Abstract
Background
Despite the introduction of anti-D prophylaxis into clinical practice, RhD alloimmunisation remains a problem, particularly in the context of transfusions and pregnancy-induced alloimmunisation. The incidence of RhD alloimmunisation among phenotypically RhD-negative individuals is unknown in most countries. We investigated RhD alloimmmunisation in RhD-negative pregnant women and transfusion recipients in south-east China in order to optimise the prevention of this phenomenon.
Methods
We analysed the RhD alloimmunisation status of RhD-negative pregnant women and transfusion recipients in south-east China. The RhD blood types of the study population were identified by standard serological methods. The D antigen was further tested with the indirect antiglobulin test to exclude or confirm weak D or partial D types. RhC, c, E and e antigens were typed in all subjects. If anti-D antibody screening was positive, the specificity and titre of the antibody were determined. The Del phenotype was investigated by the polymerase chain reaction sequence-specific primer method.
Results
An anti-D antibody was found in 61 of 416 RhD-negative pregnant women (14.66%), and in 11 of 227 RhD-negative transfusion recipients (4.85%). None of the 72 RhD-negative pregnant women or transfusion recipients with anti-D had the Del phenotype. Anti-D antibodies were not detected among Del phenotype individuals and Del phenotypes were not found in anti-D antibody producing individuals.
Discussion
Our study suggests that the risk of alloimmunity-induced neonatal haemolysis increases in true RhD-negative multipara. Perinatal protection would be necessary in these patients, while antenatal anti-D testing and Rh immune globulin prophylaxis would be unnecessary for RhDel pregnant women. Pregnant women and transfusion recipients with the Del type seldom produce anti-D antibody. RhD-negative recipients are not at risk of alloimmunisation after transfusion with Del red blood cells.
Keywords: RhD negative, pregnancy, transfusion recipient, alloimmunisation, Del phenotype
Introduction
The Rhesus (Rh) blood group system is clinically important because antibodies against Rh antigens are involved in haemolytic disease of the foetus and newborn and haemolytic transfusion reactions1. The Rh blood group system is one of the most diverse antigen systems presently known in humans and the D antigen is its most important member because D-negative individuals can be easily anti-D immunised. Indeed, each person who lacks a red blood cell antigen and is exposed to it is at risk of creating an antibody to that antigen. If RhD-positive foetal erythrocytes enter the circulation of a RhD-negative mother, the maternal immune system may be stimulated and trigger the creation of antibodies by alloimmunisation. The same immune reaction may also occur during transfusion of antigen-incompatible red blood cells.
Studies of the blood group system have shown that racial differences exist not only in the genetic background of the RhD antigen but also in the frequencies of the Rhesus D genotype (RHD). About 15% of Caucasoid people are D-negative, most of whom have deletion of RHD between the upstream and the downstream Rhesus boxes2. In contrast, the majority of D-negative black Africans have a RHD gene, with one study showing that 66% had an inactive RHD pseudogene (RHDψ) with a 37 base pair (bp) insert and a nonsense mutation3. In Asian populations, RHDψ is rare, although a certain percentage of RhD-negative individuals have a RHD-CE-DS hybrid gene and RHD1227A allele. In contrast to Caucasian population, only 0.3–0.5% of Chinese populations have a RhD-negative blood phenotype4; however, nearly 30% of the RhD-negative individuals have the RhDel allele, which is a rare variant of the Rh system with a grossly intact RHD gene, of which one is the 1227G >A mutation that probably disrupts normal intron splicing. In European populations, the reported frequency of RhDel is 1:3030, and that of the RHD1227A allele is 1:90915. There are currently no data on the frequency of Del in Africans. The most frequent RHD allele among Asian individuals is the RhDel variant RHD1227A, which serves as an important genetic marker in RhDel Asian people6–8. Although Del is the weakest D-positive phenotype, the potential danger that Del red blood cells might cause a clinical transfusion reaction cannot be completely excluded and indeed there are reports of transfusion recipients with a true D-negative phenotype having developed anti-D after transfusion with Del red blood cells9,10. Analogous cases have not as yet been reported in Chinese populations.
