Abstract
Chromium(Cr) precipitate synthesized by Cr(VI)-reducing bacterium Flexivirga alba ST13T was examined using transmission electron microscopy (TEM) and the energy dispersive X-ray (EDX). The strain showed altered-morphology after exposing to Cr(VI) in minimal medium. The resultant precipitate included bacterial pellet and needle-like structure which was similar to the structure made from Cr(OH)3 precipitate. Cr was observed in bacterial cells using TEM–EDX. Bacteria with high electron density showed the precipitation of Ca in addition to Cr. The isolated strain would be useful to precipitate Cr from Cr(VI)-containing environment.
Keywords: Chromium, Electron microscopy, Bacterium, Actinobacterium
Chromium(Cr) is an important element in industrial processes, i.e. electroplating and leather tanning. However a valence state of Cr, hexavalent Cr (Cr(VI)), is the toxic and harmful chemical form. Therefore it is important to develop technologies for the prevention of Cr(VI) from effusing into natural environment and for the detoxification of Cr(VI) by transforming the valence state into trivalent Cr (Cr(III)). In many bioreduction experiment, the most of Cr(III) was detected in water soluble form but not in insoluble precipitate. It is needed to understand Cr(III) precipitation mechanisms in Cr(VI)-reducing bacteria.
Here we describe TEM and EDX analysis of Cr-containing precipitates which were synthesized by newly isolated actinomyces, Flexivirga alba ST13T (=NBRC 107850T = DSM 24460T) as a model of Cr(III)-accumulating bacterium [1]. The strain reduced Cr(VI) efficiently in minimal medium supplemented with molasses and formed water insoluble Cr(OH)3 compound [2].
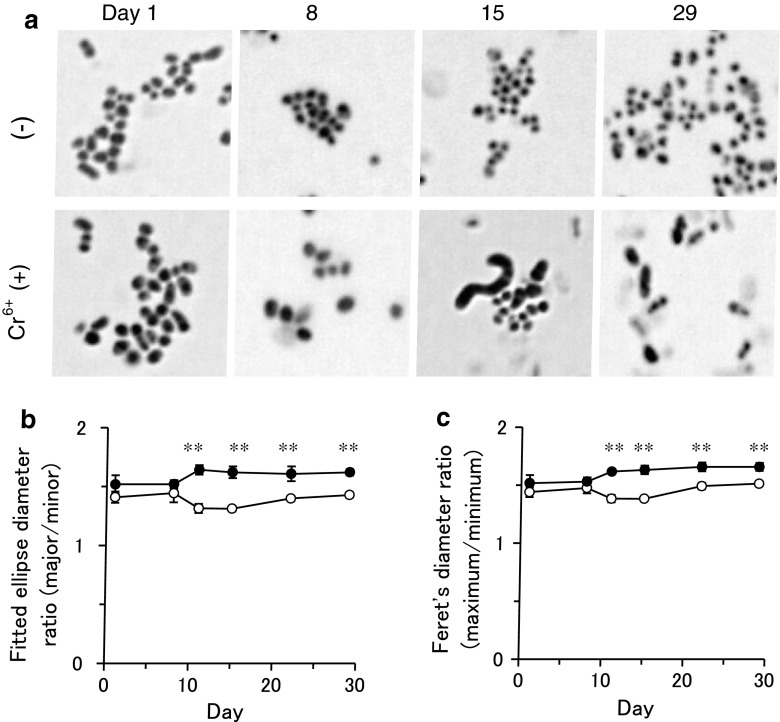
Bacteria were grown aerobically in minimal medium (6 g/L Na2HPO4, 3 g/L KH2PO4, 1 g/L NH4Cl, 0.5 g/L NaCl, 0.246 g/L MgSO4·7H2O, 0.01 g/L CaCl2, 0.4 % (w/w) molasses; pH 6.8) in a rotating bioshaker (Taitec, Saitama, Japan) at 200 rpm at 27 °C. For the preparation of Cr(VI)-containing medium, 0.4 mM CrO3 (Wako, Osaka, Japan) was added to the medium. Hexavalent and total Cr concentration were measured as described previously [2]. Strain ST13T growth in medium containing CrO3 was centrifuged (5,400×g, 5 min) to separate the culture supernatant and the cell pellet. The supernatant was used for Cr(VI) measurement. Briefly, 1 mM diphenylcarbazide (Wako) in 1.2 N H2SO4 was mixed with an equal volume of supernatant for 10 min. The absorbance at 540 nm was determined using a spectrophotometer 3100 (GE Healthcare, Buckinghamshire, England). The Cr(VI) concentration in the sample was estimated by a standard curve generated with known amounts of Cr(VI). About 48 and 5 % Cr(VI) remained in medium on days 5 and 13, respectively. To examine the effect of Cr(VI) on bacterial morphology, gram staining was performed using Fiber-G (Nissui Pharmaceutical Co., Tokyo, Japan) and observed using an upright microscope BX51 (Olympus, Tokyo, Japan). Curved-rod shaped cells were observed in bacterial culture with Cr(VI) at day 15 (Fig. 1a). Then, we fit each bacterial shape to ellipse using ImageJ software [3] to analyze the morphological change. The major to minor diameter ratio of fitted ellipse showed significant difference between bacteria cultured in medium with and without Cr(VI) after day 10 (Fig. 1b). The significant difference was also observed using the maximum to minimum Feret’s diameter ratio (Fig. 1c). These results consistent with previous report that the morphology was not depend on culture stage without Cr(VI) [1]. Interestingly, we found that morphology of F. alba ST13T was altered by exposure to Cr(VI).
