Abstract
Amniotic fluid embolism (AFE) is a rare and potentially fatal complication of pregnancy. In this case report, we highlight the successful use of sodium bicarbonate in a patient with an AFE. We present a case of a 38-year-old mother admitted for an elective caesarean section. Following the delivery of her baby, the mother suffered a cardiac arrest. Following a protracted resuscitation, transoesophageal echocardiography demonstrated evidence of acute pulmonary hypertension, with an empty left ventricle and an over-distended right ventricle. In view of these findings and no improvement noted from on-going resuscitation, sodium bicarbonate was infused as a pulmonary vasodilator. Almost instantaneous return of spontaneous circulation was noted, with normalisation of cardiac parameters. We propose that in patients suspected with AFE and who have been unresponsive to advance cardiac life support measures, and where right ventricular failure is present with acidosis and/or hypercarbia, the use of sodium bicarbonate should be considered.
Background
The deleterious and severe consequences of amniotic fluid embolism (AFE) mean that this is a disease known to most acute care practitioners, despite its rarity. It is a leading cause of maternal mortality in developed countries with a worldwide incidence of 1.9–6.1 in 100 000 deliveries and mortality rates of 11–43%.1–4 It is associated with perinatal mortality rates of 7–38% and significant morbidity in survivors.1 Early diagnosis and treatment are the most critical factors associated with survival.5
AFE is characterised clinically by a triad of parturient hypoxia, hypotension and coagulopathy. The pathophysiology is still not clearly understood. Current hypotheses suggest a systemic inflammatory response syndrome like reaction to the embolic fetal material.6 There is activation of maternal mediators resulting in pulmonary vasoconstriction, acute pulmonary hypertension and right heart failure. Concurrent activation of the coagulation cascade eventuates into disseminated intravascular coagulation. Subsequent left heart failure, acute lung injury and neurological sequelae are thought to result mainly from hypoxia.7 8
Treatment of AFE is supportive with aim to maintain cardiac output, reduce hypoxia and reverse coagulopathy and haemorrhage.7 8 Previous case reports have included the use of extracorporeal membrane oxygenation,9 10 cardiopulmonary bypass11 and pulmonary vasodilators such as prostacyclin12 and nitric oxide.13 No previous literature documents the use of sodium bicarbonate as a pulmonary vasodilator in AFE.
We report a case of AFE with cardiac arrest and severe pulmonary hypertension, immediately responsive to the administration of sodium bicarbonate as a pulmonary vasodilator.
Case presentation
A 38-year-old woman with gestational diabetes, morbid obesity and essential hypertension presented for an elective caesarean section and tubal ligation. The indication was a previous caesarean section scar and fetal) macrosomia. She was gravida 3 para 2, at 37-week gestation. She was induced with general anaesthesia following a failed attempt at spinal anaesthesia. Following the delivery of a healthy baby (weighing 3.9 kg), the patient became hypoxic and hypotensive, which rapidly progressed into pulseless electrical activity (PEA). Cardiopulmonary resuscitation was immediately started with 1 mg adrenaline every second cycle with no return of spontaneous circulation (ROSC). During this time she was also clinically coagulopathic and in total had 17 units of packed red blood cells, 8 units of fresh frozen plasma, 8 units of cryoprecipitate, three bags of platelets and 90 μg/kg of Factor VIIa.
Investigations
A transoesophageal echocardiogram (TOE) was conducted demonstrating an empty, vigorously contracting left ventricle, gross right ventricular dilation and contractile failure and a considerable amount of spontaneous echo contrast in the right atrium (figure 1).
Figure 1.
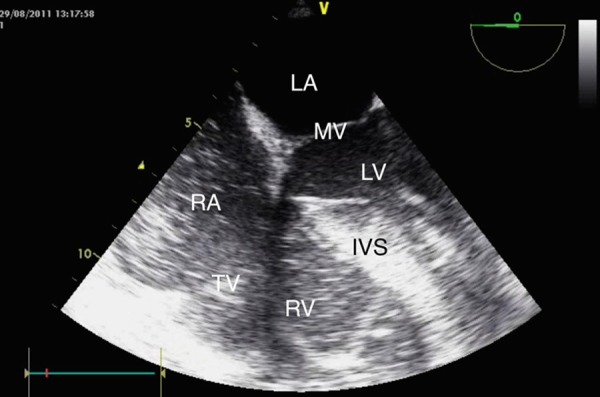
Transoesophageal echocardiogram image demonstrating severe pulmonary hypertension and right ventricular overload with a dilated right ventricle and bulging of the interventricular septum to the left. Also visible is a dilated right atrium with spontaneous echo contrast. LA, left atrium; MV, mitral valve; LV, left ventricle; RA, right atrium; TV, tricuspid valve; RV, right ventricle; IVS, interventricular septum.
