Introduction
Plasmapheresis has been proven to be an effective treatment for a variety of conditions, especially those in which circulating antibodies are known or thought to be involved in pathogenesis. The current American Society For Apheresis (ASFA) guidelines1 make recommendations on 120 specific indications encompassing a total of 68 distinct disease processes. Among the autoimmune blistering disorders of the skin, pemphigus vulgaris is the sole entity included and plasmapheresis is considered a category IV indication (disorders in which published evidence demonstrates or suggests apheresis to be ineffective or harmful). In pemphigus vulgaris auto-antibodies are directed against the adhesion molecule desmoglein2, whereas in a related entity known as bullous pemphigoid (BP) auto-antibodies are targeted against the hemidesmosome components BP1803 and BP2304.
Although no formal recommendation exists for BP, plasmapheresis has been deployed successfully as adjunctive therapy5–8. In describing our own institution’s recent experience in treating BP we add to the growing evidence supporting the use of plasmapheresis under appropriate conditions. We also include an examination of serial BP180 and BP230 titres measured by enzyme-linked immunosorbent assay (ELISA) during plasmapheresis therapy.
Case reports
In March of 2012, two consecutive patients with BP were admitted to, treated in, and discharged from our institution. The older patient, herein referred to as Patient One, was a 36-year old African-American male with a medical history of human immunodeficiency virus (HIV) infection treated with highly active anti-retroviral therapy (HAART). He was diagnosed with BP by a dermatologist about 2 months prior to hospitalisation. His work-up at the time included a skin biopsy and direct immunofluorescence testing. He then immediately began treatment with 60 mg/day of oral prednisone but due to progressively worsening symptoms over the following 2 months he was eventually admitted for higher level of care.
The younger patient, herein referred to as Patient Two, was a 23-year old Asian-American male with no significant medical history. He was definitively diagnosed with BP only after admission and inpatient evaluation by a consultant dermatologist. A skin biopsy and direct immunofluorescence testing were also performed. Three weeks previously he had presented to a primary care clinic with a localised erythematous rash and was treated with just oral antihistamines and a topical steroid cream. After rapid and continued spread of the rash along with the eruption of numerous blisters and bullae, Patient Two also presented to our institution for urgent management.
Each patient underwent a series of five plasmapheresis procedures each exchanging approximately one plasma volume using a COBE Spectra Apheresis System (centrifuge-based technology) with 5% albumin replacement fluid. For each litre of plasma processed, one gram of 10% calcium gluconate was administered as prophylaxis against hypocalcaemia. Haemoglobin concentrations, platelet counts, and coagulation levels were checked on the day of each procedure and most procedures followed an every other day schedule.
Serum samples collected 30 minutes after plasmapheresis were sent for commercial BP180 and BP230 ELISA testing (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA). Overall, there were six samples from Patient One: a pre-treatment sample and five post-procedure samples. For Patient Two there was only a pre-treatment sample and one post-treatment sample collected at the end of the fifth plasmapheresis. The titres are reported as units per millilitre (U/mL).
Results
Clinical response
In terms of symptomatology, Patient One had more severe disease with diffuse, tense bullae, erosions, crusts, and erythema involving approximately 75% of the total body surface area at admission. The mucous membranes were also involved. Patient One’s positive HIV status placed him at greater risk of opportunistic infections. His maximum dose of prednisone was, therefore, limited to approximately 1 mg/kg/day, which was an increase from his outpatient dose of 0.6 mg/kg/day. Additional medication in the form of dapsone (50 mg/day) was given as well (Table I). His first plasmapheresis procedure was performed after 12 days of treatment at the higher dose of prednisonse. Patient One’s total duration of systemic corticosteroid therapy before the initiation of plasmapheresis, inclusive of both his outpatient and inpatient regimens, was approximately 2.5 months.
Table I.
