Abstract
Introduction
Lower urinary tract symptoms (LUTS) are highly prevalent, cause an enormous economic burden on healthcare systems and significantly impair the quality of life (QoL) of affected patients. The dependence of the LUT on complex central neuronal circuits makes it unique in comparison to other visceral functions, such as the gastrointestinal tract, but also more vulnerable to neurological diseases.
Methods and analysis
This is a prospective neuroimaging study investigating the supraspinal control of LUT function in healthy controls and in patients with non-neurogenic LUTS. The clinical assessment will include medical history, neuro-urological examination, bladder diary, urine analysis, urodynamic investigations, as well as standardised questionnaires regarding LUTS and QoL. The acquisition of neuroimaging data will include structural assessments (T1-weighted imaging and diffusion tensor imaging) as well as functional investigations using blood-oxygen-level dependent sensitive functional MRI (fMRI) in a 3 T MR scanner. The fMRI will be performed during four different bladder tasks using an automated MR-compatible and MR-synchronised pump system. The first three task-related fMRIs will consist of automated, repetitive filling of 100 mL warm (37°C) saline starting with (1) an empty bladder, (2) a low prefilled bladder volume (100 mL) and (3) a high prefilled bladder volume (persistent desire to void). The fourth task-related fMRI will comprise of automated, repetitive filling of 100 mL cold (4–8°C) saline starting with an empty bladder.
Ethics and dissemination
The local ethics committee approved this study (KEK-ZH-Nr. 2011–0346). The findings of the study will be published in peer-reviewed journals and presented at national and international scientific meetings.
Trial registration number
This study has been registered at clinicaltrials.gov (http://www.clinicaltrials.gov/ct2/show/NCT01768910).
Strengths and limitations of this study.
This will be the first study to identify brain networks of supraspinal LUT control in healthy subjects and abnormalities within such brain networks in patients with non-neurogenic LUTS using structural and functional MRI techniques in correlation with clinical measurements.
Investigation of test-retest reliability has not yet been performed for neuroimaging of LUT tasks. However, this is important for the interpretation of participants' activations in regard to the validity of these activations, i.e. absolute and consistent agreement (ICC) from visit to visit.
Comparison of clinical correlates of treatment efficacy in patients with non-neurogenic LUTS with the associated changes in brain activity and connectivity.
Introduction
Lower urinary tract symptoms (LUTS) are highly prevalent, that is, about 11% in the worldwide population in 2008, and forecasted to increase up to 20% until 2018.1 2 Moreover, LUTS cause an enormous economic burden on each healthcare system,3 4 which is comparable to diabetes mellitus,5 and significantly impair the quality of life (QoL) of affected patients.6 7
For proper functioning, LUT structures, that is, bladder, bladder neck, urethra and urethral sphincter, rely on intact neuronal innervations that are under the control of a complex supraspinal network.8–10 The dependence of the LUT on such complex central neuronal circuits makes it unique in comparison with other visceral functions, for example, gastrointestinal tract, but also more vulnerable to neurological diseases.10
Recent neuroimaging studies have shown that patients with neurological disorders such as Parkinson's disease11–13 and spinal cord injury14 demonstrate different supraspinal activity patterns compared with healthy controls in response to LUT stimulation tasks, which might represent a neural correlate to their LUTS.
Although there are several concepts regarding the human LUT function and neuronal control in normal and pathological conditions, the exact pathophysiological mechanisms involved remain largely unknown.9 Despite the popularity of resting-state functional MRI (RS-fMRI)15–20 and diffusion tensor imaging (DTI)21 in other fields in neuroscience, these techniques have not been applied in the context of supraspinal LUT control. There are only two DTI studies published in regard to LUT control in general: (1) a case report by Théaudin et al22 studying spinal cord infarctions and clinical symptoms and (2) a prospective study by van der Jagt et al23 investigating architectural configuration and microstructural properties of the sacral plexus.
