Abstract
By using EST database from a full-length cDNA library of Curvularia lunata, we have isolated a 2.9 kb cDNA, termed PKAr. An ORF of 1,383 bp encoding a polypeptide of 460 amino acids with molecular weight 50.1 kDa, (GeneBank Acc. No. KF675744) was cloned. The deduced amino acid sequence of the PKAr shows 90 and 88 % identity with cAMP-dependent protein kinase A regulatory subunit from Alternaria alternate and Pyrenophora tritici-repentis Pt-1C-BFP, respectively. Database analysis revealed that the deduced amino acid sequence of PKAr shares considerable similarity with that of PKA regulatory subunits in other organisms, particularly in the conserved regions. No introns were identified within the 1,383 bp of ORF compared with PKAr genomic DNA sequence. Southern blot indicated that PKAr existed as a single copy per genome. The mRNA expression level of PKAr in different development stages were demonstrated using real-time quantitative PCR. The results showed that the level of PKAr expression was highest in vegetative growth mycelium, which indicated it might play an important role in the vegetative growth of C. lunata. These results provided a fundamental supporting research on the function of PKAr in plant pathogen, C. lunata.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-013-0439-3) contains supplementary material, which is available to authorized users.
Keywords: Curvularia lunata, PKAr, Southern blot, Real-time PCR
Introduction
Curvularia leaf spot is caused by Curvularia lunata, a pathogenic fungus to maize, and was responsible for one of most economically devastating diseases in Northern China during the 1990s [4]. It is currently one of most widely distributed maize leaf diseases nationwide, whereas the damage caused by the disease has been significantly reduced by the increased application of numerous resistant cultivars in China. However, most studies found that the pathogenic variation and physiological differentiation were very obvious, which is a potential risk of outbreak [5, 20]. Therefore, the molecular mechanism of pathogens was studied in depth, which benefits to establish for the effective prevention and control technology, and to ensure the security and stability of maize production.
For uncovering of molecular mechanisms of the pathogenicity, previous works have focused on the identification of related genes with virulence factors, such as pigment (brn1, scd and PKA) [11, 20], toxin biosynthesis genes and a series of signal transduction related genes through mutant [9, 19] and homology cloning methods. However, as the pathogen genome is not sequenced, there are still more difficulties in identify enormous genes relative to virulence factors synthesis, for example, the toxin and pigment synthesis is accomplished through the secondary metabolic pathways involving interaction of many regulatory networks. Therefore, the research group established cDNA library of pathogen, and obtained a number of EST information through large-scale EST sequencing [10]. Among of these, An EST that has high similarity with cAMP-dependent protein kinase A regulatory subunit from Alternaria alternata was obtained.
The cAMP as second messenger was widely studied in intracellular signal transduction pathways in fungi [15]. Encoding cAMP-dependent protein kinase A PKA gene is a key component in the downstream of cAMP. Recently, a number of reports have demonstrated that a homolog of the PKA protein plays a major role in controlling cell growth and metabolism in response to nutrients and stress conditions in eukaryotic organisms [3, 6, 14]. But there was no any report about PKA gene, and its expression in development of C. lunata.
In this study, we described the cloning, characterization, and expressions of a PKA regulation subunit gene in different development stage (designated as PKAr) from C. lunata. The main objectives of this study are (1) to clone ORF of PKAr from C. lunata and compare it with other pathogens, (2) to investigate the molecular characterization of PKAr in C. lunata by analysis of bioinformation, (3) to detect the level of PKAr expression by Real-time PCR in different development stages. The results of this study may be useful to explore the function of PKAr for further research.
Materials and Methods
Fungal Strains and Culture Conditions
Curvularia lunata strain CX-3 kindly provided by Professor Jie Chen (Shanghai Jiaotong University, Shanghai, China), which causes typical leaf spots in maize, was used as the wild type strain. The strain was maintained on potato dextrose agar (PDA) at 28 °C.