The specific medications used to treat bullous pemphigoid in our two patients and any additional medications that have potential immunomodulatory effects.
| Clinical and Medication Profile | ||
|---|---|---|
|
| ||
| Patient One | Patient Two | |
| Age | 36 | 23 |
| Weight (kg) | 106 | 61 |
| Bullous pemphigoid medications | Prednisone 100 mg | Prednisone 120 mg |
| Dapsone 50 mg | Azathioprine 150 mg | |
| Other immunomodulatory drugs | Darunavir | None |
| Etravirine | ||
| Raltegravir | ||
| Ritonavir | ||
Once plasmapheresis was begun, Patient One reported immediate relief of pruritus. Over the course of five procedures, there was unequivocal evidence of healing of the blisters and erosions and, for the first time since the patient’s admission, there was complete resolution in certain areas. Until plasmapheresis, Patient One required protective sterile dressings over large parts of his thighs, chest, arms, and neck, whereas by the end of the fifth procedure there was no longer any need for such dressings. Based on clinical impression, the reduction in blisters, erosions, and erythema reflected at least 50% less skin involvement than before plasmapheresis was started.
Patient Two had both discrete and confluent erythematous patches and plaques but with fewer tense bullae than Patient One. Skin involvement was localised primarily to the neck, axillae, antecubital fossae, abdomen, and inguinal regions, but was spreading far more rapidly. Patient Two’s prednisone was aggressively titrated from a starting dose of 0.3 mg/kg/day up to 2 mg/kg/day over the span of 5 days. In his case, azathioprine (150 mg/day) was added as a second-line drug (Table I). He began systemic steroids upon admission and therefore received 8 days of treatment before plasmapheresis, although he had been using a topical steroid cream at home for approximately 3 weeks.
Patient Two also experienced significant and immediate relief of pruritus once placed on plasmapheresis therapy. However, the defining clinical feature in his case was how the continual development of new blisters and bullae from the time of admission was effectively halted. In fact, once the cycle of plasmapheresis was initiated no new blister formation was ever reported by the patient or documented by the clinicians. As in Patient One, the overall clinical improvement after plasmapheresis was an approximately 50% reduction in blisters and erythema distribution.
All procedures were tolerated well and without complications in either patient.
Serological response
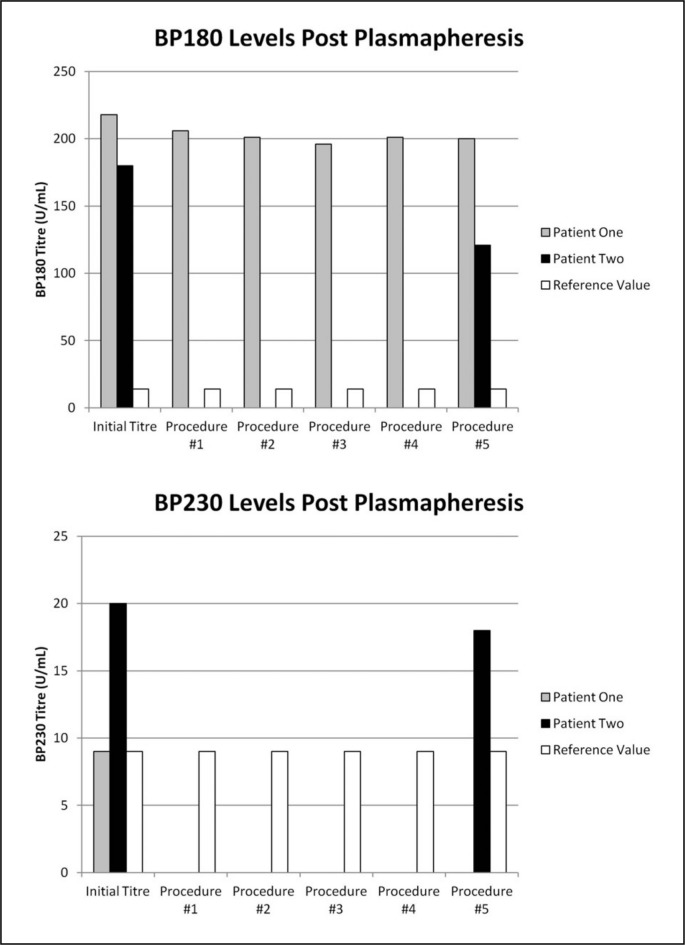
Just prior to his first plasmapheresis Patient One had an elevated BP180 titre of 218 U/mL (reference range ≤14 U/mL). Conversely, his BP230 titre was not increased (reference range <9 U/mL). Serial titres of BP180 are shown in Figure 1. Apart from a slight drop after the first procedure, there was minimal fluctuation in levels following the remaining procedures. The total change in the BP180 titre after all procedures was an 8% decrease from 218 U/mL to 200 U/mL.
