Abstract
Emerging drug resistance in Salmonella coupled with the recent poor success rate of antibiotic discovery programs of the pharmaceutical industry is a cause for significant concern. It has forced the scientific community to look for alternative new classes of antimicrobial compounds. In this context, combinations of antimicrobial peptides (AMPs) and conventional antibiotics have gained interest owing to their versatile applications. The present study was therefore planned to evaluate the synergistic effects, if any, of cryptdin-2, a mouse Paneth cell alpha-defensin, in combination with four different antibiotics i.e. ciprofloxacin, ceftriaxone, cefotaxime and chloramphenicol, which are conventionally used against Salmonella. Minimum bactericidal concentrations of the selected antimicrobial agents were determined by micro and macro broth dilution assays. In-vitro synergy between the agents was evaluated by fractional bactericidal concentration index (checkerboard test) and time-kill assay. Cryptdin-2-ciprofloxacin, cryptdin-2-ceftriaxone and cryptdin-2-cefotaxime combinations were found synergistic as evident by in vitro assays. This synergism provides an additional therapeutic choice by allowing the use of conventional antibiotics in conjunction with AMPs against MDR Salmonella.
Keywords: Conventional antibiotics, FBC, Salmonella, Synergy, Time-kill assay
Introduction
Salmonellae are Gram-negative bacterial pathogens that are capable of infecting a wide range of animals, which can result in several manifestations of disease [1]. Antibiotics have been the mainstay of therapy to combat these infections. However, a disturbing increase in the prevalence of antibiotic resistant bacteria, coupled with the recent poor success rate of the pharmaceutical industry’s antibiotic discovery programs, is a cause for significant concern. Further, there are reports about Salmonella strains showing increased MICs to antibiotics of choice [2, 3]. Therefore, there is an urgent need for alternative new classes of antimicrobial compounds that can be used in the management of Salmonella infections.
In this context, development of antimicrobial peptides (AMPs) for the treatment of salmonellosis has recently become a major area of investigation [4], because of their broad spectrum of action and a lower risk of resistance acquisition [5]. Among naturally occurring AMPs, defensins form a unique and principle family of cysteine rich cationic polypeptides with 3 or 4 disulfide bridges [6]. Mouse enteric alpha-defensins called cryptdins are expressed in the granules of Paneth cells and are secreted into the lumen of intestinal crypts in response to bacterial stimuli [7]. Cryptdins are broad-spectrum AMPs due to their ability to kill various bacteria [8–10], parasites [11] and enveloped viruses [12] in vitro.
Synergy is rapidly gaining grounds at the forefront of antimicrobial therapy, especially for the most worrisome infectious diseases. This is due to the scarcity of antibiotics compared with the huge diversity of demanding clinical care scenarios currently encountered in antimicrobial therapy [13]. Earlier, the combinations of AMPs and conventional drugs have been successfully employed to overcome antibiotic resistance in selected cases [13–15]. Recently, we demonstrated that cryptdin-2 possesses a strong in vivo therapeutic potential against murine salmonellosis and it can be used as a possible adjunct to ampicillin for superior treatment of salmonellosis [16, 17]. However, possibility of using this peptide as an adjunct to the currently recommended conventional antibiotics (in the present scenario) remains to be explored.
The present study was therefore, planned to evaluate the synergistic effects, if any, of cryptdin-2, a mouse Paneth cell alpha-defensin, in combination with four different antibiotics (From first generation, second generation and third generation antibiotics), which are conventionally used against Salmonella.
Materials and Methods
Bacterial Strain and Growth Medium
Standard strain of S. enterica serovar Typhimurium NCTC74, originally procured from Central Research Institute, Kasauli, India, was used in the present study. This strain has been maintained in our laboratory for the last several years and has also been used in recent studies. Stock cultures were prepared and stored at −80 °C in glycerol (20 %). Purity of the strain was confirmed biochemically as well as serologically.
