Abstract
The effectiveness of the metal oxide nanoparticles viz. CuO and Fe2O3 as antibacterial agents against multidrug resistant biofilm forming bacteria was evaluated. CuO nanoparticles were also experimented for antibiofilm and time kill assay. The CuO displayed maximum antibacterial activity with zone of inhibition of (22 ± 1) mm against methicillin resistant Staphylococcus aureus (MRSA) followed by Escherichia coli (18 ± 1) mm. The Fe2O3 showed the zone of inhibition against MRSA of (14 ± 1) mm followed by E. coli (12 ± 1) mm. CuO proved to be more toxic than Fe2O3 nanoparticles showing significantly high antibacterial activity and found to possess dose dependent antibiofilm properties.
Keywords: Biofilm, Uropathogens, Nanoparticles, CuO, Fe2O3
Biofilm can be defined as the microbial-derived sessile communities characterized by the cells that remain attached to any surface. Quorum sensing among the biofilm forming organisms plays significant role in the formation of biofilm [4, 6]. Urinary catheters when inserted into the human body parts, may readily acquire biofilm. Eradication of the biofilm is very hard and cause many chronic infections. Recent research work reveals that use of quorum sensing inhibitors could be useful for restricting biofilm formation [3, 5]. Nanoparticles have good antibacterial activity and they could be used as an effective bactericidal agent [8, 10, 11]. Considering the above facts, experiment was conducted for detection of biofilm producing uropathogens and antibacterial/antibiofilm activity of iron oxide and copper oxide nanoparticles against multidrug resistant biofilm forming uropathogens was investigated.
A total of 50 samples and 213 isolates were screened for the biofilm production. All the clinical samples were collected from hospitalized female patients with urinary catheter inserted for more than 2 days from government hospitals in Sonitpur (Tezpur) (Lat. 26.63°N Long. 92.8°E) and Dibrugarh (Lat. 27.48°N Long. 95°E) districts of Assam, India.
The biofilm formation was analyzed by different standard methods [9]. Table 1 represented biofilm formation by uropathogens isolated from urine of normal and hospitalised females with urinary catheter inserted. Among 168 clinical samples maximum 88 isolates (52.4 %) of uropathogens were detected as biofilm former in Microtitre plate method. Among different biofilm formers Escherichia coli detected as maximum biofilm producer with 24 isolates (80 %) followed by Proteus mirabilis, 16 isolates (72.73 %); Staphylococcus aureus, 16 isolates (57.1 %); Enterococcus faecalis, 14 isolates (53.8 %); Pseudomonas species 14 isolates (43.8 %) and Staphylococcus epidermidis 4 isolates (40 %).
Table 1.
Biofilm formation by uropathogens isolated from urine of hospitalised females with urinary catheter inserted
| Methods | Biofilm formers | |||
|---|---|---|---|---|
| Total biofilm formers | Strong | Moderate | Weak | |
| Microtitre plate method | 88 (52.4 %) | 10 (11.36 %) | 22 (25 %) | 56 (63 %) |
| Pellicle assay | 49 (29.17 %) | 13 (26.53 %) | 16 (32.65 %) | 20 (40.81 %) |
| Tube method | 57 (33.93 %) | 16 (28.07 %) | 20 (35.08 %) | 21 (36.84 %) |
| Congo red agar method | 21 (12.5 %)a | – | – | – |
| Coverslip assay | 57 (33.93 %) | 16 (28.07 %) | 20 (35.08 %) | 21 (36.84 %) |
aWith the Congo red method differences among the biofilm formers could not be detected, so the distribution of strong, moderate and weak biofilm formers could not be tabulated
Antibiotic resistance of different kinds of uropathogens was studied by Kirby–Bauer disc diffusion method on MHA plates, which revealed that resistance was higher in biofilm formers than in non-biofilm formers. Three antibiotics namely penicillin, ampicillin and piperacillin were found to be resistant equally in both biofilm formers (100 %) and non-biofilm formers (100 %). Among Gram-positive biofilm formers, oxacillin (82.35 %) and erythromycin (70.6 %) were found to be most resistant antibiotics. In case of Gram-negative biofilm formers, very high resistance was observed for ceftazidime (79.6 %), cefixime (70.3 %), meropenem (72.22 %), ciprofloxacin (68.5 %) and polymyxin-B (63.5 %). E. coli showed maximum resistance to most of the antibiotics.
Antibacterial activity analysis of commercial CuO and Fe2O3 nanoparticles (Sigma Aldrich) of 25–30 nm size were performed by agar well diffusion method as described by Das et al. [2]. The CuO displayed greater antibacterial activity with zone of inhibition (in mm) against MRSA (22 ± 1) followed by E. coli (18 ± 1) (Table 2). The Fe2O3 showed the zone of inhibition (14 ± 1) mm against MRSA followed by E. coli (12 ± 1) mm. Antibiotic chloramphenicol was run as positive control in this experiment where maximum 23 mm zone of inhibition was recorded. MIC for MRSA were found to be 30 and 40 μg/ml for CuO and Fe2O3 nanoparticles respectively while for E. coli, MIC were found to be 35 and 45 μg/ml for CuO and Fe2O3 nanoparticles respectively.