In the present study, we analysed the RhD alloimmunisation status of RhD-negative pregnant women and transfusion recipients in a south-eastern region of China. There were no Del phenotype individuals among the RhD-negative pregnant women and transfusion recipients who produced anti-D antibody. Among Chines populations, RhD-negative pregnant women and transfusion recipients with the Del blood type seldom produce the anti-D antibody. On the basis of our analysis, we advocate that a programme to prevent Rh immunisation and a transfusion strategy be implemented to avoid anti-D alloimmunisation among RhD-negative Chinese populations.
Materials and methods
Blood samples and DNA isolation
Peripheral blood samples (5.0 mL) were collected from 227 transfusion recipients and RhD-negative pregnant women during routine antenatal care visits at the Anhui Provincial Hospital, the Jiangsu Provincial Maternal & Children Health Care Hospital, the Shaoxing Hospital of China Medical University and the Affiliated Hospital of Guilin Medical College. Subjects were excluded from participation in this study if they: were RhD-positive, were RhD-negative but already immunised against the RhD antigen, had a history of anaphylactic or other severe systemic reaction to immunoglobulins, had been administered anti-D immunoglobulin, had IgA deficiency, had been transfused with RhD-positive blood or been given any other blood-derived product within the 6 months prior to enrolment, when the biological father of the child was RhD-negative, had clinically relevant abnormal laboratory parameters (haematology, biochemistry, coagulation, urinalysis) which, in the opinion of the investigator, were not acceptable.
Plasma was separated by centrifugation at 2,000×g for 10 minutes. Genomic DNA was extracted with commercially supplied kits following the manufacturer’s instructions (QIAamp blood mini kit, Qiagen, Hilden, Germany), transferred to new propylene tubes and stored at −20 °C until use. The buffy coat from each blood sample was also collected and stored at −20 °C.
This study was approved by the institutional Ethics Review Board of Nanjing Medical University and all subjects provided written informed consent to their participation in it.
RhD phenotyping
Blood samples were collected into Vacutainer blood tubes containing EDTA. The RhD blood phenotype status was determined by classical serological techniques with direct agglutination. Two commercial anti-D were used (Immucor, Norcross, Georgia, USA; Gamma Biologicals, Houston, Texas, USA). Anti-C (Novaclone, Dominion Biologicals, Dartmouth, Canada), anti-E (Gamma Biologicals), anti-c (Immucor), and anti-e (Immucor) were also used. Samples that were negative with anti-D in the direct agglutination test were re-examined using the indirect antiglobulin test. An adsorption elution test was also performed on all the samples (including those from all RhD-negative donors), which were negative with the indirect antiglobulin test in order to determine the presence of RhDel.
Polymerase chain reaction - sequence-specific primer assay for the RHD1227A allele
A polymerase chain reaction -sequence-specific primer (PCR-SSP) assay was used to screen for the RHD1227A allele, which is the most common Del allele reported in the Chinese population. Del-U and D-int9-L, designed by Shao et al.11, were used to specifically genotype RHD1227A. Multiplex PCR amplifications were performed with 1.0 μL of genomic DNA and 0.5 U DNA polymerase (Applied Biosystems, Foster City, California, USA), 200 μM dNTPs, RH primers, primers for the internal control (IC), 2.5 mM MgCl2 in a buffer supplied by the manufacturer in a 12.5 μL reaction volume. The PCR consisted of denaturation at 95 °C for 5 minutes and then 35 cycles of 10 seconds at 94 °C, 40 seconds at 62 °C, and 30 seconds at 72 °C were carried out with a thermocycler (PE 9700 GeneAmp PCR system, Applied Biosystems). PCR products were separated and visualized in 5% acrylamide gels.
Statistical analysis
The SPSS software package, version 11.0 (SPSS Inc., Cary, North Carolina, USA) was used for all statistical tests.
Results
RhD phenotyping
Four hundred and sixteen blood samples from pregnant women and 227 blood samples from transfusion recipients were collected, which were determined to be RhD-negative by routine serological testing. An indirect antiglobulin test was performed on all samples typed as D-negative, showing that there were no cases of weak D or partial D. About 98% of the individuals were of Chinese Han ethnicity and lived in the south-eastern area of China, in Jiangsu Province, Zhejiang Province, or Anhui Province.