Figure 1.
Serial BP180 and BP230 titres determined by ELISA following each plasmapheresis procedure. The reference cut-off values for BP180 (<14 U/mL) and BP230 (<9 U/mL) are also shown.
Patient Two’s initial titres of BP180 and BP230 were both elevated at 180 U/mL and 20 U/mL, respectively. At the conclusion of five plasmapheresis procedures, there was a 33% reduction in BP180 (from 180 U/mL to 121 U/mL) and a 10% reduction in BP230 (from 20 U/mL to 18 U/mL), as shown in Figure 1.
Discussion
Clinical efficacy
This study was limited to a retrospective review of each patient’s medical record. Our attempt to measure and monitor disease severity does, therefore, rely heavily on subjective descriptions of the patients entered by physicians and nurses into the records. Nevertheless, there was sufficient information from which to make correlations in clinical status and trajectory to plasmapheresis therapy.
In considering plasmapheresis, the prevailing issue was the patients’ unremitting disease that showed minimal response to medical management at the time. This was especially evident in Patient One who had been treated with systemic corticosteroids for well over 2 months with little to no improvement. In comparison, Patient Two did not have as long a trial period on systemic corticosteroids but his dosing was far more aggressive (2 mg/kg/day vs 1 mg/kg/day).
Neither patient had suffered any negative side effects as a consequence of high dose steroids with dapsone or azathioprine but, in the judgement of the clinical teams, the risk-benefit profile of further dosage up-titration was not favourable. The advantage of plasmapheresis lies in its ability to clear existing auto-antibodies and cytokines from the circulation rapidly. Since auto-antibodies in BP are thought to cause the skin lesions directly or to precipitate them, the rapid and substantial clinical response displayed in our patients strongly suggests that significant quantities were indeed removed. This effect was evident despite the fact that plasma-protein bound drugs such as prednisone were being removed as well.
In terms of our desired primary outcome -achieving clinical improvement either by stopping disease progression or by reversing it- our experience with plasmapheresis in the treatment of BP has been quite successful. The magnitude of skin lesion healing and its temporal relationship to the cycle of five procedures are in favour of a direct correlation between plasmapheresis and the reduction in disease burden. Although it is true that oral immunosuppressant medications may need days to weeks before producing noticeable results, we believe that Patient One’s prolonged and unsuccessful prior treatment with corticosteroids argues in favour of his BP being at least partially refractory to the drug. Furthermore, both patients exhibited weeks to months of steadily increasing skin involvement that was suddenly and substantially reversed in fewer than 10 days, which was the time required to complete the cycle of plasmapheresis.
While our understanding of the pathogenesis of BP continues to progress, current data support the role of auto-antibodies in the recruitment of leucocytes and activation of inflammatory mediators such as complement9,10. An alternative or complementary mechanism may involve the binding of antibodies to BP180 leading to internalisation of the BP180 immune-complex along the surface of basal cells causing impaired hemidesmosome formation11. Even though we certainly are not discounting the concomitant beneficial effects of systemic steroids and immunosuppressants on each patient’s clinical condition, nor are we advocating treatment with plasmapheresis alone, we do contend that it was the addition of plasmapheresis that marked the tipping point in favour of disease control. In all likelihood, clinical improvement would not have occurred at the time it did and to the same extent it did without both steroids and plasmapheresis.
In comparing our results with previously published information in the literature we found a number of reports that also advocated the use of plasmapheresis as an adjunctive therapy alongside immunosuppression in controlling severe or refractory cases of BP.
The first published report on the matter was by Roujeau and colleagues12 in 1979. Subsequently in 1984, Roujeau and colleagues5 published the first randomised controlled trial involving 37 BP patients comparing the efficacy of treatment with prednisolone against prednisolone combined with plasmapheresis (eight 1.5 volume exchanges over 4 weeks). Their results showed that by the end of 4 weeks disease control was achieved in a far greater number of patients treated with plasmapheresis (13 of 22 patients vs 0 of 15). The cumulative dose of prednisolone was also lower in the group of patients who underwent plasmapheresis (average daily dose of 0.52 mg/kg/day vs 0.97 mg/kg/day).