Arterial blood glass taken during the arrest revealed a severe mixed acidosis with hypoxaemia and anaemia: haemoglobin (Hbg) 67 g/L (normal: 115–160), pH 6.8 (normal: 7.35–7.45), pO2 52 mm Hg (normal: 80–100), pCO2 86 mm Hg (normal: 35–45) and lactate 8.3 mmol/L (normal: 0.5–2.0). Laboratory results after the arrest revealed a Hbg of 105 g/L (normal: 115–160), platelets of 291×109/L, International Normalised Ratio of 1.2 (normal: 0.9–1.2), activated partial thromboplastin time of 59 s (normal: 26–41) and a drop in fibrinogen from 6.3 to 2.4 g/L (normal: 1.7–4.5).
Treatment
After 30 min with no ROSC, a decision was made to go onto cardiopulmonary bypass. While that was being set up, a 100 mL bolus of 8.4% sodium bicarbonate was infused via the central line. There was an immediate effect, with ROSC.
The subsequent TOE, approximately 60 min after the onset of cardiac arrest showed a full, strongly contracting left ventricle and minimal dilation of the right ventricle (figure 2).
Figure 2.
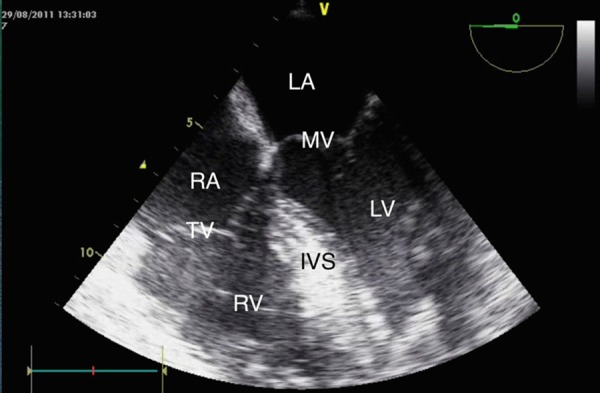
Transoesophageal echocardiogram demonstrating normalisation of left and right ventricular size immediately after administration of sodium bicarbonate. LA, left atrium; MV, mitral valve; LV, left ventricle; RA, right atrium; TV, tricuspid valve; RV, right ventricle; IVS, interventricular septum.
Outcome and follow-up
The patient was subsequently transferred to our intensive care unit where she was extubated successfully the following day. One year later she has resumed work and is a capable mother to her three children. The only neurological defect noted is a mild defect in her short-term memory.
Discussion
There are currently no reports describing the use of sodium bicarbonate as a pulmonary vasodilator in life-threatening AFE. It has, however, been used in several cases of AFE with ROSC following cardiovascular collapse.14 15 These case reports have never attributed the ROSC to sodium bicarbonate because they never used TOE during the initial resuscitation. In the case report by Shechtman et al,14 the use of TOE occurred only after the mother suffered a second cardiac arrest. She was not reported to have had any further doses of sodium bicarbonate and subsequently died. In the case report by Mashid et al,15 the patient died 2 h after ROSC because of coagulopathy.
Sodium bicarbonate was once a mainstay of cardiac arrest algorithms. In recent times there has been an increased focus on its possible adverse effects, which include hypernatraemia, hyperosmolality, volume overload, hypokalaemia, hypercapnia, intracellular acidosis and left shift of the oxyhaemoglobin dissociation curve. These factors coupled with a lack of evidence have seen it being excluded from routine administration during a cardiac arrest. Despite this, it still has an important role in defined clinical scenarios including hyperkalaemia, documented metabolic acidosis, tricyclic antidepressant overdose and protracted arrest.
Sodium bicarcabonate is postulated to have direct and indirect mechanism of actions on the pulmonary vasculature. Its direct mechanism of action as a pulmonary vasodilator is unknown. Potential explanations include a reduction in ionised calcium leading to pulmonary smooth muscle relaxation,16 hypertonicity causing fluid migration from intracellular and interstitial compartments to arteriolar vessels, resulting in pulmonary vasodilation, or by inhibiting endogenous vasopressor substances.17
The postulated indirect mechanism is thought to be through alkalosis and hypocarbia with several animal studies demonstrating the attenuation of hypoxic pulmonary vasoconstriction.18–20 These studies found that metabolic and respiratory alkalosis significantly reduced mean pulmonary artery pressure (MPAP) and pulmonary vascular resistance (PVR) compared with controls. Conversely, hypocapnia alone increased MPAP, suggesting that pH was more important than changes in CO2. In one human study, acidaemia was induced via hypoventilation and then alkalaemia with the administration of 4 mmol/kg of intravenous sodium bicarbonate.21 Hypercapnia caused an increase in MPAP and PVR and conversely sodium bicarbonate reduced MPAP and PVR to levels lower than baseline.
Alternative pulmonary vasodilators employed in the setting of AFE have featured in several case reports. These include the administration of inhaled prostacyclin12 and nitric oxide.13 These agents, however, require a degree of familiarity and experience in their use. Other more selective pulmonary vasodilators such as sildenafil and bosentan are not readily available in parenteral form.