Of all 643 D-negative samples received, 379 samples were typed as ccee. The remaining 264 samples had the C antigen or E antigen, with the majority exhibiting the Ccee or CCee phenotype (237/643; 36.9%). Of the 643 samples determined to be D-negative by the indirect antiglobulin test, 155 cases were actually Del as shown by adsorption/elution testing (Tables I and II).
Table I.
Anti-D immunisation analysis in 416 RhD-negative pregnant women.
| Pregnancy history | Number of cases | Saline test | IAT | Adsorption and elution test | IgG-D ( % ) | RHD1227A | Foetus or newborn RhD phenotype | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| − | + | − | + | ||||||
| G2P1 | 153 | – | – | 121 | 32 | 12* (7.8) | 122 | 31 | 148(+), 4(Del), 1(−) |
| G2P2 | 57 | – | – | 45 | 12 | 6* (10.5) | 45 | 12 | + |
| G3P1 | 98 | – | – | 78 | 20 | 9* (9.2) | 79 | 19 | 97(+), 1(−) |
| G3P2 | 22 | – | – | 18 | 4 | 6* (27.3) | 18 | 4 | 21(+), 1(Del) |
| G3P3 | 9 | – | – | 6 | 3 | 3* (33.3) | 6 | 3 | + |
| GnP2 | 47 | – | – | 35 | 12 | 13* (27.7) | 35 | 12 | 46(+), 1(Del) |
| GnP3 | 30 | – | – | 25 | 5 | 12* (40.0) | 25 | 5 | + |
G: gestation; P: parturition; n: number of time (n ≥4); IAT: indirect antiglobulin test;
pregnant woman with non-RhDel phenotype;
“+”: positive; “−”: negative.
Table II.
Anti-D immunisation analysis in 227 RhD-negative transfusion recipients.
| Transfusion history | Number of cases | Saline test | IAT | Adsorption and elution test | IgG-D ( % ) | RHD1227A | Donors RhD phenotype | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| − | + | − | + | ||||||
| Second times | 156 | – | – | 108 | 48 | 4* (2.6) | 108 | 48 | 142(−), 14(Del) |
| Multiple | 71 | – | – | 52 | 19 | 7* (9.9) | 54 | 17 | 63(−), 8(Del) |
recipient with non-RhDel phenotype;
“+”: positive; “−”: negative; Multiple: at least three times; IAT: indirect antiglobulin test.
Anti-D immunisation analysis
Among the 416 RhD-negative pregnant women, 61 produced an anti-D antibody, for a production rate of 14.66% (61/416) (Table I). Among the 227 RhD-negative transfusion recipients, 11 produced an anti-D antibody, for a production rate of 4.85% (11/227) (Table II).
To investigate the immunogenicity of Del red blood cells in RhD-negative recipients, we investigated the alloimmunisation status of 19 RhD-negative recipients transfused with Del red blood cells. Of these 19 Rh-negative recipients transfused with Del variants, ten were males and the other nine were females with a history of pregnancy. The results of adsorption-elution tests for these 19 subjects showed that five cases were Del and 14 were true Rh negative. Three men and two women identified as Del individuals showed no reactions after transfusion, and irregular antibodies were not detected in their serum. Among the remaining 14 true Rh-negative samples, all subjects gave the same results as the Del samples (Table III).
Table III.
Anti-D immunisation analysis in 19 RhD-negative transfusion recipients of RhDel red blood cells.
| SN | Sex | Age | Saline test | IAT | Adsorption and elution test | Amount of Del (U) | Phenotype | IgG-D |
|---|---|---|---|---|---|---|---|---|
| 11005 | M | 31 | – | – | − | 2 | Ccee | – |
| 11012 | M | 22 | – | – | + | 4 | CCee | – |
| 11032 | M | 31 | – | – | − | 1 | ccee | – |
| 11053 | F | 28 | – | – | − | 3 | ccEe | – |
| 11060 | M | 69 | – | – | − | 6 | ccee | – |
| 11070 | F | 35 | – | – | − | 2 | ccee | – |
| 11075 | F | 57 | – | – | − | 2 | ccee | – |
| 11079 | M | 53 | – | – | − | 2 | ccEe | – |
| 11082 | M | 48 | – | – | + | 2 | Ccee | – |
| 11089 | F | 58 | – | – | − | 2 | ccee | – |
| 11099 | F | 27 | – | – | + | 1 | Ccee | – |
| 11103 | F | 69 | – | – | − | 3 | ccee | – |
| 11117 | M | 31 | – | – | − | 4 | ccee | – |
| 11134 | M | 32 | – | – | − | 2 | Ccee | – |
| 11171 | F | 30 | – | – | − | 4 | ccee | – |
| 12204 | M | 34 | – | – | + | 2 | Ccee | – |
| 12220 | M | 46 | – | – | − | 4 | ccee | – |
| 12225 | F | 41 | – | – | + | 4 | CCee | – |
| 12227 | F | 27 | – | – | − | 2 | ccee | – |
SN: specimen number; F: Female; M: Male; “+”: positive; “−”: negative; IAT: indirect antiglobulin test; 1 U: red blood cells prepared from 200 mL whole product.
