of donors; among those who did find a difference, 67% reported fewer side effects with the UPP (p=0.189).
Discussion
When a new device is introduced into clinical or laboratory practice, it is important to validate its efficacy compared with that of the device previously in use. In this observational study in 135 donors, we compared the new UPP with the previous CSDP to evaluate both performance and impact on donors’ comfort. We tested the hypothesis of whether the new protocol can increase efficiency by combining higher collection efficiency with shorter procedure time, while reducing the volume of processed whole blood and the citrate load to the donor. We found a mean collection efficiency for the UPP of 58%, which was more than 10% higher than that of the CSDP. This was due to the fact that the UPP led to a higher number of platelets collected per bag and a lower volume of processed blood compared with CSDP. The UPP was also faster than the CSDP, with a 13% reduction in procedure time, which translated in about 10 minutes saved per donation. The combined increase in collection efficiency and reduction in procedure time led to an increase of 30% in the collection rate for the UPP compared to the CSDP. Our study confirms previous findings obtained by comparing data from the CE-marking studies of the two protocols (31 and 30 SDP donations, respectively), which showed higher collection efficiency (59% vs 48%) and lower procedure time (91 vs 96 minutes) for the UPP, resulting in a higher collection rate (5.1 vs 4.2×109) (Haemonetics Corporation, unpublished internal data).
Collection efficiency is a simple parameter used to evaluate apheresis systems, comparing the number of platelets collected vs the number that pass through the instrument. Different collection efficiencies are reported in the literature for single-needle devices: 55–58% for the COBE Spectra LRS8 (Caridian BCT, Lakewood, CO, USA), 63–67% for the COBE Spectra LRS-Turbo8, 51–66% for the Fresenius AS1049–12 (Fresenius Kabi AG, Bad Homburg, Germany), 51–54% for the Fresenius AS.TEC 20413,14, 63% for the Haemonetics MCS+15,16, 63–74% for the Baxter Amicus15,17,18 (Baxter Transfusion Therapies, Deerfield, IL, USA) and 76% for the Gambro Trima Accel17,18 (Gambro BCT, Lakewood, CO, USA). However, collection efficiencies reported by different centres might not be directly comparable because of different populations of donors and different collection targets17–19. In our study this issue was overcome by using a within-subject design, in which the efficiency of the two protocols was compared within the same donor who had used both protocols at different times. By eliminating subject-to-subject variation in the comparison of the two protocols, this study design provides higher statistical power compared with classical designs in which each subject experiences only one procedure and comparisons are performed between subjects.
Since collection efficiencies in different centres might not be directly comparable because of different populations of donors and different collection targets, the collection rate appears to be a more practical way to compare platelet collection devices across studies. The collection rate is not influenced by differences in volume of processed whole blood or flow rates associated with different collection protocols17–19. The collection rate for the UPP was slightly higher in our study than that reported in the UPP CE-marking study (Haemonetics Corporation, unpublished internal data). which might be explained by greater operator experience associated with the higher number of procedures performed in our study (135 vs 30 SDP donations).
Due to the Super Surge® technology, which allows further concentration of platelets, the UPP collects SDP in less volume than does the CSDP, and the presence of plasma is negligible. In fact, we observed red blood cell contamination in 4% of the CSDP donations, in line with Ranganathan’s previous finding of a 3% occurrence14, but in less than 1% of the UPP donations, although the difference was not statistically significant. Red blood cell contamination could be ascribed to an accidental bug of the line’s optical reader calibration, which did not stop collections at the right time. The negligible presence of plasma is advantageous in routine transfusion practice, since concentrated SDP could reduce the risk of allergic reactions20. Unexpectedly, the minimum of 350 mL of plasma for the production of fresh-frozen plasma was not reached in nine UPP donations, so the fresh-frozen plasma unit was subsequent discarded, while this was not observed with CSDP. We hypothesised that a software bug in the extracorporeal volume monitoring of the UPP donations might have caused this problem, but this requires further evaluation and it may be resolved in the new software version A.1-IT of the UPP protocol (www.haemonetics.com).
The satisfaction questionnaire showed that most donors found donations with the UPP equal to donations with CSDP in terms of duration, comfort and citrate-related side effects. Among the remaining donors who reported a difference, there was a statistically significant preference for the UPP with regards to comfort and duration. In terms of side effects, there was some preference, albeit not statistically significant, for the UPP, which might be due to the new UPP plasma allocation during reinfusion which reduces the ACD-A return flow rate and potential donor citrate reactions. In general, during the study period we observed few adverse events with both protocols (<5%), none of which was serious. This disagrees with previous findings from Chaudhary et al., who reported a 18% prevalence of adverse events in 67 donations with the CSDP16. Saline infusion was required in four CSDP donations but not in UPP donations, although this difference did not reach statistical significance. The European Blood Alliance Europheresis Group has recommended avoiding saline infusion21, since deaths have been reported as a result of a mistake by nursing staff who confused saline bags with similar-looking ACD-A bags22. The UPP may reduce the need to resort to saline infusion because of the extracorporeal volume reduction obtained with its new fractionated reinfusion programme, but this needs to be confirmed by further investigation.