Another randomised controlled study was completed by Guillaume and colleagues13 in 1993. Their patients were divided into three arms in order to evaluate whether the addition of plasmapheresis or azathioprine improved outcomes. All patients received prednisolone at a dose of 1 mg/kg/day. Plasmapheresis consisted of only four 1.5 plasma volume exchanges over the first 2 weeks. After 4 weeks of treatment the percentage of patients who achieved disease control was the same in the prednisolone alone and the prednisolone with plasmapheresis group. At 6 months the percentage of patients who maintained disease control was slightly higher in the prednisone alone group (42% vs 29%). There were, however, slightly fewer deaths and fewer reports of major complications in the plasmapheresis group (3 deaths and 6 major side effects vs 5 deaths and 10 major side effects).
Based on these findings Guillaume concluded that plasmapheresis offered no additional benefit. The disparate outcomes seen between the studies by Guillaume and Roujeau are likely explained by differences in the plasmapheresis regimens. Although the volume and timing of each procedure was similar, the total number of procedures performed in Guillaume’s was half that used previously. Whether this difference affected the outcome is unknown but is worth mentioning.
To date, Roujeau and Guillaume have performed the only randomised controlled trials evaluating plasmapheresis in the treatment of BP. However, there have been a number of smaller series6–8 and case reports14–18 supporting Roujeau’s conclusion of improved outcomes with plasmapheresis.
One such study was performed by Egan and colleagues6. Their group retrospectively reviewed 15 years of experience at a single institute and found ten patients with BP who had undergone plasmapheresis. All patients were determined to have received plasmapheresis only as a last resort due to either poor response to or intolerable side effects of oral immunosuppression (prednisone, azathioprine, and cyclophosphamide). Patients underwent an average of 7.2 procedures (range, 3–10). Nine patients continued treatment with steroids after their course of plasmapheresis and of those seven received supplemental azathioprine or cyclophosphamide therapy.
All the patients were followed up for 6 months. One patient had died by the end of that time period but all remaining patients achieved and maintained clinical remission. What is especially noteworthy was the dramatic reduction in the amount of steroids required at 6 months. Seven of the patients were able to be weaned off steroids completely while another achieved a 75% reduction in dose. Presumably, many of the adverse side effects associated with prolonged corticosteroid usage that begat plasmapheresis diminished accordingly. The group did report two significant complications related to plasmapheresis. One patient developed a pneumothorax during catheter placement while another developed staphylococcal bacteraemia after prolonged catheter usage.
A separate study by Yamada and colleagues7 retrospectively reviewed all patients at their institute who underwent plasmapheresis for very similar indications. Plasmapheresis was performed using centrifugation, double filtration, as well as a combination of the two modalities. Overall efficacy was measured by a decrease in antibody titre determined by indirect immunoflourescence, a decrease in clinical severity score, a decrease in corticosteroid dose, induction of remission, or by adverse effects related to plasmapheresis.
Eleven of 12 patients achieved improvement in all criteria. Only one patient, who received double filtration plasmapheresis, was unable to have a corticosteroid dose decrease but still achieved lower titres, improved clinical score, and remission without suffering any adverse effects from the procedure.
Mazzi and colleagues8 reported on a cohort of four patients. Again, the indication for plasmapheresis was essentially identical to those described in other studies. The average number of procedures performed in each patient was 10.4 (range, 7–14). All four patients had been treated with both prednisone and azathioprine prior to plasmapheresis and two patients subsequently received a 5-day course of intravenous immunoglobulins at a dose of 0.4 mg/kg/day beginning after their final procedure.
All four patients achieved a clinical remission. Three remained disease-free at the time of publication (range, 3–75 months) with only a single patient experiencing a relapse at 15 months after the cessation of plasmapheresis. Additionally, all patients achieved reductions in their prednisone and azathioprine doses including one patient who was able to be weaned off both drugs altogether by 6 months and another who could be taken off azathioprine. Interestingly, these two particular patients did not receive any adjunctive treatment with intravenous immunoglobulins. The only adverse outcome related to plasmapheresis was one reported incidence of hypotension at the end of a single procedure that resolved without the need for aggressive intervention or pharmacotherapy.
In the three studies described above and in several additional case reports14–18, plasmapheresis was undertaken under comparable circumstances, especially when the disease was severe and the initial response to oral medications was limited. A secondary indication has been as a method of achieving and maintaining remission with less and lower doses of immunosuppressive drugs thereby decreasing the incidence or extent of negative side effects.