Genomic DNA and Total RNA Isolation
For DNA or total RNA extraction, the fungal mycelia were grown in potato dextrose (PD) at 28 °C in the condition of continuous shaking (120 rpm) for 5 days. The CTAB method was used for genomic DNA isolation [16]. Total RNA was extracted by TrizolTM Reagent (Invitrogen, USA) and then treated with DNase I (Takara, Japan) following the manufacturer’s instructions.
cDNA Library Construction and EST Analysis
Total RNA was quantified by ultraviolet absorbance at A260/280 (Eppendorff AG 22331, Germany). A cDNA library construction was carried out according to SMARTer™ cDNA Library Construction Kit with SMART (switching mechanism at 5′ end of RNA transcript) technique. Random EST of the library using T7 primer was sequenced. The methods were described as Liu et al. [9].
Cloning ORF and DNA of PKAr
Based on the identified EST sequence by BLAST analysis,an EST of 2.9 kp has high similarity with cAMP-dependent protein kinase A regulatory subunit from Alternaria alternata. The ORF of gene was predicted by the DNAMAN software. To obtain the ORF and DNA of the PKAr gene, the primers A 5′-ATGTCCCTCCCTCCCGACTACG-3′ and B 5′-TTACGAAGCGTGCGAGAGAGGATC-3′ was designed by Primer Premier 5.0 with the template of cDNA and Genomic DNA respective. The amplification profile was as follows: 1 cycle at 95 °C for 2 min; 30 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min. After a final extension of 5 min at 72 °C, the samples were stored at 4 °C. The amplification products were gel purified and cloned into pMD 19 T-Vector (Takara, Japan) and sequenced.
Sequence Analysis and Phylogenetic Analysis
Nucleotide sequence and deduced amino acid sequence were analyzed by DNAMAN software. The protein sequence of the PKAr was compared to its counterpart sequences currently available in GenBank, retrieved using the BLAST program (http://www.ncbi.nlm.nih.gov). The percentage of similarity and identity of the known sequences was calculated by the MatGAT program. Molecular weight and isoelectric point predication was conducted by ExPASy Compute pI/Mw tool (http://www.expasy.ch/tools/pi_tool.html), as well as conserved domains of the deduced protein were performed by ScanProsite tool. The phylogenetic analysis was conducted using the program MEGA 5.2. The phylogenetic tree was constructed by the neighbor joining method based on the PKAr amino acid sequences distances. The phylogenetic tree was tested for reliability using bootstrap replications.
Southern Blotting
Southern blotting was performed as previously described [9] to assay PKAr copy number in genome. Genomic DNA from CX-3 was digested with HindIII, BamHI and XbaI for 24 h at 37 °C, respectively. The digested genomic DNA were electrophoresed at 70 V for 5 h, blotted and hybridized with the probes at 55 °C for 16 h. The PKAr DNA fragment amplified with primer A and B was labeled with digoxigenin (DIG) as probe.
PKAr Expression in Difference Development Stages
PKAr expression in difference growth stages was analyzed by Real-time quantitative PCR. Samples from different development stages of C. lunata were collected. For stage one, conidia, the conidia were harvested in aqueous solution, and the conidia suspension was filtered through three layers of sterile cheesecloth to remove mycelia and agar particles. For stage two, the vegetative growth mycelia, the conidia suspension was cultured the PD medium for 72 h at 28 °C, then the mycelia was collected and mixed by sterile cheesecloth. For stage three, the germination of conidia, conidia suspension were grown on PDA-cellophane and collected at 3, 6 and 9 h respectively by washing the cellophane paper with physiological salt solution. Primer C 5′-TACAACACCAACAGACGCACTTC-3′ and D 5′-CGCCCTGCTGAATGACCTTTAT-3′ were employed for PKAr amplification based on the cDNA sequence of PKAr. Another pair of primers E 5′-GACGGCAACAACCTGACT-3′ and F 5′-CAGTGCTGCTGGGAATGA-’ for GAPDH nucleotide fragments was designed using PRIMER Premier 5.0 (Premier Biosoft, CA, US) as an internal positive control [15]. PCRs was carried out in 25 μl reactions with 1 μl of cDNA, 2.5 μl of 10 × Reaction Buffer, 2.5 μl MgCl2, 0.4 μl of dNTPs Mixture(10 mM), 16.9 μl of ddH2O, 0.4 μl each of primers (10 mM), 0.4 μl of E-Taq(5 U/μl), and 0.4 μl of 50 × SYBR Green. The PCR cycle was as follows: 95 °C for 1 min, 40 cycles of 95 °C for 20 s, 60 °C for 20 s and 72 °C for 40 s, followed by one cycle of 72 °C for 5 min. The results of RT-PCR in the form of fluorescence curves and threshold cycle (Ct) values were analyzed by 2−ΔΔCt method.