For preparation of bacterial cell suspension, bacterial cells grown overnight (at 37 °C, 150 rpm) in nutrient broth (5.0 g/l peptone, 5.0 g/l NaCl, 1.5 g/l beef extract, 1.5 g/l yeast extract, pH 7.4 ± 0.2) were harvested by centrifugation (8,000 rpm, 15 min), washed once with 10 mM sodium phosphate-buffered saline (PBS, pH 7.2) and resuspended in PBS to a final concentration of ~107 CFU/ml.
Synthetic Cryptdin-2 and Antibiotics
Based on amino acid sequence identical to mouse Paneth cell cryptdin-2 (LRDLVCYCRTRGCKRRERMNGTCRKGHLMYTLCCR), chemically synthesized peptide with three disulfide linkages (at CysI–CysVI, CysII–CysIV and CysIII–CysV) obtained from Genpro Biotech, New Delhi, India was used in the present study. It was dissolved in 0.01 % acetic acid, stored as a stock solution at 100 mg/l at −20 °C and used within 3 weeks. Ciprofloxacin (cipro), ceftriaxone (cef), cefotaxime (cefo) and Chloramphenicol (chlor) powder were procured from Sigma Aldrich (St Louis, MO, USA). Ceftriaxone, cefotaxime and chloramphenicol were dissolved in distilled water whereas, ciprofloxacin was dissolved in 0.1 N HCl. Stock solutions of 1,000 mg/l were prepared and used within 1 week.
Radial Diffusion Assay
The qualitative determination of antimicrobial activity of cryptdin-2 and tested antibiotics alone and in combination was evaluated by radial diffusion assay [18]. Following the addition of test agents alone (20 μg/ml) in each well, combinations of cryptdin-2-ciprofloxacin, cryptdin-2-ceftriaxone, cryptdin-2-cefotaxime and cryptdin-2-chloramphenicol were added separately in wells (10 μg/ml of cryptdin-2 + 10 μg/ml of tested antibiotics). The plates were then incubated at 37 °C for 24 h and observed for the zones of inhibition around the wells. Anti-bacterial activity was evaluated by measuring the size of the clear zone of inhibition of bacterial growth around the wells.
MBC Determination
The minimum bactericidal concentrations (MBCs) of all the agents against serovar Typhimurium were determined by macro and micro broth dilution assay methods as described by us earlier [17]. In brief, various antimicrobial agents (cryptdin-2 and antibiotics) were added to the bacterial suspension containing ~107 CFU/ml of log phase cells of the bacterial strain. Overnight growth was monitored by measuring the optical density at 620 nm. MBC was determined by plating serial dilutions of bacteria preincubated with or without various antimicrobial agents on agar plates and enumerating colony forming units after overnight incubation at 37 °C. The MBC was defined as the concentration at which there was >99 % inhibition of growth.
Fractional Bactericidal Concentrations (FBCs)
To evaluate synergy, the fractional inhibitory concentration (FIC) index was determined by checkerboard microtitre test, using an 8-by-8 well configuration at 64 different combinations as described earlier [15, 17]. Briefly, twofold serial dilutions of cryptdin-2 and each antibiotic were prepared, with concentrations ranging from 0.016 to 2 times the MBC. Ten microliters of each cryptdin-2 dilution was added to the wells of a 96-well plate in a vertical orientation and same amount of each antibiotic dilution was added in a horizontal orientation so that the plate contained various combinations of the two agents. Each well was then supplemented with 80 μl (107 CFU/ml) of serovar Typhimurium and the plate was incubated at 37 °C. Wells not containing any antibacterial agent were used as positive growth controls.
The FBC was calculated after dividing the MBC of the tested agent in combination by the MBC of tested agent alone separately. The FBC index, obtained by adding both FBCs, was interpreted as indicating a synergistic effect when it was ≤0.5, an additive or indifferent effect when it was >0.5 and ≤2.0 and an antagonistic effect when it was >2.0 [15].
Time-Kill Assay
The in vitro synergy as estimated by checkerboard technique was further confirmed by time-kill assay. The antimicrobial action of all the antibacterial agents (cryptdin-2 and antibiotics) was determined at their respective MBCs, when the agents were used alone or in combination. Briefly, all the agents (alone and in combination) were added to 3 ml of nutrient broth containing 107 CFU of serovar Typhimurium and then incubated at 37 °C. For each agent, five flasks of nutrient broth were prepared corresponding to various time intervals i.e. 0, 3, 6, 12 and 24 h. 100 μl aliquots were withdrawn at the given time intervals, spread plated on MacConkey agar plates and the viable counts were monitored after overnight incubation at 37 °C.