In conclusion, our study endorses the use of the new UPP protocol in daily transfusion practice, since it substantially improves performance in SDP collection while guaranteeing equal or greater comfort for the donor compared with the previous CSDP protocol.
Acknowledgements
The authors thank Ivone Varinelli, Bruno Meani and Julie Baron from Haemonetics Corporation for their technical support, the apheresis medical and nursing staff, and the donors.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Schrezenmeier H, Seifried E. Buffy-coat-derived pooled platelet concentrates and aphaeresis platelet concentrates: which product type should be preferred? Vox Sang. 2010;99:1–15. doi: 10.1111/j.1423-0410.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 2.Wiltbank TB, Giordano GF. The safety profile of automated collections: an analysis of more than 1 million collections. Transfusion. 2007;47:1002–5. doi: 10.1111/j.1537-2995.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 3.Despotis GJ, Goodnough LT, Dynis M, et al. Adverse events in platelet apheresis donors: a multivariate analysis in a hospital-based program. Vox Sang. 1999;77:24–32. doi: 10.1159/000031070. [DOI] [PubMed] [Google Scholar]
- 4.Burgstaler EA, Pineda AA, Wollan P. Plateletapheresis: comparison of processing times, platelet yields, and white blood cell content with several commonly used systems. J Clin Apher. 1997;12:170–8. doi: 10.1002/(sici)1098-1101(1997)12:4<170::aid-jca3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Bonomo P, Alfano G, Gandini G, et al. Standard of Transfusion Medicine. second edition. Edizioni SIMTI; Jun, 2010. [Google Scholar]
- 6.Council of Europe. Guide to the preparation, use, and quality assurance of blood components. 15th edn. Strasbourg: Council of Europe Publishing; 2010. [Google Scholar]
- 7.Schoendorfer DW, Hansen LE, Kenney DM. The surge technique: a method to increase purity of platelet concentrates obtained by centrifugal aphaeresis. Transfusion. 1983;23:182–9. doi: 10.1046/j.1537-2995.1983.23383224892.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones DA, Drillat P, Verheyden R, et al. Multicenter evaluation of the Cobe Spectra LRSTM Turbo for collecting leukoreduced platelets (Abstract) J Clin Apher. 1998;13:87. [Google Scholar]
- 9.Zeiler T, Weisbach V, Zingsem J, et al. Initial experiences with routine use of the AS-104 cell separator in a single needle procedure. Beitr Infusionsther. 1993;31:100–3. [PubMed] [Google Scholar]
- 10.Moog R. Single-needle results with cell separator A-201 and AS-104. Beitr Infusionsther. 1993;31:104–6. [PubMed] [Google Scholar]
- 11.Moog R, Müller N. Evaluation of the single needle procedure in plateletapheresis with Fresenius AS104 blood cell separator. J Clin Apher. 1995;10:90–5. doi: 10.1002/jca.2920100208. [DOI] [PubMed] [Google Scholar]
- 12.Zeiler T, Zingsem J, Weisbach V, et al. Eighteen months experience with a new single-needle cytapheresis system (Fresenius AS 104 SNR) Infusionsther Transfusionsmed. 1994;21:91–5. [PubMed] [Google Scholar]
- 13.Moog R, Müller N, Goergens D. Platelet collection with the Amicus and AS.TEC 204 blood cell separators. Transfusion. 1998;38:285–9. doi: 10.1046/j.1537-2995.1998.38398222873.x. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan S. Comparison of plateletaphaeresis on the Fresenius AS.TEC 204 and Haemonetics MCS 3p. J Clin Apher. 2007;22:1–4. doi: 10.1002/jca.20108. [DOI] [PubMed] [Google Scholar]
- 15.Moog R, Müller N. White cell reduction during plateletapheresis: a comparison of three blood cell separators. Transfusion. 1999;39:572–7. doi: 10.1046/j.1537-2995.1999.39060572.x. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary R, Das SS, Khetan D, et al. Comparative study of automated plateletpheresis using five different aphaeresis systems in a tertiary care hospital. Transfus Apher Sci. 2009;40:99–103. doi: 10.1016/j.transci.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Picker SM, Radojska SM, Gathof BS. Prospective comparison of high-dose plateletpheresis with the latest apheresis systems on the same donors. Transfusion. 2006;46:1601–8. doi: 10.1111/j.1537-2995.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 18.Burgstaler EA, Winters JL, Pineda AA. Paired comparison of Gambro Trima Accel versus Baxter Amicus single-needle plateletpheresis. Transfusion. 2004;44:1612–20. doi: 10.1111/j.0041-1132.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 19.Moog R. Implementation of concurrent red blood cell and platelet collection by aphaeresis in a university haemapheresis unit. Transfus Med. 2004;14:145–50. doi: 10.1111/j.0958-7578.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160:434–44. doi: 10.1111/bjh.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Blood Alliance Europheresis Group report 15 th June 2010. [Accessed on 23/02/2013]. Available at: www.basg.gv.at/uploads/media/100720_Report_from_WG_apheresis.pdf.
- 22.Recall of CS3000 Apheresis Kits. US Food and Drug Administration; Jun 21, 2007. [Accessed on 23/02/2013]. Available at: www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Recalls/ucm053390.htm. [Google Scholar]