Results
Cloning and Characterization of PKA
A 2.9 kp fragment from Random sequencing of cDNA library showed that it shared high similarity with cAMP-dependent protein kinase A regulatory subunit, implying that it was probably a sequence of PAK regulatory subunit, and designated as PKAr. A ORF of sequence was predicted by DNAMAN software. Two pair of specific primers was designed to amplify the ORF of PKAr base on the sequence. A 1,383 bp fragment was amplified by PCR technology. The PKAr sequence was deposited in GenBank under accession no. KF675744.
The open reading frame encoded a peptide of 460 amino acids with molecular weight 50.1 kDa and a theoretical isoelectric point of 5.51. No introns were found within the 1,383 bp of ORF compared with PKAr genomic DNA sequence. ScanProsite analysis indicated that PKAr included 2 highly conserved cAMP binging sites domain (Electronic Supplementary Material 1).
Homology and Phylogenetic Analysis
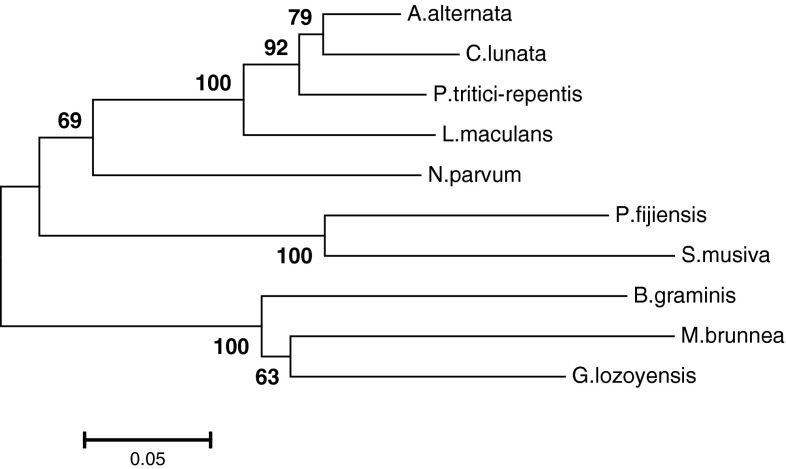
Homology analysis of the deduced amino acid sequence of the PKAr was analyzed by MatGAT software. The result revealed that the PKAr was very high homology with the other plant pathogenic cAMP-dependent protein kinase A regulatory subunit proteins. The homology analysis of the PKAr shows 90 and 88 % identity with cAMP-dependent protein kinase A regulatory subunit from Alternaria alternate and Pyrenophora tritici-repentis Pt-1C-BFP, respectively. The phylogenetic tree was constructed by the neighborjoining method based on PKAr amino acid sequences and 9 cAMP-dependent protein kinase A regulatory subunit from other fungi. The results showed in Fig. 1.
Fig. 1.
Phylogenetic analysis of PKAr on the deduced amino acid sequences using Mega 5.2 software based on neighbour-joining method. The values shown above were bootstrap values. Abbreviations: A. alternata: Alternaria alternate; C. lunata: Curvularia lunata; P. tritici-repentis: Pyrenophora tritici-repentis Pt-1C-BFP; L. maculans: Leptosphaeria maculans JN3; N. parvum: Neofusicoccum parvum UCRNP2; P. fijiensis: Pseudocercospora fijiensis CIRAD86; S. musiva: Sphaerulina musiva SO2202; B. graminis: Blumeria graminis f. sp. hordei DH14; M. brunnea: Marssonina brunnea f. sp. ‘multigermtubi’ MB_m1; G. lozoyensis: Glarea lozoyensis 74030
The Copy Number of PKAr
The copy number of PKAr in the C. lunata genome was determined by Southern blot analysis. Only one band hybridized to the PKAr probe with any of three enzymes (BamHI, HindIII and XbaI) which did not digest the DNA of PKAr (Electronic Supplementary Material 2), which implies that the gene is present as a single copy in the C. lunata genome.