Statistical Analysis
Data were expressed as means ± standard deviations for three to five independent experiments. Statistical analysis was done by Student’s unpaired t test and one-way analysis of variance (ANOVA) using Jandel Sigma Stat statistical software, version 2.0. In all cases, statistical significance was defined as having a P value of <0.05.
Results
Radial Diffusion Assay
When the wells were filled with 20 μg/ml of cryptdin-2, cipro, cef, cefo and chlor alone, the zone sizes obtained were 22, 28, 19, 20 and 8 mm, respectively. However, in combination at half the concentrations of both agents i.e. cryptdin-2 (10 μg/ml) + cipro (10 μg/ml), cryptdin-2 (10 μg/ml) + cef (10 μg/ml), cryptdin-2 (10 μg/ml) + cefo (10 μg/ml) and cryptdin-2 (10 μg/ml) + chlor (10 μg/ml), the zone sizes were 35, 27, 31 and 18 mm respectively. The observed zone of growth-inhibition of serovar Typhimurium indicated synergy in all the tested combinations as the dose required to give the same antibacterial effect in terms of size of zone of inhibition was reduced to approximately half when agents were used in combination as compared to when used individually at different concentrations (Fig. 1).
Fig. 1.
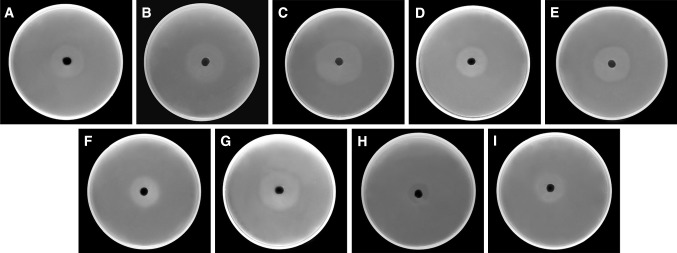
Zone of inhibition of growth of serovar Typhimurium produced by a cryptdin-2 (20 μg/ml): 22 mm b cipro (20 μg/ml): 28 mm c cryptdin-2 (10 μg/ml) + cipro (10 μg/ml): 35 mm d cef (20 μg/ml): 19 mm e cryptdin-2 (10 μg/ml) + cef (10 μg/ml): 27 mm f cefo (20 μg/ml): 20 mm g cryptdin-2 (10 μg/ml) + cefo (10 μg/ml): 31 mm h chlor (20 μg/ml): 8 mm and i cryptdin-2 (10 μg/ml) + chlor (10 μg/ml): 18 mm
MBC Determination
MBC for various agents was determined and confirmed by both macro and micro broth dilution assay. Cryptdin-2 and antibiotics decreased the CFU of serovar Typhimurium in vitro in a concentration-dependent manner. When bacterial cells were incubated with 0.5, 1, 2, 4, 8, 16 and 24 μg/ml of cef, no visible growth was observed at 16 μg/ml, indicating this concentration as the MBC of cef against serovar Typhimurium. Similarly, the MBCs of cryptdin-2, cipro and cefo against Salmonella were evaluated to be 19, 6 and 12 μg/ml respectively. Using chlor, MIC was observed to be 80 μg/ml but MBC was not obtained up to 128 μg/ml (Table 1).
Table 1.