As cortical and subcortical (eg, brainstem) brain regions are crucial for voluntary LUT control,10 24 25 investigation of the supraspinal regions with high-resolution imaging techniques, such as fMRI, can significantly contribute to increase our understanding of the effects of supraspinal lesions and alterations related to LUTS.10 26
In this study, we are aiming to identify supraspinal areas associated with LUT control in healthy controls and in patients with non-neurogenic LUTS. Task-related blood-oxygen-level dependent (BOLD) and RS-fMRI will be used along with structural MRI (T1-weighted MRI and DTI). Hence, we will examine whether bladder processing is already altered on the structural level and on baseline (RS-fMRI) functional connectivity (FC). For example, multiple repetition of the RS-fMRI will help to understand whether manipulation of sensory perception (induced by infusion and withdrawal) will alter the default mode network20 of the brain. Furthermore, we can examine volumetric parameters (eg, grey matter concentration) by voxel-based morphometry (VBM),27 structural integrity and connectivity of white matter tracts (DTI) as well as FC.
This unique and detailed multimodal imaging protocol should pinpoint to structural and functional processing units involved during supraspinal LUT control and should identify all dysfunctional neuronal components in patients with disturbed LUT control.
Importantly, we will investigate the reliability28 of BOLD signals in task-related fMRI and RS-fMRI in healthy controls and patients with non-neurogenic LUTS. The test–retest validation, that is, the intraclass correlation coefficient (ICC) for absolute or consistent agreement of subject activations from visit to visit, has not been evaluated in regard to the supraspinal LUT control yet.
Methods and analysis
Study design
This prospective research study will be conducted at the University of Zürich, Zürich, Switzerland.
Study population and recruitment
According to the inclusion and exclusion criteria (table 1), we will investigate patients with non-neurogenic LUTS and healthy controls with an unimpaired LUT function. Participants of both groups will be matched according to age and gender.
Table 1.
Inclusion and exclusion criteria for all participants
| Groups | Inclusion criteria | Exclusion criteria |
|---|---|---|
| All participants |
|
|
| Healthy controls |
|
|
| Patients with non-neurogenic LUTS |
|
|
ISC, intermittent self-catheterisation; LUTS, lower urinary tract symptoms; PVR, postvoid residual; SUI, stress urinary incontinence.
Patients with non-neurogenic LUTS will be recruited from our own department (Neuro-Urology, Balgrist University Hospital, Zürich, Switzerland) and through our partners at the University Hospital Zürich and the Triemli Hospital Zürich. Eligible patients with non-neurogenic LUTS and healthy controls will be invited to a first visit (screening) during which detailed information about the study, in particular the aims, methods, possible risks and side effects, will be given. After obtaining written informed consent, the following data will be collected: medical history, a 3-day bladder diary, urine sample to exclude urinary tract infection (UTI) and pregnancy in female participants, urodynamic parameters and postvoid residual measured by ultrasound as well as standardised questionnaires regarding LUTS and QoL. The validated German versions of these questionnaires will be used with permission of the International Consultation on Incontinence Modular Questionnaire ((ICIQ), Bristol Urological Institute, Southmead Hospital Bristol, UK) and will address the LUTS (ICIQ-LUTS) in women (ICIQ-FLUTS) and men (ICIQ-MLUTS), whereas the ICIQ-LUTSQoL will display the QoL in regard to LUTS.
Determination of sample size
A power analysis was conducted using G*Power (http://www.gpower.hhu.de). In order to have sufficient power (0.80) to detect a large effect size (0.80) between healthy controls and patients with non-neurogenic LUTS (significance level 0.05), at least 21 participants per group need to be recruited. To demonstrate post-treatment effects in patients with non-neurogenic LUTS compared with their baseline using the same power, effect size and significance level, at least 12 participants for each treatment option are necessary. These sample sizes are in line with earlier studies22 23 including our own,14 29 which provided statistical evidence using small collectives, that is, between 12 and 21 participants.
Study location
Neuro-Urology, Spinal Cord Injury Centre and Research, University of Zürich, Balgrist University Hospital, Zürich, Switzerland.
MR-Centre, University Hospital Zürich, Zürich, Switzerland.
Partners
Institute of Neuro-Radiology, University of Zürich, University Hospital Zürich, Zürich, Switzerland.
Departments of Urology and Gynaecology, University Hospital Zürich, Zürich, Switzerland.
Department of Urology and Gynaecology, Triemli Hospital, Zürich, Switzerland.