Table 2.
Antibacterial activity of nanoparticles against uropathogens
| Uropathogens | CuO nanoparticles (mean ± SD) mm | Fe2O3 nanoparticles (mean ± SD) mm | Chloramphenicol (mm) |
|---|---|---|---|
| MRSA | 22 ± 1 | 14 ± 1 | 23 |
| MRSE | 20 ± 1 | 12 ± 1 | 22 |
| VRE | 21 ± 1 | 13 ± 1 | 22 |
| E. coli | 18 ± 1 | 12 ± 1 | 23 |
| K. pneumoniae | 14 ± 1 | 10 ± 1 | 23 |
| Pseudomonas sps. | 15 ± 1 | 10 ± 1 | 23 |
| P. mirabilis | 15 ± 1 | 9 ± 1 | 22 |
MRSA methicillin resistant S. aureus, MRSE methicillin resistant S. epidermidis, VRE vancomycin resistant E. faecalis
CuO nanoparticles showed remarkable antibacterial activity against both Gram-positive and Gram-negative bacteria. For Gram-positive bacteria, MRSA showed maximum activity while E. coli showed the highest activity among Gram-negative bacteria against both CuO and Fe2O3 nanoparticles. The variation in the sensitivity or resistance to both Gram-positive and Gram-negative bacteria could be due to the differences in the cell structure, physiology, metabolism, or degree of contact of organisms with nanoparticles. In our experiments, CuO was found to be more effective antibacterial agent than Fe2O3. Gram-positive bacteria such as MRSA, MRSE and VRE were found to be more sensitive to the CuO nanoparticles which might directly attribute to the greater abundance of amines and carboxyl groups on their cell surface and greater affinity of copper towards these groups [7].
Figure 1 represented the SEM images of MSRA and E. coli biofilm. The effect of CuO nanoparticles was studied with time-kill assay against MRSA and E. coli as presented in Fig. 2a. MRSA and E. coli cells were replicating without any hindrance when CuO nanoparticles were not added into the media. But when the cells of MRSA and E. coli were allowed to grow in the presence of CuO nanoparticles, the growth was found to be slowed down after 1 h of incubation as evident from decreased cfu/ml.
Fig. 1.
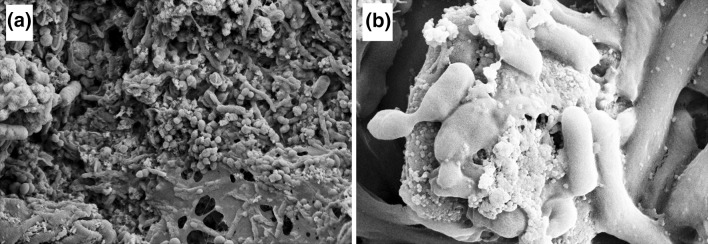
SEM micrograph of a MRSA biofilm, b E. coli biofilm
Fig. 2.
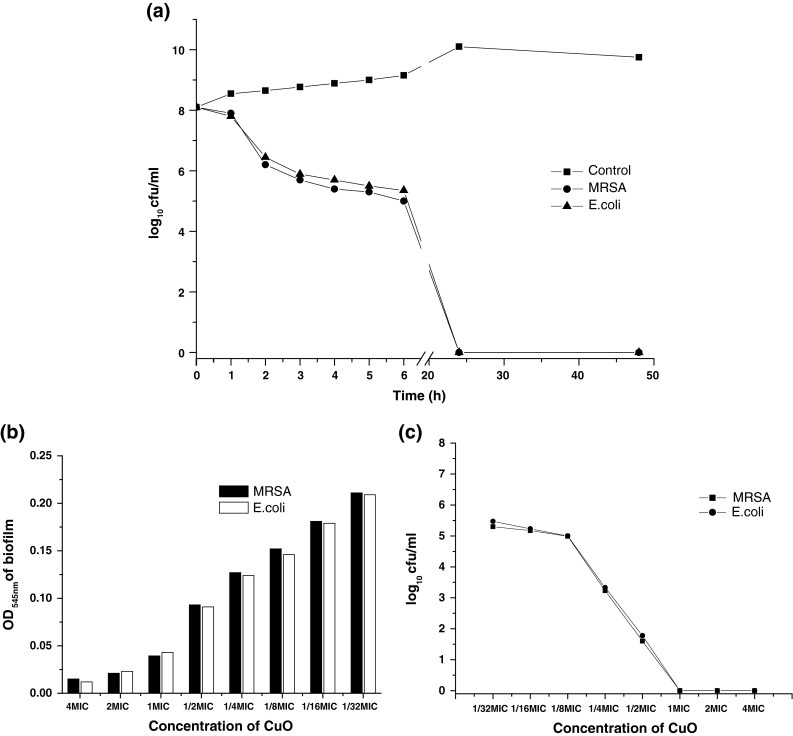
Antibiofilm activity of CuO nanoparticles against MRSA and E. coli. a time-kill assay of CuO nanoparticles against MRSA and E. coli., b a plot of sub-MIC value (4MIC to 1/32MIC) of CuO nanoparticles vs. OD545 nm of biofilm, c a plot of sub-MIC values of CuO against log10 cfu/ml
The efficiency of the clinical isolates MRSA and E. coli, were screened for their ability to form biofilm on the surface of Foley’s catheters and polyurethane catheters in vitro. As presented in Fig. 2b it was found that both of these two organisms were equally capable of forming biofilm on the catheter surfaces as indicated by the increased OD545nm of biofilm and was found to be >0.3.