MBC values and FBC index of various antimicrobial agents alone and in combinations against S. enterica serovar Typhimurium as determined by Broth dilution technique and checkerboard method
| Antimicrobial agents | Conc. used (μg/ml) | MBC (μg/ml) | MBC in combination | FBC | FBC index | ||
|---|---|---|---|---|---|---|---|
| Cryptdin-2 (μg/ml) | Antibiotic (μg/ml) | Cryptdin-2 | Antibiotic | ||||
| Cryptdin-2 | 5–20 | 19 | – | – | – | – | – |
| Ciprofloxacin | 0.1–16 | 6 | 2.5 | 0.75 | 0.132 | 0.125 | 0.257 |
| Ceftriaxone | 0.1–32 | 16 | 5 | 1 | 0.263 | 0.063 | 0.326 |
| Cefotaxime | 0.1–32 | 12 | 2.5 | 3 | 0.132 | 0.25 | 0.382 |
| Chloramphenicola | 1–128 | Till 128 no MBC | 5 | 32 | 0.263 | 0.25 | 0.513 |
aMBC of chloramphenicol was considered as 128 μg/ml
Fractional Bactericidal Concentrations (FBCs)
The combinations of cryptdin-2-cipro, cryptdin-2-cef and cryptdin-2-cefo were found to be highly synergistic as indicated by FBC indices, which were ≤0.5 for serovar Typhimurium. However, cryptdin-2-chlor combination was found to have additive effects against serovar Typhimurium as indicated by FBC indices, which was observed to be 0.513 (Table 1).
Time-Kill Assay
All synergistic interactions inferred from checkerboard analysis were reassessed by time-kill assay performed with cryptdin-2 in combination with cipro, cef, cefo and chlor. Out of these four combinations, cryptdin-2-cipro combination showed synergy after 3 h which was evident by a ≥2 log10 unit higher rate of killing compared with either single agent. Both cryptdin-2-cef and cryptdin-cefo combinations showed synergy after 6 h which was evident by a ≥2 log10 unit reduction as compared to the killing of same magnitude by each agent alone, which seems to occur at 24 h (approximately) indicating that the combinations could kill bacteria more rapidly than each single agent against serovar Typhimurium. Cryptdin-2-chlor combination did not exhibit synergy (Fig. 2).
Fig. 2.
Log10 CFU/ml of S. enterica serovar Typhimurium at different time intervals in presence of antibiotic alone (MBC) and in presence of cryptdin-2 + antibiotic (1 × MBC + 1 × MBC). Values are expressed as mean ± standard deviation of three individual values. *P < 0.01 versus log10 CFU of serovar Typhimurium after 3 h in the in the presence of cryptdin-2 (19 μg/ml); # P < 0.01 versus log10 CFU of serovar Typhimurium after 3 h in the presence of cipro (6 μg/ml); † P < 0.05 versus log10 CFU of serovar Typhimurium after 6 h in the in the presence of cryptdin-2 (19 μg/ml); ‡ P < 0.01 versus log10 CFU of serovar Typhimurium after 6 h in the presence of cipro (6 μg/ml); § P < 0.01 versus log10 CFU of serovar Typhimurium after 6 h in the presence of cef (16 μg/ml); α P < 0.01 versus log10 CFU of serovar Typhimurium after 6 h in the presence of cefo (12 μg/ml); β P < 0.01 versus log10 CFU of serovar Typhimurium after 6 h in the presence of chlor (128 μg/ml)
Discussion
Earlier, chloramphenicol, ampicillin and cotrimoxazole (first generation antibiotics) were used to treat Salmonella infections [19]. Later on with the emergence of MDR Salmonella strains, ciprofloxacin (second generation antibiotics) became the drug of choice. However, reports of increasing MIC values and subsequent increase in the occurrence of typhoidal salmonellae resistant to ciprofloxacin suggest that it will not remain effective for much longer [20, 21]. Furthermore, extended-spectrum cephalosporins such as ceftriaxone are now being used specially for treating children [22, 23]. These therapeutic failures have prompted lively research on possible synergism of AMPs with conventional antibiotics as a means of clearing difficult-to treat infections. These synergistic combinations of antimicrobials are gaining ground in clinical therapy, not only as an effort to overcome the spreading phenomenon of multidrug resistance, but also as a way of exploring new ways to improve the clinical usefulness and safety of effective antibiotics [13]. Paneth cell cryptdins are reported to act as natural pore-forming peptides and may be capable of mediating the transport of therapeutic molecules inside the target cell [24]. In light of these facts, the present study aimed at evaluating the synergetic effect of conventionally used antibiotics from various generations (first generation-chlor, second generation-cipro, third generation-cef and cefo) in conjunction with cryptdin-2 against S. enterica serovar Typhimurium.