Expression Analysis of PKAr
To investigate PKAr expression pattern in different development stages, total RNA was extracted from the condia, germinating conida and vegetative growth mycelium, and subjected to Real-time PCR analysis. The results showed that PKAr expression level was significantly difference with different development stages, and highest in vegetative growth mycelium, implying that the expression of PKAr correlates with the vegetative growth of pathogen (Electronic Supplementary Material 3).
Discussion
The cAMP singal transduction pathways play important role in the growth, development and pathogenicity of fungi. The cAMP signal pathway includes two main components: Protein kinase A (protein kinase A, PKA) and adenosine Cyclase (Adenylyl cyclase, AC). cAMP mainly activated the protein kinase A, which regulated the downstream signal pathway. Protein kinase A is also known as cAMP-dependent protein kinase including two catalytic subunits (PKA-C) and two regulatory subunits (PKA-R). The four subunits consisting of a heterologous protein tetramer commonly shows no catalytic activity. Only when the cAMP binds to the regulatory subunit of PKA kinase, PKA catalytic subunit can release and be activated. Activation of the catalytic subunit phosphorylation cascade may activate or migrate into the nucleus phosphorylation target labeled proteins [15].
Numerous studies confirmed that many fungal hyphae growth, virulence, the formation of melanin, pathogenicity, and so on, have a close relationship with PKA [7, 8, 12, 13, 18]. For example, PKA regulatory subunit regulates the mycelium polar growth in Candida albicans and Neurospora crassa [1, 2]. In S. cerevisiae, BCY1 genes encoding the regulatory subunit of PKA was disrupted, leading to loss to of cyclic AMP-dependent protein kinase activity in vitro, fail to grow on many carbon sources, and are exquisitely sensitive to heat shock and starvation [17]. In Mycosphaerella graminicola, the regulatory subunit MgBcy1 of protein kinase A mutated showed altered phenotypes in vitro when grown under different growth conditions, and altered osmosensitivity, reduced melanization and virulent, but were unable to produce the asexual fructifications, which was particularly due to inappropriate differentiation during the late stage of this morphogenesis-related process [12].
In this study, we have successfully cloned and characterized the PKAr gene from C. lunata, and found that the level of PKAr expression was highest in vegetative growth mycelium which implying that the regulatory subunit of protein kinase A in C. lunata plays an important role in regulating mycelium vegetative growth. In order to further understand and uncover the function of the PKAr, over-expression and disruption of PKAr vector have been constructed and the genetic transformation are undergoing. The cloning and characterization of PKAr will be helpful in functional analysis of PKAr in C. lunata.
Electronic supplementary material
Acknowledgments
This work was supported by NSFC (Grant Nos. 31101407, 31272026 and 31301611), Doctoral Fund of Ministry of Education of China (20112305120001), Open Project of State Key Laboratory of Crop Stress Biology in Arid Regions (CSBAA2011-18), Doctoral Fund of Heilongjiang Bayi Agricultural University (B2011-03).