In the present study, PCR-SSP and sequencing showed that none of the 72 RhD-negative pregnant women or transfusion recipients with anti-D antibodies carried the RHD1227A allele. Anti-D antibodies were not detected among Del phenotype individuals (Tables I and II). Further investigations found no cases of anti-D alloimmunisation in D-negative recipients after the transfusion of Del variants (Table III).
Samples with the RHD1227A allele
In 151 of the 155 Del phenotyped cases, RHD1227A alleles were detected by PCR-SSP (Figure 1). RHD1227A alleles were identified in 85 of the D-negative Ccee samples and 66 of the CCee samples. For confirmation, all 151 samples were determined to have the RHD1227A alleles by sequencing analysis (Figure 2).
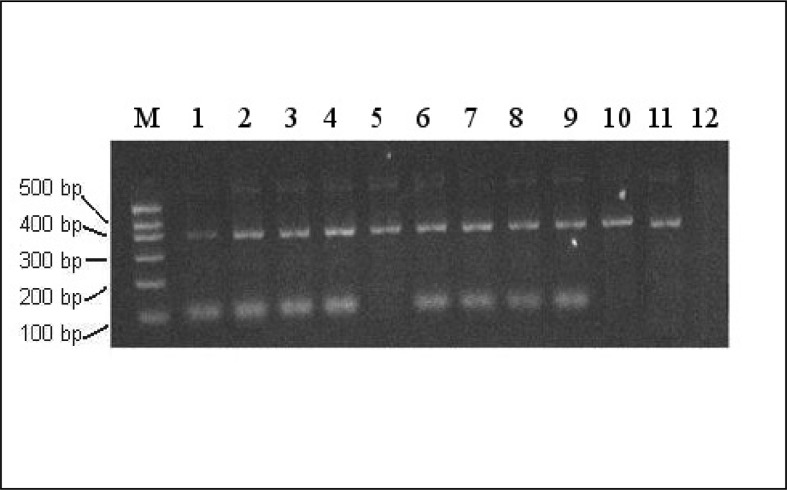
Figure 1.
PCR-SSP for RHD1227A (109 bp band).
Lanes 1–4, 6–9, RHD1227A allele detected in Del phenotype samples; Lane 5, non- RHD1227A allele gene detected in Del phenotype samples; lane 10, Rh-negative control sample; lane 11, sample with a normal RHD gene; lane 12, H2O control. A representative example of 155 Del phenotyped cases is shown.
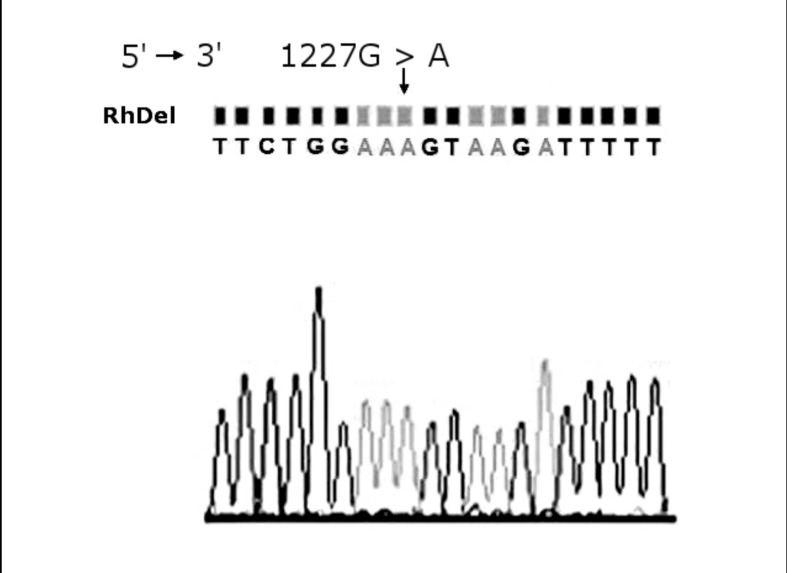
Figure 2.