Fig. 1.
Effect of Cr(VI) exposure on morphology of F. alba ST13T. a Gram staining of bacteria. Bacterial shape analysis using ellipse diameters (b) and Feret’s diameters (c) obtained by fitting each bacterium with ellipse. Data are presented as the mean ± SEM; **P < 0.01, n = 350–3,000
Figure 2a shows TEM image of F. alba ST13T aerobically exposed to 0.5 mM Cr(VI) in minimal medium at 27 °C for 20 days. Briefly, a part of the bacterial pellet was placed on Exel support film 200 mesh Cu grid (Nisshin EM Co., Tokyo, Japan) without fixation. The specimen was dried in desiccator and examined in H-7650 (Hitachi Co., Tokyo, Japan) operated at 100 kV. About 19 % of total Cr was precipitated in the pellet. We observed elongated-rod shaped cells with un-uniform electron dense area (Fig. 2a). Needle-like structure which was similar to Cr(OH)3 precipitate (Fig. 2b) was also observed in the pellet (arrow in Fig. 2a). Next, we performed EDX analysis of bacteria showing both high and low electron density (Fig. 2c). Each bacterial population showed the association of Cr (Fig. 2d, e). The Cr was not observed in control area (Fig. 2f). Interestingly, Ca was observed only with bacteria showing higher electron density (Fig. 2d). The presence of different population in terms of element association was confirmed (Fig. 2g) by quantitation using Cliff–Lorimer thin ratio section method [4]. In sulfate-reducing bacterium Desulfovibrio vulgaris, which was known to reduce Cr(VI) into Cr(III), insoluble Ca-bearing Cr(III) phosphate was accumulated in the membrane [5]. Ca might tend to co-precipitate with Cr(III) in Cr(VI)-reducing bacteria. Further study is needed on the relation between Ca accumulation and Cr(VI) reduction.
Fig. 2.
TEM of F. alba ST13T exposed to Cr(VI). a TEM of bacterial after 20 days exposure of Cr(VI). Bar 5 μm. b TEM of Cr(OH)3. Bar 2 μm. c TEM for EDX analysis. Each circle indicates area for EDX analysis. Bar 2 μm. d TEM–EDX in area 1 indicated by c. e TEM–EDX in area 2 indicated by c. f TEM–EDX in area 3 indicated by c. g TEM–EDX element analysis shown by wt% using peak area showing each element in (d) and (e)
Electron microscopic observations of Cr precipitation using Cr(VI)-reducing bacteria have shown the usefulness of those bacteria for the detoxification of Cr(VI)-contaminated environment [6–9]. However we and others result need more investigations to establish a method for an efficient precipitation of Cr(III) synthesized by Cr(VI)-reducing bacterium.
Acknowledgments
We thank Mr. Koichi Higashimine and Ms. Madoka Katayama for TEM analysis.
References
- 1.Anzai K, Sugiyama T, Sukisaki M, Sakiyama Y, Otoguro M, Ando K. Flexivirga alba gen. nov., sp. nov., an actinobacterial taxon in the family Dermacoccaceae. J Antibiot. 2011;64:613–616. doi: 10.1038/ja.2011.62. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama T, Sugito H, Mamiya K, Suzuki Y, Ando K, Ohnuki T. Hexavalent chromium reduction by an actinobacterium Flexivirga alba ST13T in the family Dermacoccaceae. J Biosci Bioeng. 2012;113:367–371. doi: 10.1016/j.jbiosc.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorimer GW, Cliff G. Analytical electron microscopy of minerals. In: Wenk HR, Wenk HR, editors. Electron microscopy in mineralogy. Berlin: Springer; 1976. pp. 506–519. [Google Scholar]
- 5.Goulhen F, Gloter A, Guyot F, Bruschi M. Cr(VI) detoxification by Desulfovibrio vulgaris strain Hildenborough: microbe–metal interactions studies. Appl Microbiol Biotechnol. 2006;71:892–897. doi: 10.1007/s00253-005-0211-7. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Yang L, Luo M, Liang X, Wei X, Zhao J, Liu H. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 coated with polyethylenimine-functionalized magnetic nanoparticles under alkaline conditions. J Hazard Mater. 2011;189:787–793. doi: 10.1016/j.jhazmat.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Naik UC, Srivastava S, Thakur IS. Isolation and characterization of Bacillus cereus IST105 from electroplating effluent for detoxification of hexavalent chromium. Environ Sci Pollut Res Int. 2011;19:3005–3014. doi: 10.1007/s11356-012-0811-6. [DOI] [PubMed] [Google Scholar]
- 8.Dogan NM, Kantar C, Gulcan S, Dodge CJ, Yilmaz BC, Mazmanci MA. Chromium(VI) bioremoval by Pseudomonas bacteria: role of microbial exudates for natural attenuation and biotreatment of Cr(VI) contamination. Environ Sci Technol. 2011;45:2278–2285. doi: 10.1021/es102095t. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava S, Thakur IS. Evaluation of biosorption potency of Acinetobacter sp. for removal of hexavalent chromium from tannery effluent. Biodegradation. 2007;18:637–646. doi: 10.1007/s10532-006-9096-0. [DOI] [PubMed] [Google Scholar]