Investigations
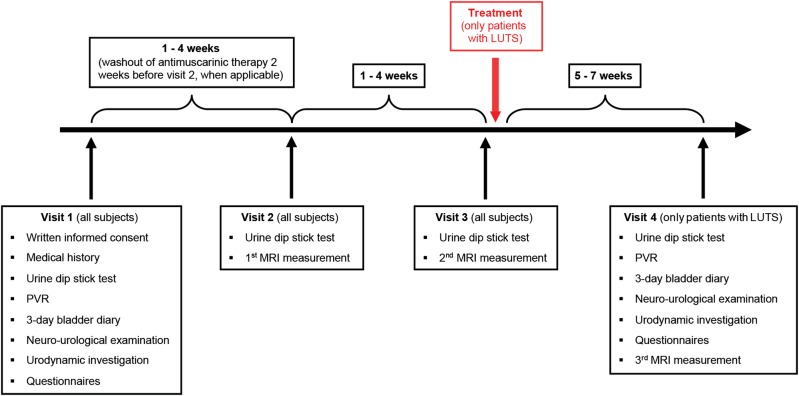
Following screening and study inclusion, all participants will be scheduled for the second and third visits (first and second MRI measurements) at the MR-Centre. Patients with non-neurogenic LUTS will return for a fourth visit (third MRI measurement), either after receiving treatment for LUTS or without treatment acting as a direct control group within the patient cohort (figure 1).
Figure 1.
Timetable and characteristics of all four visits. LUTS, lower urinary tract symptoms; MRI, magnetic resonance imaging; PVR, post void residual; QoL, quality of life.
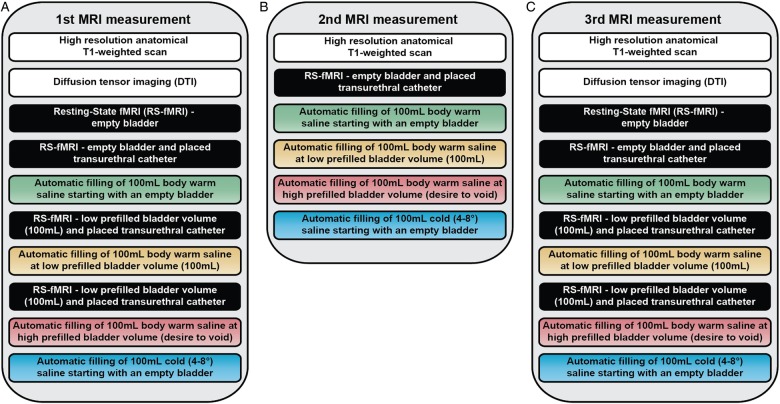
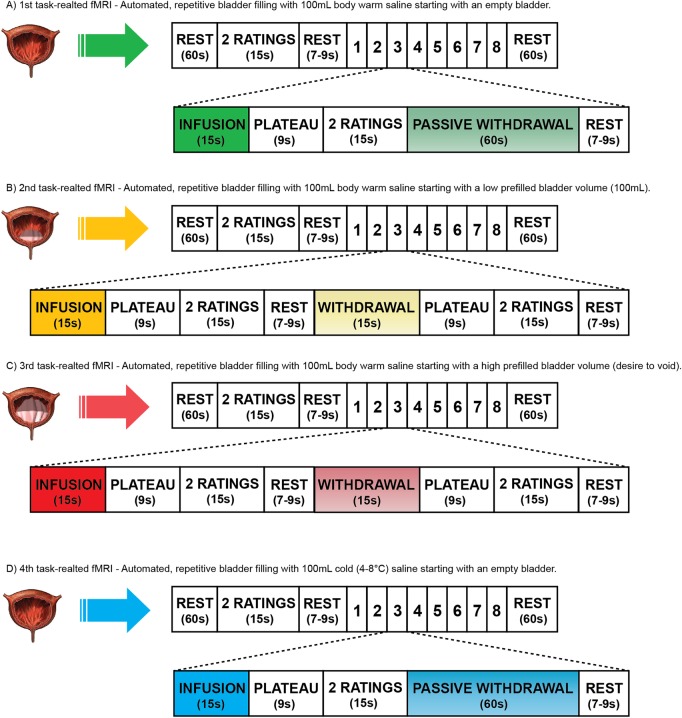
All MRI measurements (figure 2) will be performed using a Philips Ingenia 3 T MR scanner (Philips Medical Systems, Best, The Netherlands) with a 16-channel head coil. During the second visit, we will acquire the following neuroimaging data. Structural measurements will contain T1-weighted MRI and DTI. Functional measurements will comprise RS-fMRIs and task-related fMRIs. Four different RS-fMRIs will be applied, that is, (1) at baseline with an empty bladder, (2) with an empty bladder plus transurethral catheter, (3) with a low prefilled bladder (100 mL saline, body warm) prior to task-related fMRI and (4) after a task-related fMRI, to understand whether manipulation of sensory perception (induced by catheter or prefilling) will alter the default mode network.20 The task-related fMRI will be acquired during four different bladder tasks (figure 3). In order to precisely fill and drain the bladder (ie, specific volume and duration of time), we designed an automated MR-compatible and MR-synchronised pump system. In the first three task-related fMRIs, we will examine the effect of visceral bladder sensation by automated, repetitive filling with 100 mL body warm saline starting with (1) an empty bladder, (2) a low prefilled bladder and (3) a high prefilled bladder (persistent desire to void). The fourth task-related fMRI will consist of automated, repetitive filling of 100 mL cold (4–8°C) saline starting with an empty bladder to investigate the neural correlates of cold bladder sensation (figure 3). Participants will rate their desire to void and their level of pain using a displayed visual analogue scale and an fMRI-compatible handheld response system.30
Figure 2.
Schematic protocol of operational sequences of MRI measurements including functional MRI (fMRI): (A) first MRI measurement, (B) second MRI measurement and (C) third MRI measurement.
Figure 3.