References
- 1.Bruno KS, Aramayo R, Minke PF, Metzenberg RL, Plamann M. Loss of growth polarity and mislocalization of septa in a neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 2.Cassola A, Parrot M, Silberstein S, Magee BB, Passeron S, Giasson L, Cantore M. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot Cell. 2004;3:190–199. doi: 10.1128/EC.3.1.190-199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YE, Xu JR. The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol Plant Microbe Interact. 2010;23:522–533. doi: 10.1094/MPMI-23-4-0522. [DOI] [PubMed] [Google Scholar]
- 4.Dai FC, Gao WD, Wu RJ, Jin XH. A noticeable corn disease: curvularia leaf spot. Acta Phytopathol Sin. 1995;25:330. [Google Scholar]
- 5.Gao SG, Liu T, Li YY, Wu Q, Fu KH, Chen J. Understanding resistant germplasm-induced virulence variation through analysis of proteomics and suppression subtractive hybridization in a maize pathogen Curvularia lunata. Proteomics. 2012;12:1–12. doi: 10.1002/pmic.201200105. [DOI] [PubMed] [Google Scholar]
- 6.Giacometti R, Kronberg F, Biondi M, Hernández IA, Passeron S. Cross regulation between Candida albicans catalytic and regulatory subunits of protein kinase A. Fungal Genet Biol. 2012;49:74–85. doi: 10.1016/j.fgb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Park SY, Lee S, Adams EL, Czymmek K, Kang S. Loss of cAMP-dependent protein kinase A affects multiple traits important for root pathogenesis by fusarium oxysporum. Mol Plant Microbe Interact. 2011;24:719–732. doi: 10.1094/MPMI-11-10-0267. [DOI] [PubMed] [Google Scholar]
- 8.Kronstad JW, Hu G, Choi J. The cAMP/protein kinase A pathway and virulence in Cryptococcus neoformans. Mycobiology. 2011;39:143–150. doi: 10.5941/MYCO.2011.39.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Liu LX, Jiang X, Hou JM, Fu KH, Zhou FH, Chen J. Agrobacterium-Mediated transformation as a useful tool for molecular genetic study of phytopathogen Curvularia lunata. Eur J Plant Pathol. 2010;126:363–371. doi: 10.1007/s10658-009-9541-0. [DOI] [Google Scholar]
- 10.Liu T, Liu LX, Liu ZC, Hou JM, Gao SG, Zhou FH, Chen J. Construction and characterization of a normalized full length cDNA library of Curvularia lunata. ACTA Phytopathol Sin. 2010;40:250–257. [Google Scholar]
- 11.Liu T, Xu SF, Liu LL, Zhou FH, Hou JM, Chen J. Functional analysis of multi-copy Brn1 gene from the phytopathogenic fungus Curvularia lunata. Eur Plant Pathol. 2011;131:211–219. doi: 10.1007/s10658-011-9800-8. [DOI] [Google Scholar]
- 12.Mehrabi R, Kemt GHJ. Protein kinase A subunits of the ascomycete pathogen Mycosphaerella graminicola regulate asexual fructification, filamentation, melanization and osmosensing. Mol Plant Pathol. 2006;7:565–577. doi: 10.1111/j.1364-3703.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Priyatno TP, Abu Bakar FD, Kamaruddin N, Mahadi NM, Abdul Murad AM. Inactivation of the catalytic subunit of cAMP-dependent protein kinase A causes delayed appressorium formation and reduced pathogenicity of Colletotrichum gloeosporioides. Sci World J. 2012;2012:545784. doi: 10.1100/2012/545784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaldi J, Wu J, Yang J, Ralston YC, Sankaran B, Moreno S, Taylor SS. Structure of yeast regulatory subunit: a glimpse into the evolution of PKA Signaling. Structure. 2010;18:1471–1482. doi: 10.1016/j.str.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen S, Hao ZM, SGu HQ, Wang JJ, Cao ZY, ZY LI, Wang Q, LI P, Hao J, Dong J. The catalytic subunit of cAMP-dependent protein kinase A StPKA-c contributes to conidiation and early invasion in the phytopathogenic fungus Setosphaeria turcica. FEMS Microbiol Lett. 2013;343:135–144. doi: 10.1111/1574-6968.12150. [DOI] [PubMed] [Google Scholar]
- 16.Stewart CN, Via EL. A Rapid CTAB DNA Isolation Technique Useful for RAPD fingerprinting and other PCR applications. Biotechniques. 1993;14:748–749. [PubMed] [Google Scholar]
- 17.Toda T, Cameron S, Sass P, Zoller M, Scott DJ, McMullen B, Hurwitz M, Krebs EG, Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzima A, Paplomatas EJ, Rauyaree P, Kang S. Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet Biol. 2010;47:406–415. doi: 10.1016/j.fgb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang YJ, Liu T, Hou JM, Zuo YH. Isolation and identification of pathogenicity mutant of Curvularia lunata via restriction enzyme-mediated integration. Indian J Microbiol. 2013;53:303–307. doi: 10.1007/s12088-013-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu SF, Chen J, Liu LX, Wang X, Huang XL, Zhai YH. Proteomics associated with virulence differentiation of Curvularia lunata in maize (Zea mays) in China. J Integr Plant Biol. 2007;49:487–496. doi: 10.1111/j.1744-7909.2007.00469.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.