Sequencing analysis of the RHD1227A allele.
The arrow indicates the position of the nucleotide mutation between RHD exon 9 and intron 9. A representative example of 151 RHD1227A genotyped cases is shown.
Discussion
In this study, we investigated the RhD alloimmunisation rate among RhD-negative pregnant women and transfusion recipients in the south-eastern region of China. Despite the fact that the prevalence of Rh-negative phenotypes is significantly lower among Asians than among Caucasians, China is a populous country, and spouses of RhD-negative women are almost all RhD-positive individuals. We have previously reported the non-invasive determination of foetal RhD blood type using free foetal DNA from Chinese RhD-negative maternal plasma12. If the foetus is RhD negative, it is unnecessary to administer IgG anti-D at gestation or institute prophylaxis of RhD alloimmunisation in the case of potentially sensitising events, but its application is still very limited. More than 98% of RhD-negative pregnant Chinese women give birth to RhD-positive infants (Table I).
Most RhD alloimmunisation status studies have been conducted in Caucasian and African populations and their results are not entirely applicable to the Chinese population as there are significant differences in the frequencies and molecular characteristics of RhD variants in different populations. Our previous studies had shown that East Asian populations who carry the Del can safely receive transfusions from RhD-positive donors13. However, alloimmunisation associated with the RhD- negative phenotype has been poorly studied among the Chinese population. Many questions remain unanswered and there is an urgent need for the development of a management protocol for this condition among the Chinese population. This study was conducted to evaluate the state of secondary anti-D immunisation among Chinese individuals with the RhD-negative phenotype.
Several studies have revealed that RhD-negative individuals may have a partial or intact RHD gene, and this genotype occurs at different frequencies depending on the ethnic population14,15. The immunogenic impact of D antigen depends mainly on the quantity of D antigen sites on the surface of red blood cells. Red blood cells with weak D express less D and are less immunogenic than red blood cells with normal expression of D antigen16. However, previous studies have demonstrated that red blood cells from subjects with the weak D type 1 phenotype may cause primary alloimmunisation of anti-D17, as may those of the weak D type 2 phenotype with a D antigen density of approximately 500 per cell18, although Del is defined by expression of trace amounts of the antigen that can be detected only by an absorption-elution study. Shao and associates11 deduced that Del is a type of weak D, and different Del individuals might have different amounts of D antigen sites on their red blood cells. Although the Del phenotype is very rare in Caucasians, it is very common in the Rh-negative Chinese population as shown by indirect antiglobulin testing. The reported rates in Chinese Hans (the major ethnic group, accounting for more than 91% of the Chinese population) are from 26% to 30%11,19,20. Moreover through genomic DNA analysis, the studies by Shao et al. and Chen et al. in Shenzhen, Southern China, and in Taiwan6, showed that the RHD1227A allele is a dominant genetic marker for Chinese and Taiwanese individuals with Del. These individuals possessed one or two of these alleles with Ccee or CCee phenotypes11. The Del allele, RHD1227A has a synonymous mutation at the end of exon 9 compared to the consensus RHD. This single nucleotide polymorphism causes complete exon 9 mis-splicing, which has been confirmed through sequence analysis of mRNA transcripts. The exon 9 corresponding region in mRNA codes for transmembranous amino acids21,22. Although Del is the weakest D-positive phenotype, the potential danger that Del red blood cells might cause a clinical transfusion reaction cannot be completely excluded. Indeed, recipients with a true D-negative phenotype have developed anti-D after transfusion with Del red blood cells9,10, although no such cases have yet been reported in the Chinese population. Previous studies demonstrated that “Asian type” Del displays the complete repertoire of RhD antigen epitopes23. However, carriers of Del can still be RhD-negative donors, since there are fewer than 22 membrane RhD antigens per Del red cell, as compared with thousands of RhD antigens on a normal RhD-positive red cell23.