Efficiency of CuO nanoparticles to inhibit biofilm formation at sub-MIC concentrations was evaluated against MRSA and E. coli. Figure 2b showed the effect of CuO nanoparticles on the biofilm formed by MRSA and E. coli and clearly revealed that inhibition of biofilm formation was concentration dependent. From 2MIC to 1/2MIC of CuO nanoparticles, biofilm was almost completely eradicated as the OD545nm of biofilm was <0.1 and also the cfu/ml was decreased indicating that established biofilm was eradicated. But as soon as the concentration further decreased, removal of the biofilm was also decreased. At the 1/8MIC, 1/16MIC, 1/32MIC, the OD545nm of biofilm was >1 and ≥2, that means the biofilm was not completely eradicated and similar result was also observed when established biofilm was incubated with CuO nanoparticles at sub-MIC values as evident from Fig. 2c.
Antibiofilm activity study of CuO nanoparticles showed that it efficiently reduced the biofilm formation by both MRSA and E. coli. It was noticed that when the concentration of the nanoparticles was increased, the inhibition of biofilm was also enhanced which can be attributed directly to the dose dependent activity. Almost all MRSA and E. coli biofilm cells died within 4 days of exposure to CuO nanoparticles. However, on the 10th day of cultivation a few persisting biofilm bacteria re-grew to lesser bacterial counts, probably because of low amounts of the antimicrobial (Cu) agent after its absorption by the initially seeded bacteria. Toxicity of copper to the microorganisms is exerted by several parallel mechanisms, which lead to the death of the microorganisms. It seems that the first target site of copper that it damages is the microorganism’s envelope. Copper ions also damage nucleic acids. It has been suggested that subsequent to the specific binding of copper to DNA, repeated cyclic redox reactions generate several OH radicals near the binding site causing multiple damage to the nucleic acids. However, it still may be that in some microorganisms, copper oxidative damage to the genetic material may occur through Fenton mechanism [1].
Catheter associated biofilm formation by the multiple drug resistant uropathogens is a great concern and it is very difficult to eradicate biofilm causing serious health related complications to the patients undergoing catheterization. As compared to Fe2O3 nanoparticles CuO is found to be highly effective as an antibacterial material against multidrug resistant biofilm forming uropathogens. CuO nanoparticles have high potential of absorption, adsorption, penetration and availability which make it an essential antibiofilm agent.
Acknowledgments
M. Agarwala thanks Rup Agarwala Foundation for financial support. Financial support from DBT, India is gratefully acknowledged. Authors also thank IASST, Guwahati for XRD and SEM analyses.
Ethical Standards
The present study was approved by the Ethics Committee of Dibrugarh University, Assam, India.
References
- 1.Borkow G, Gabbay J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol. 2009;3:272–278. [Google Scholar]
- 2.Das A, Raychaudhuri U, Chakraborty R. Antimicrobial effect of edible plant extract on the growth of some foodborne bacteria including pathogens. Nutra Foods. 2012;12:83–88. [Google Scholar]
- 3.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 4.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2013 doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Sun M, Li L, Qiao R, Chen K, Xiao Q, Xu F. Preparation and antibacterial activities of polyaniline/Cu0.05Zn0.95O nanocomposites. Dalton Trans. 2012;4:2804–2811. doi: 10.1039/c2dt11823h. [DOI] [PubMed] [Google Scholar]
- 8.Mahanty A, Mishra S, Bosu R, Maurya UK, Netam SP, Sarkar B. Phytoextracts-synthesized silver nanoparticles inhibit bacterial fish pathogen Aeromonas hydrophila. Indian J Microbiol. 2013;53:438–446. doi: 10.1007/s12088-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2011;1:1–18. doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravikumar S, Gokulakrishnan R, Boomi P. In vitro antibacterial activity of the metal oxide nanoparticles against urinary tract bacterial pathogens. Asia Pac J Trop Dis. 2012;2:85–89. doi: 10.1016/S2222-1808(12)60022-X. [DOI] [Google Scholar]
- 11.Sales EH, Sedeh FM, Rajabifar S. Effects of gamma irradiation and silver nano particles on microbiological characteristics of saffron, using hurdle technology. Indian J Microbiol. 2012;52:66–69. doi: 10.1007/s12088-011-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