The large majority of patients in the above studies underwent plasma exchange by centrifugation, as did ours, compared to only a few who received double filtration plasmapheresis. Another related modality that removes existing antibodies from circulation and has been employed in the treatment of BP is immunoadsorption. Although the number of cases reported to date19–22 (n=8) is much smaller than for plasmapheresis, the patients treated in this way have also demonstrated significant clinical improvement that was not observed with conventional oral immunosuppression. In aggregate, all of these studies show that the mechanical removal of pathological auto-antibodies is effective in helping to control symptoms of BP.
Utility of serology
The second major goal of our study was to determine whether auto-antibody titres against BP180 and BP230 could be used as a surrogate measure of disease severity during plasmapheresis. Both have been shown to be highly sensitive and specific when used to diagnose BP. The results from one study23 involving 127 patients showed a sensitivity of 95% and specificity of 94% for BP180. The sensitivity and specificity for BP230 alone was 82% and 65%, respectively, but almost 80% of cases had concordant reactivity with BP180.
One advantage of ELISA is that of being able to provide quantifiable data. Beyond a set cut-off used for diagnosis, the question remains whether absolute values can help guide treatment decisions such as when to taper medications. Several authors have begun to investigate the possible correlation between ELISA titres and disease activity24–33. Most focused upon BP180 antibodies although a few included BP230 antibodies in their analysis29,31,32. Two studies even differentiated between the IgG and IgE antibody subtypes of BP18025,29. The time frame for titre evaluation varied ranging from simply before treatment and after complete remission to more regular sampling on weekly and monthly bases. Clinical activity was typically assessed based on the percent of total body surface area involved, the number of blisters present, or a combination of the above.
Overall results showed a positive correlation between the level of BP180 antibodies and disease activity. Despite this general trend, titres were found to remain above cut-off values after complete resolution of skin lesions in the vast majority of reported cases. Based on the findings of their study, Izumi and colleagues27 further commented that there appears to be a greater discrepancy in the short-term, which they described as the first 2 weeks of therapy, when titres demonstrated far less dramatic declines compared to disease activity scores and in some cases were found to increase despite clinical improvement.
In contrast to BP180, published data indicate that BP230 titres do not parallel disease activity29,31,32. Di Zenzo and colleagues31 even suggested that there may be an inverse relationship. When they compared BP230 IgG levels among two groups of patients, one with limited-to-moderate disease versus another with extensive disease, they found relatively higher levels of BP230 IgG in the former group.
One important distinction in the analysis of titres is the fact that almost all patients were exclusively receiving oral treatment. Only four patients among those in all the studies reviewed here were treated with plasmapheresis26,29,33 at any point. In the only other study to our knowledge that presented sequential post-plasmapheresis ELISA data as we have, Lee and colleagues33 followed a single patient with extensive recurrent BP who underwent two rounds of plasmapheresis (comprising 5 and 8 procedures) while being treated with prednisolone, minocycline, and niacinamide. Immediate pre- and post-procedure BP180 titres were available for all but three instances. Although exact values were not provided, their data appeared to show an approximate overall decline in ELISA titre of about 50% after the first round of plasmapheresis whereas after the second round the decrease was about 25%. Titres generally followed disease activity according to the authors but they too found that the final post-procedure titre remained relatively high despite the almost complete disappearance of skin lesions. Of note, their method of plasmapheresis was via double filtration rather than centrifugation.
Like other study patients, ours were being treated with oral corticosteroids in addition to various secondary immunosuppressive drugs when serum samples for BP180 and BP230 ELISA were obtained. In broad terms our data also showed that the trajectory of titres paralleled disease activity, although this seemed most apparent only when comparing the baseline and final values. Additionally, the magnitude of clinical improvement was greater than any serological changes and titres always remained far above reference ranges.
As in the study by Izumi27, the lack of initial serological response in our study, as measured by BP180 and BP230 titres, was a surprising finding. The kinetics of plasmapheresis predicts that the removal of large macromolecules after consecutive single plasma volume exchanges achieves more than a 95% reduction from initial concentration after five procedures34. As highlighted by Patient One’s serial titres, this did not occur. The patient in Lee’s33 study also failed to reach 95% clearance by a wide margin. Even by factoring in the division of IgG between intravascular and extravascular compartments, the 24–48 hour interval between procedures should still allow sufficient time for redistribution. Assuming immunosuppressive medications are limiting continued antibody production, the modest decline in titres remains puzzling.