In-vitro synergy was evidenced for the combinations of cryptdin-2-cipro, cryptdin-2-cef and cryptdin-2-cefo from the radial diffusion assay, FBC index and the time-kill assay, as a larger antibacterial effect was exhibited when the two agents were used against Salmonella in combination at concentrations much lower than their individual MBCs. Synergy between cryptdin-2 and β-lactams (ceftriaxone and cefotaxime) could be attributed to an increased permeability of the Salmonella cell membrane created by cryptdin-2 that might have helped increase permeabilization of these antibiotics to the bacterial cell membrane, thereby leading to increased antimicrobial activity. Ciprofloxacin is a fluoroquinolone, which interferes with the DNA gyrase and inhibits DNA synthesis. The observed synergy between cryptdin-2 and cipro might be attributed to the fact that cryptdin-2 makes pores in the cell membranes, which allows increased amounts of ciprofloxacin to enter the cell and block DNA synthesis.
The most potent combination was found to be that of cryptdin-2 and ciprofloxacin in terms of FBC index and time-kill assay. It could be attributed to the fact that besides acting directly on bacterial cell, quinolones have also been reported to sensitize Gram-negative bacteria to cationic AMPs by displacement of divalent cations from their LPS-binding sites thereby increases the binding of AMPs to outer membrane [25]. These two mechanisms might have acted mutually leading to make this very combination to be the most effective amongst all the combinations tested. These observations are consistent with previous studies pertaining to the synergetic actions of α-helical peptide p18, reproductive tract beta-defensins, HNP-1, magainin, cathelicidins and several other novel cationic peptides with commonly used antibiotics against Escherichia coli, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Klebsiella pneumoniae and Enterobacter cloacae [26, 27].
The results of current study exhibited that cryptdin-2 has the potential to augment the activity of conventional anti-Salmonella antibiotics as all these four combinations could reduce Salmonella growth significantly. This synergism provides an additional therapeutic choice by allowing the use of conventional antibiotics in conjunction with AMPs against MDR Salmonella. However, in order to have the clinical importance of the combinations, in vivo studies remain worth exploring, which are presently being undertaken in the lab.
Acknowledgments
The authors express their gratitude to Mr. Navtej Singh at Central Instrumentation Laboratory, Panjab University, Chandigarh, India, for providing assistance in photographic analysis of the samples. The authors also wish to acknowledge the Council of Scientific and Industrial Research, New Delhi, for providing financial assistance to carry out this work.
References
- 1.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 2.Singh AP, Preet S, Rishi P. Augmentation of antimicrobial activity of conventional antibiotics by cell free extract of L. plantarum. J Antibiot. 2011;64:795–798. doi: 10.1038/ja.2011.92. [DOI] [PubMed] [Google Scholar]
- 3.Chalon MC, Acuña L, Morero RD, Minahk CJ, Bellomio A. Membrane-active bacteriocins to control Salmonella in foods: are they the definite hurdle? Food Res Int. 2012;45:735–744. doi: 10.1016/j.foodres.2011.08.024. [DOI] [Google Scholar]
- 4.Wiradharma N, Khoe U, Hauser CA, Seow SV, Yang YY, Zhang S. Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents. Biomaterials. 2011;32:2204–2212. doi: 10.1016/j.biomaterials.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Mastroianni JR, Ouellette AJ. α-Defensins in enteric innate immunity: functional Paneth cell α-defensins in mouse colonic lumen. J Biol Chem. 2009;284:27848–27856. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 8.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 9.Masuda K, Sakai N, Nakamura K, Yoshioka S, Ayabe T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J Innate Immun. 2010;3:315–326. doi: 10.1159/000322037. [DOI] [PubMed] [Google Scholar]