BOLD signal intensity changes during task-related fMRI in relation to the specific condition, that is, infusion or to a contrast, that is, low versus full bladder volume, during two (healthy controls) or three (patients with non-neurogenic LUTS) visits. Investigation of these changes will focus on supraspinal regions of interest (ROI) that are known from the existing literature, for example, pons, insula, anterior cingulate cortex, thalamus, hypothalamus, supplementary motor area and prefrontal cortex. However, the precise selection of ROIs will be based on the coordinates of the peak activations during task-related fMRI taken from the Montreal Neurological Institute (MNI) space.
During the second MRI measurement (third visit, 1–4 weeks later), we will utilise a selection of MRI measurements for the purpose of reliability analysis,28 that is, RS-fMRI (baseline with an empty bladder plus transurethral catheter) and task-related fMRI to compare within and between groups.
The third MRI measurement will be identical to the first MRI measurement to evaluate post-treatment versus pretreatment effects in patients with non-neurogenic LUTS. The time from the start of treatment to the fourth visit, that is, 5–7 weeks (figure 1), is necessary to let clinical improvements develop.31 32
Safety
The staff involved in this study will be instructed and trained according to the safety regulations of the MR-Centre of the University Hospital Zürich. All participants will be asked to remove any ferromagnetic items, for example, bra, earrings, chains, rings and piercings prior to entering the scanner room. All participants will be provided with standardised clinical scrubs instead of wearing their own clothes. Before every MRI measurement (visits 2, 3 and 4), urine samples will be analysed from every participant in order to exclude UTI or pregnancy. In case of pregnancy, the participant will be excluded from the study and referred to a gynaecologist. In case of UTI, the participant will not undergo the experiment, but will receive immediate antibiotic treatment if the UTI is symptomatic or be treated depending on further microbiological urine analysis in the absence of UTI symptoms. The participant can be reassigned to the study if the microbiological urine analysis shows no evidence of a UTI or the UTI has been successfully treated.
In the situation of an adverse event (AE) or a severe AE (SAE), as defined by the International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines (E6)33 and International Organization for Standardization (ISO, 14155),34 appropriate actions will be executed and the according body (principle investigator, ethics committee) will be informed. All AEs and SAEs will be followed as long as medically indicated.