In conclusion, we are able to demonstrate signs consistent with acute pulmonary hypertension and severe right heart failure leading to a PEA cardiac arrest in this case of AFE. The most important factor for survival after AFE is the overall management of cardiovascular collapse. However, despite the immediate institution of appropriate advanced cardiac life support measures in our patient, there was no clinical improvement until the administration of sodium bicarbonate. Even at this late stage, this resulted in immediate restoration of a spontaneous circulation with clear benefit to the cardiac function as directly observed by TOE. We propose that the bicarbonate may have had a direct effect by reducing the PVR, allowing the failing right ventricle to restore forward flow. This is in agreement with previous small studies of interventional alkalosis conducted in animals and predominantly cardiac patients with pulmonary hypertension.
Learning points.
Amniotic fluid embolism is a rare but potentially fatal complication of pregnancy associated with high morbidity and mortality.
The pathophysiology of amniotic fluid embolism likely involves a systemic inflammatory response syndrome-like response resulting in pulmonary vasoconstriction and acute pulmonary hypertension with right heart failure.
Sodium bicarbonate should be considered in the treatment of a patient with suspected amniotic fluid embolism that has been unresponsive to normal advanced life support measures, and where right ventricular failure is evident on transoesophageal echocardiogram, and acidosis and/or hypercarbia is present.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Knight M, Berg C, Brocklehurst P, et al. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth 2012;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark SL, Hankins GD, Dudley DA, et al. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol 1995;172:1158–67 [DOI] [PubMed] [Google Scholar]
- 3.Tuffnell DJ. United Kingdom amniotic fluid embolism register. BJOG 2005;112:1625–9 [DOI] [PubMed] [Google Scholar]
- 4.Morgan M. Amniotic fluid embolism. Anesthesia 1979;34:20–32 [DOI] [PubMed] [Google Scholar]
- 5.Matsuda Y, Kamitomo M. Amniotic fluid embolism: a comparison between patients who survived and those who died. J Int Med Res 2009;37:1515–21 [DOI] [PubMed] [Google Scholar]
- 6.Steiner PE, Lushbaugh C. Maternal pulmonary embolism by amniotic fluid as a cause of obstetric shock and unexplained death in obstetrics. JAMA 1994;117:1245–54 [DOI] [PubMed] [Google Scholar]
- 7.Clark SL. Amniotic fluid embolism. Obstet Gynecol 2014;123:337–48 [DOI] [PubMed] [Google Scholar]
- 8.Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidenced based review. Am J Obstet Gynecol 2009;201:445e1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh YY, Chang CC, Li PC, et al. Successful application of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. Am J Obstet Gynecol 2000;183:496–7 [DOI] [PubMed] [Google Scholar]
- 10.Shen HP, Chang WC, Yeh LS, et al. Amniotic fluid embolism with emergency extracorporeal membrane oxygenation: a case report. J Reprod Med 2009;54:706–8 [PubMed] [Google Scholar]
- 11.Stanten RD, Iverson LI, Daugharty TM, et al. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol 2003;102:496–8 [DOI] [PubMed] [Google Scholar]
- 12.Van Heerden P, Webb S, Hee G, et al. Inhaled aerosolized prostacyclin as a selective pulmonary vasodilator for the treatment of severe hypoxaemia. Anaesth Intensive Care 1996;24:87–90 [DOI] [PubMed] [Google Scholar]
- 13.McDonnell N, Chan B, Frengley R. Rapid reversal of critical haemodynamic compromise with nitric oxide in a parturient with amniotic fluid embolism. Int J Obstet Anesth 2007;16:269–73 [DOI] [PubMed] [Google Scholar]
- 14.Shechtman M, Ziser A, Markovits R, et al. Amniotic fluid embolism: early findings of transesophageal echocardiography. Anesth Analg 1999;89:1456–8 [DOI] [PubMed] [Google Scholar]
- 15.Mahshid N, Ahmad S, Nahid M, et al. Sudden cardiac arrest during caesarean section—a possible case of amniotic fluid embolism. Middle East J Anesthesiol 2009;20:315–17 [PubMed] [Google Scholar]
- 16.Kennedy T, Summer W. Inhibition of hypoxic pulmonary vasoconstriction by nifedipine. Am J Cardiol 1982;50:864–8 [DOI] [PubMed] [Google Scholar]
- 17.Croxato H, Vera R, Roblero J, et al. Polypeptides formed by acidification of blood serum of normal and hypertensive rats. Can Med Assoc J 1964;90:313–21 [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber M, Heymann M, Soifer S. Increased arterial pH, not decreased PaCO2, attenuates hypoxia-induced pulmonary vasoconstriction in newborn lambs. Pediatr Res 1986;20:113.– [DOI] [PubMed] [Google Scholar]
- 19.Lloyd T. Influence of blood pH on hypoxic pulmonary vasoconstriction. J Appl Physiol 1966;21:358–64 [DOI] [PubMed] [Google Scholar]
- 20.Malik AB, Kidd B. Time course of pulmonary vascular response to hypoxia in dogs. Am J Physiol 1973;224:1–6 [DOI] [PubMed] [Google Scholar]
- 21.Chang A, Zucker H, Hickey P, et al. Pulmonary vascular resistance in infants after cardiac surgery: role of carbon dioxide and hydrogen ion. Crit Care Med 1995;23:568–74 [DOI] [PubMed] [Google Scholar]