- 10.Preet S, Rishi P. Antimicrobial activity of Paneth-cell derived cryptdin-2 against selected pathogens. Am J Biomed Sci. 2010;2:13–22. doi: 10.5099/aj100100013. [DOI] [Google Scholar]
- 11.Foureau DM, Mielcarz DW, Menard LC, et al. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J Immunol. 2010;184:7022–7029. doi: 10.4049/jimmunol.0901642. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe H, Ouellette AJ, Cocco MJ, Robinson WE. Differential effects on human immunodeficiency virus type 1 replication by α-defensins with comparable bactericidal activities. J Virol. 2004;78:11622–11631. doi: 10.1128/JVI.78.21.11622-11631.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassone M, Otvos L., Jr Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev Anti Infect Ther. 2010;8:703–716. doi: 10.1586/eri.10.38. [DOI] [PubMed] [Google Scholar]
- 14.Giacometti A, Cirioni O, Barchiesi F, Fortuna M, Scalise G. In-vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J Antimicrob Chemother. 1999;44:641–645. doi: 10.1093/jac/44.5.641. [DOI] [PubMed] [Google Scholar]
- 15.Yenugu S, Narmadha G. The human male reproductive tract antimicrobial peptides of the HE2 family exhibit potent synergy with standard antibiotics. J Pept Sci. 2010;16:337–341. doi: 10.1002/psc.1246. [DOI] [PubMed] [Google Scholar]
- 16.Preet S, Verma I, Rishi P. Cryptdin-2: a novel therapeutic agent for experimental Salmonella typhimurium infection. J Antimicrob Chemother. 2010;65:991–994. doi: 10.1093/jac/dkq066. [DOI] [PubMed] [Google Scholar]
- 17.Rishi P, Preet S, Bharrhan S, Verma I. In-vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob Agents Chemother. 2011;55:4176–4182. doi: 10.1128/AAC.00273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rishi P, Preet S, Kaur P. Effect of L. plantarum cell-free extract and co-trimoxazole against Salmonella typhimurium: a possible adjunct therapy. Ann Clin Microbiol Antimicrob. 2011;10:9. doi: 10.1186/1476-0711-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birosova L, Mikulasova M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar typhimurium. J Med Microbiol. 2009;58:436–441. doi: 10.1099/jmm.0.003657-0. [DOI] [PubMed] [Google Scholar]
- 20.Mandal S, Mandal MD, Pal NK. Synergism of ciprofloxacin and trimethoprim against Salmonella enterica serovar typhi isolates showing reduced susceptibility to ciprofloxacin. Chemotherapy. 2004;50:152–154. doi: 10.1159/000077890. [DOI] [PubMed] [Google Scholar]
- 21.Aarestrup FM, Wiuff C, Molbak K, Threlfall EJ. Is it time to change fluoroquinolone breakpoints for Salmonella spp.? Antimicrob Agents Chemother. 2003;47:827–829. doi: 10.1128/AAC.47.2.827-829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fey PD, Safranek TJ, Rupp ME, et al. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342:1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- 23.Endt K, Maier L, Käppeli R, Barthel M, Misselwitz B, Kremer M, Hardt WD. Peroral ciprofloxacin therapy impairs the generation of a protective immune response in a mouse model for Salmonella enterica serovar Typhimurium diarrhea, while parenteral ceftriaxone therapy does not. Antimicrob Agents Chemother. 2012;56:2295–2304. doi: 10.1128/AAC.05819-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencer WI, Cheung G, Strohmeier GR, Currie MG, Ouellette AJ, Selsted ME, Madara JL. Induction of epithelial chloride secretion by channel-forming cryptdins-2 and 3. Proc Natl Acad Sci USA. 1997;94:8585–8589. doi: 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos MA, Morey P, Bengoechea JA. Quinolones sensitize Gram-negative bacteria to antimicrobial peptides. Antimicrob Agents Chemother. 2006;50:2361–2367. doi: 10.1128/AAC.01437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darveau RP, Cunningham MD, Seachord CL, Cassiano-Clough L, Cosand WL, Blake J, Watkins CS. Beta-lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob Agents Chemother. 1991;35:1153–1159. doi: 10.1128/AAC.35.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulvatne H, Karoliussen S, Stiberg T, Rekdal O, Svendsen JS. Short antibacterial peptides and erythromycin act synergically against Escherichia coli. J Antimicrob Chemother. 2001;48:203–208. doi: 10.1093/jac/48.2.203. [DOI] [PubMed] [Google Scholar]