Study outcome measures
Primary
BOLD signal intensity changes in a priori supraspinal regions of interest (ROI), for example, pons, insula, anterior cingulate cortex, thalamus, hypothalamus, supplementary motor area and prefrontal cortex, during task-related fMRI in relation to the specific condition, that is, infusion or to a contrast, that is, low versus full bladder volume, during two (healthy controls) or three (patients with non-neurogenic LUTS) visits. The precise selection of ROIs will be based on the coordinates of the peak activations during task-related fMRI taken from the Montreal Neurological Institute (MNI) space.
Reliability of BOLD signal changes during RS-fMRI and task-related fMRI across visits (eg, second and third visits) in healthy controls and patients with non-neurogenic LUTS.
BOLD signal changes in supraspinal ROIs during task-related fMRI in patients with non-neurogenic LUTS before and after treatment to quantify the link between BOLD signal changes and treatment efficacy.
Secondary
Structural differences between healthy controls and patients with non-neurogenic LUTS, and changes in the post-treatment versus pretreatment state of grey matter concentration using VBM.
Structural connectivity (SC) and FC between supraspinal ROIs to identify specific alterations with DTI35 between healthy controls and patients with non-neurogenic LUTS (including post-treatment versus pretreatment changes). This will include whole-brain fractional anisotropy (FA) and mean diffusivity (MD) comparison as well as probabilistic tractography between ROIs (white matter fibre structure).
Differences in BOLD signals during RS-fMRI between healthy controls and patients with non-neurogenic LUTS (including post-treatment versus pretreatment changes) in regard to intrahemispheric and interhemispheric connectivity.36 Investigation will focus on whether these signals differ (1) already at baseline or are influenced by the (2) presence of a catheter, (3) by prefilling and/or (4) by task-related fMRI.
Clinical scores (eg, bladder volume, urodynamic parameters and level of desire to void during fMRI) will be correlated to BOLD signal changes as well as to structural markers (eg, grey matter volume or number of white matter tracts between ROIs) using regression analyses.
Data analysis
Clinical data, for example, urodynamic parameters, 3-day bladder diary outcome and questionnaire scores, will be statistically analysed and compared between groups using IBM's Statistical Package for the Social Sciences (SPSS) V.19.0 or newer (Armonk, New York, USA) and will be presented with means and SDs or with medians and IQRs as appropriate.
The neuroimaging data will be analysed using statistical parametric mapping (SPM) V.8 or newer (Wellcome Department of Imaging Neuroscience, University College London, UK). Preprocessing of functional data from each task-related fMRI will be carried out for each participant individually. The images will be realigned to the first scan, unwarped to control for movement-induced and susceptibility-induced image distortions,37 spatially coregistered to the T1-weighted image and normalised to the MNI anatomical standard space. At last, the functional data will be smoothed spatially with an isotropic Gaussian kernel. Thereafter, first-level analysis using the general linear model will be performed to create contrasts of interest, for example, low versus full bladder or infusion versus withdrawal.38 The six movement parameters will be modelled as additional regressors to control for potential head motion.
Second-level factorial design will include at least (1) one-sample t test to compute a mean for each group, (2) two-sample t tests to compare healthy controls and patients with non-neurogenic LUTS and (3) paired t tests to evaluate post-treatment versus pretreatment effects in patients with non-neurogenic LUTS. The ICC for task-related and RS-fMRI reliability will be analysed using the SPM-compatible ICC toolbox (http://www.kcl.ac.uk/iop/depts/neuroimaging/research/imaginganalysis/Software/ICC-Toolbox.aspx).
Association of individual (clinical) variables with BOLD signal changes will be assessed by whole-brain and ROI-based correlation analyses (correlation coefficients will be reported).
DTI data will be analysed using TBSS (http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/tbss/index) and BrainVoyager (http://www.brainvoyager.com/downloads/downloads.html) with the following established DTI analyses: whole-brain FA and MD comparison between groups as well as probabilistic tractography between ROIs. Seed and target regions, that is, ROIs will be defined (1) a priori using anatomic coordinates, for example, from the SPM toolbox WFU_PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas) and (2) from peak activations acquired from task-related fMRI and RS-fMRI on the standard MNI space. Volumetric changes in grey and white matter will be analysed using VBM, for example, the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm) in SPM.