In North America, approximately 9% to 10% of all pregnancies involve a Rh-negative woman carrying a Rh-positive infant. Rh alloimmunisation occurs antenatally in 0.4% to 2.0% of first pregnancies involving Rh incompatibility. The risk of alloimmunisation is approximately 3% to 5% after abortion, with the risk increasing with increasing gestational age, and 2.1% to 3.4% after amniocentesis. A cross-sectional retrospective study to determine the prevalence of anti-D immunoglobulin among Cameroonian women of reproductive age indicated an anti-D prevalence of 4% among Rh-negative African women24. In our study of Chinese women, we observed that 18.60% (61/328) of the true RhD-negative pregnant women carrying a RhD-positive foetus produced anti-D IgG antibody. These pregnant women had a history of pregnancy or childbirth. D-antigen may cause primary alloimmunisation that cannot be detected and may not cause a haemolytic immune response, but is responsible for an immune recall response. Abortion and other invasive procedures can lead to maternal RhD primary immunisation. Considering our overall cohort of phenotypically RhD-negative pregnant women, the anti-D detection rate was 14.66% (61/416). Further analysis of the women who had been twice or more, showed an anti-D detection rate of 31.48% (34/108). Among 227 RhD-negative transfusion recipients, 11 produced the anti-D antibody for a production rate of 4.85%. To further investigate the immunogenicity of Del red blood cells in Rh negative recipients, we investigated the alloimmunisation status of 19 RhD-negative recipients transfused with Del red blood cells. No anti-D was detected in any of these 19 RhD-negative recipients. All 72 of the RhD-negative pregnant women and transfusion recipients with anti-D were excluded from carrying the Del phenotype through PCR-SSP analysis and sequencing. Anti-D antibodies were not found among Del phenotype individuals and Del phenotypes were not found in anti-D antibody-producing individuals. Körmöczi et al. suggested that the Del phenotypes might be subdivided into two groups, partial Del and complete Del23. We wondered whether all of the Del found in our Chinese subjects were complete Del, as no Del sample was found to have anti-D in the serum.
Our study supports the biochemical observations that Del variants express normal RhD and pose virtually no risk of inducing anti-RhD antibodies. We suggest that people in East Asian populations who carry Del variants can safely receive transfusions from RhD-positive donors. Our findings would apply to East Asian transfusion recipients in Europe, North America, and elsewhere and could be implemented easily when genetic cross-matching becomes a reality25.
Footnotes
Contribution
Qing-ping Wang and Guang-tao Dong contributed equally to this work.
Financial disclosure
This research was supported by the Medical Research Foundation of Wuxi Municipal Health Bureau (Grant N. ML201203) and supported by the Scientific Technology Development Foundation of Nanjing Medical University (Grant N. 2012NJMU071). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
References
- 1.Westhoff CM. The Rh blood group system in review: a new face for the next decade. Transfusion. 2004;44:1663–73. doi: 10.1111/j.0041-1132.2004.04237.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner FF, Flegel WA. RHD gene deletion occurred in the Rhesus box. Blood. 2000;95:3662–8. [PubMed] [Google Scholar]
- 3.Singleton BK, Green CA, Avent ND, et al. The presence of an RHD pseudogene containing a 37 bp duplication and a nonsense mutation in Africans with the Rh D-negative blood group phenotype. Blood. 2000;95:12–8. [PubMed] [Google Scholar]
- 4.Peng CT, Shih MC, Liu TC, et al. Molecular basis for the RhD negative phenotype in Chinese. Int J Mol Med. 2003;11:515–21. [PubMed] [Google Scholar]
- 5.Wanger FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JC, Lin TM, Chen YL, et al. RHD1227A is an important genetic marker for RhD(el) individuals. Am J Clin Pathol. 2004;122:193–8. doi: 10.1309/3XMF-2NV5-707T-JE7X. [DOI] [PubMed] [Google Scholar]