In brief, ELISA titres have also generally decreased in the small number of BP patients who have undergone immunoadsorption. Herrero-González20 demonstrated declines in BP180 ranging from 65–77% immediately after consecutive immunoadsorption procedures in two patients, although an exceedingly large rebound effect was observed after the first procedure in one of them. Conversely, Müller21 reported on a single case with a 90% drop in BP180 and a 58% drop in BP230 after a cycle of three procedures. Again, it is significant that ELISA titres in these cases also did not fall below reference cut-off values.
Conclusions
Corticosteroids will likely remain the preferred first-line choice for BP given their low cost, ease of administration, and generally favourable risk-benefit ratio at typical doses. Yet, through our own experience and a review of the medical literature, there is growing evidence suggesting plasmapheresis is a legitimate option in the treatment of BP. The data do not show that plasmapheresis is superior to traditional medications or that it is effective at achieving or maintaining clinical remission when used alone. Nevertheless, we believe that when the acute clinical condition appears refractory to oral immunosuppression or when further titration and additional second-line drugs produce an increasingly unfavourable side effect profile, it is then appropriate to consider a trial of plasmapheresis. Depending on each individual patient’s comorbidities affecting their ability to tolerate the procedure, the use of plasmapheresis may in fact be a safer and more effective option.
As far as it concerns BP180 and BP230 ELISA titres, their role in guiding clinical management has not yet been clearly established. Combined with the fact that titres remained elevated, often markedly so, even when a complete remission had been achieved, it could be argued that knowing absolute quantitative results, whether by ELISA or other methods, has limited consequences over that of a mere qualitative value. Indeed, when samples are taken during acute periods involving plasmapheresis there appears to be even less clinical utility given day-to-day fluctuations and the, at best, loose correlation with clinical severity or activity. In all likelihood, if titres are to be used as more than a diagnostic tool, they may potentially be employed during long-term follow-up to identify risk of relapse.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Apheresis Applications Committee of the American Society for Apheresis. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 2.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 3.Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- 4.Stanley JR, Tanaka T, Mueller S, et al. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roujeau JC, Guillaume JC, Morel P, et al. Plasma exchange in bullous pemphigoid. Lancet. 1984;2:486–8. doi: 10.1016/s0140-6736(84)92565-0. [DOI] [PubMed] [Google Scholar]
- 6.Egan CA, Meadows KP, Zone JJ. Plasmapheresis as a steroid saving procedure in bullous pemphigoid. Int J Dermatol. 2000;39:230–5. doi: 10.1046/j.1365-4362.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamada H, Yaguchi H, Takamori K, Ogawa H. Plasmapheresis for the treatment of pemphigus vulgaris and bullous pemphigoid. Ther Apher. 1997;1:178–82. doi: 10.1111/j.1744-9987.1997.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazzi G, Raineri A, Zanolli FA, et al. Plasmapheresis therapy in pemphigus vulgaris and bullous pemphigoid. Transfus Apher Sci. 2003;28:13–8. doi: 10.1016/S1473-0502(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Giudice GJ, Swartz SJ, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–44. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Giudice GJ, Zhou X, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–63. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiroyasu S, Ozawa T, Kobayashi H, et al. Bullous pemphigoid IgG induces BP180 internalization via a macropinocytic pathway. Am J Pathol. 2013;182:828–40. doi: 10.1016/j.ajpath.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roujeau JC, Revuz J, Touraine R, et al. Cortico-resistant bullous pemphigoid. Favorable results with plasmapheresis. Nouv Presse Med. 1979;8:3362. [In French] [PubMed] [Google Scholar]