- 7.Luettringhaus TA, Cho D, Ryang DW, et al. An easy RHD genotyping strategy for D- East Asian persons applied to Korean blood donors. Transfusion. 2006;46:2128–37. doi: 10.1111/j.1537-2995.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasekura H, Ota M, Ito S, et al. Flow cytometric studies of the D antigen of various Rh phenotypes with particular reference to Du and Del. Transfusion. 1990;30:236–8. doi: 10.1046/j.1537-2995.1990.30390194344.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner T, Körmöczi GF, Buchta C, et al. Anti-D immunization by Del red blood cells. Transfusion. 2005;45:520–6. doi: 10.1111/j.0041-1132.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Ohto H, Sakuma S, et al. Secondary anti-D immunization by Del red blood cells. Transfusion. 2005;45:1581–4. doi: 10.1111/j.1537-2995.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 11.Shao CP, Maas JH, Su YQ, et al. Molecular background of Rh D-positive, D-negative, D(el) and weak D phenotypes in Chinese. Vox Sang. 2002;83:156–61. doi: 10.1046/j.1423-0410.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang XD, Wang BL, Ye SL, et al. Non-invasive foetal RHD genotyping via real-time PCR of foetal DNA from Chinese RhD-negative maternal plasma. Eur J Clin Invest. 2009;39:607–17. doi: 10.1111/j.1365-2362.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- 13.Shao CP. Transfusion of RHD-positive blood in “Asia type” DEL recipients. New Engl J Med. 2010;362:472–3. doi: 10.1056/NEJMc0909552. [DOI] [PubMed] [Google Scholar]
- 14.Yan LY, Wu JJ, Zhu FM, et al. Molecular basis of D variants in Chinese person. Transfusion. 2007;47:471–7. doi: 10.1111/j.1537-2995.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 15.Machado IN, Castilho L, Pellegrino J, Jr, et al. Fetal RHD genotyping from maternal plasma in a population with a highly diverse ethnic background. Rev Assoc Med Bras. 2006;52:232–5. doi: 10.1590/s0104-42302006000400022. [DOI] [PubMed] [Google Scholar]
- 16.Brecher ME. Blood Groups. In: Brecher ME, editor. Technical manual. 14th ed. Bethesda, MD: American Association of Blood Banks; 2003. pp. 301–4. [Google Scholar]
- 17.Mota M, Fonseca NL, Rodrigues A, et al. Anti-D alloimmunization by weak D type 1 red blood cells with a very low antigen density. Vox Sang. 2005;88:130–5. doi: 10.1111/j.1423-0410.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 18.Flegel WA, Khull SR, Wagner FF. Primary anti-D immunization by weak D type 2 RBCs. Transfusion. 2000;40:428–4. doi: 10.1046/j.1537-2995.2000.40040428.x. [DOI] [PubMed] [Google Scholar]
- 19.Mak KH, Yan KF, Cheng SS, et al. Rh phenotypes of Chinese blood donors in Hong Kong, with special reference to weak D antigens. Transfusion. 1993;33:348–51. doi: 10.1046/j.1537-2995.1993.33493242645.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun CF, Chou CS, Lai NC, et al. RHD gene polymorphisms among RhD-negative Chinese in Taiwan. Vox Sang. 1998;75:52–7. [PubMed] [Google Scholar]
- 21.Shao CP, Xiong W, Zhou Y. Multiple isoforms excluding normal RHD mRNA detected in Rh blood group Del phenotype with RHD1227A allele. Transfus Apheresis Sci. 2006;34:145–52. doi: 10.1016/j.transci.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Jayachandran S, Nguyen HH, et al. Structure of the Nitrosomonas europaea Rh protein. Proc Natl Acad Sci USA. 2007;104:19279–84. doi: 10.1073/pnas.0709710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Körmöczi GF, Gassner C, Shao CP, et al. A comprehensive analysis of Del types: partial Del individuals are prone to anti-D alloimmunization. Transfusion. 2005;45:1561–7. doi: 10.1111/j.1537-2995.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 24.Belinga S, Ngo Sack F, Bilong C, et al. High prevalence of anti-D antibodies among women of childbearing age at Centre Pasteur of Cameroon. Afr J Reprod Health. 2009;13:47–52. [PubMed] [Google Scholar]
- 25.Denomme GA, Flegel WA. Applying molecular immunohematology discoveries to standards of practice in blood banks: now is the time. Transfusion. 2008;48:2461–75. doi: 10.1111/j.1537-2995.2008.01855.x. [DOI] [PubMed] [Google Scholar]