- 13.Guillaume JC, Vaillant L, Bernard P, et al. Controlled trial of azathioprine and plasma exchange in addition to prednisolone in the treatment of bullous pemphigoid. Arch Dermatol. 1993;129:49–53. [PubMed] [Google Scholar]
- 14.Goldberg NS, Robinson JK, Roenigk HH, Jr, et al. Plasmapheresis therapy for bullous pemphigoid. Arch Dermatol. 1985;121:1484–5. doi: 10.1001/archderm.121.12.1484a. [DOI] [PubMed] [Google Scholar]
- 15.Guillot B, Donadio D, Guilhou JJ, Meynadier J. Long term plasma exchange therapy in bullous pemphigoid. Acta Derm Venereol. 1986;66:73–5. [PubMed] [Google Scholar]
- 16.Konstadt JW, Remlinger K, Schild J, et al. Refractory bullous pemphigoid leading to respiratory arrest and successfully treated with plasmapheresis. Arch Dermatol. 1990;126:1241–2. [PubMed] [Google Scholar]
- 17.Lee MW, Lee WS, Choi JH, et al. A case of severe bullous pemphigoid treated with plasmapheresis. J Dermatolog Treat. 2001;12:59–60. doi: 10.1080/095466301750163617. [DOI] [PubMed] [Google Scholar]
- 18.Kitabata Y, Sakurane M, Orita H, et al. Double filtration plasmapheresis for the treatment of bullous pemphigoid: a three case report. Ther Apher. 2001;5:484–90. doi: 10.1046/j.1526-0968.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 19.Ino N, Kamata N, Matsuura C, et al. Immunoadsorption for the treatment of bullous pemphigoid. Ther Apher. 1997;1:372–6. doi: 10.1111/j.1744-9987.1997.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 20.Herrero-González JE, Sitaru C, Klinker E, et al. Successful adjuvant treatment of severe bullous pemphigoid by tryptophan immunoadsorption. Clin Exp Dermatol. 2005;30:519–22. doi: 10.1111/j.1365-2230.2005.01853.x. [DOI] [PubMed] [Google Scholar]
- 21.Müller PA, Bröcker EB, Klinker E, et al. Adjuvant treatment of recalcitrant bullous pemphigoid with immunoadsorption. Dermatology. 2012;224:224–7. doi: 10.1159/000339071. [DOI] [PubMed] [Google Scholar]
- 22.Kolesnik M, Becker E, Reinhold D, et al. Treatment of severe autoimmune blistering skin diseases with combination of protein A immunoadsorption and rituximab: a protocol without initial high dose or pulse steroid medication. J Eur Acad Dermatol Venereol. doi: 10.1111/jdv.12175. [DOI] [PubMed] [Google Scholar]
- 23.Thoma-Uszynski S, Uter W, Schwietzke S, et al. BP230- and BP180-specific autoantibodies in bullous pemphigoid. J Invest Dermatol. 2004;122:1413–22. doi: 10.1111/j.0022-202X.2004.22603.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–8. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 25.Dopp R, Schmidt E, Chimanovitch I, et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42:577–83. [PubMed] [Google Scholar]
- 26.Amo Y, Ohkawa T, Tatsuta M, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2001;26:14–8. doi: 10.1016/s0923-1811(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 27.Izumi T, Ichiki Y, Esaki C, Kitajima Y. Monitoring of ELISA for anti-BP180 antibodies: clinical and therapeutic analysis of steroid-treated patients with bullous pemphigoid. J Dermatol. 2004;31:383–91. doi: 10.1111/j.1346-8138.2004.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji-Abe Y, Akiyama M, Yamanaka Y, et al. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005;37:145–9. doi: 10.1016/j.jdermsci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Iwata Y, Komura K, Kodera M, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. 2008;144:41–8. doi: 10.1001/archdermatol.2007.9. [DOI] [PubMed] [Google Scholar]
- 30.Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225–8. doi: 10.1111/j.1365-4632.2008.03473.x. [DOI] [PubMed] [Google Scholar]
- 31.Di Zenzo G, Thoma-Uszynski S, Fontao L, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128:415–26. doi: 10.1016/j.clim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Lee EH, Kim YH, Kim S, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant BP180 and BP230 for serodiagnosis and monitoring disease activity of bullous pemphigoid. Ann Dermatol. 2012;24:45–55. doi: 10.5021/ad.2012.24.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JB, Fumimori T, Kurose K, et al. A case of bullous pemphigoid successfully treated by plasmapheresis: assessment of the change in titers of circulating antibodies by immunoblotting and enzyme-linked immunosorbent assay. J Dermatol. 2003;30:326–31. doi: 10.1111/j.1346-8138.2003.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 34.Brecher ME, editor. Technical Manual. 15th edition. Bethesda, MD: ABB; 2005. p. 149. [Google Scholar]