Ethics and dissemination
This cohort study will be performed in accordance with the World Medical Association Declaration of Helsinki39 and the guidelines of the Swiss Academy of Medical Sciences.40
Furthermore, handling of all personal data will strictly comply with the federal law of data protection in Switzerland.41
This study has been registered at clinicaltrials.gov (http://www.clinicaltrials.gov/ct2/show/NCT01768910).
Discussion
This study will investigate supraspinal LUT control in healthy controls and patients with non-neurogenic LUTS using a multimodal imaging protocol, that is, structural (T1-weighted and DTI) and fMRI (RS-fMRI and task-related fMRI), to examine haemodynamic responses to LUT stimulation. From the acquired neuroimaging data, SC and FC, and structural integrity evaluation of white matter tracts and grey matter concentration (using VBM) will be analysed to identify specific alterations of the supraspinal LUT control. In addition, effects on the supraspinal LUT control after treatment for LUTS (eg, antimuscarinergics or botulinum toxin) will be investigated. It is expected that this study will provide new insights into the supraspinal neuronal mechanisms and networks responsible for LUT control. The findings will help to verify, amend or adjust neuronal circuitry models established from findings in healthy controls, now in the context of patients with non-neurogenic LUTS. The use of newer imaging and evaluation techniques has the potential to serve as quantifiable outcome measures for therapy success and provide evidence for non-responders of LUTS treatment.
Supplementary Material
Footnotes
Acknowledgements: The authors would like to acknowledge the Swiss National Science Foundation, SwissLife Jubiläumsstiftung and Swiss Continence Foundation for financial support. Furthermore, they would like to thank the partners contributing to this study: Departments of Urology and Gynaecology of the University Hospital Zürich and Departments of Urology and Gynaecology, Triemli Hospital, Zürich, Switzerland. They also thank Behnaz Jarrahi for granting figure contents.
Contributors: All authors participated in creating the study design. MW and UM drafted the manuscript. LM, SK, PEvK and TMK critically reviewed the manuscript. UM, SK and TMK obtained the funding of this study. All the authors read and approved the final manuscript.
Funding: Swiss National Science Foundation (grant number: 135774), SwissLife Jubiläumsstiftung, Swiss Continence Foundation.
Competing interests: None.
Ethics approval: This study has been approved by the local ethics committee (Kantonale Ethikkommission Zürich, KEK-ZH-Nr. 2011-0346).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Irwin DE, Kopp ZS, Agatep B, et al. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011;108:1132–8 [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306–14; discussion 14–5 [DOI] [PubMed] [Google Scholar]
- 3.Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology 2010;75:526–32, 32 e1–18 [DOI] [PubMed] [Google Scholar]
- 4.Klotz T, Bruggenjurgen B, Burkart M, et al. The economic costs of overactive bladder in Germany. Eur Urol 2007;51:1654–62; discussion 62–3 [DOI] [PubMed] [Google Scholar]
- 5.Hampel C, Gillitzer R, Pahernik S, et al. Epidemiology and etiology of overactive bladder. Urologe A 2003;42:776–86 [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 2008;101:1388–95 [DOI] [PubMed] [Google Scholar]
- 7.Irwin DE, Milsom I, Kopp Z, et al. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int 2006;97:96–100 [DOI] [PubMed] [Google Scholar]
- 8.Blok BF. Brain control of the lower urinary tract. Scand J Urol Nephrol Suppl 2002:11–15 [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 2008;9:453–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn 2010;29:49–55 [DOI] [PubMed] [Google Scholar]
- 11.Herzog J, Weiss PH, Assmus A, et al. Subthalamic stimulation modulates cortical control of urinary bladder in Parkinson's disease. Brain 2006;129(Pt 12):3366–75 [DOI] [PubMed] [Google Scholar]
- 12.Herzog J, Weiss PH, Assmus A, et al. Improved sensory gating of urinary bladder afferents in Parkinson's disease following subthalamic stimulation. Brain 2008;131(Pt 1):132–45 [DOI] [PubMed] [Google Scholar]
- 13.Kitta T, Kakizaki H, Furuno T, et al. Brain activation during detrusor overactivity in patients with Parkinson's disease: a positron emission tomography study. J Urol 2006;175(3 Pt 1):994–8 [DOI] [PubMed] [Google Scholar]
- 14.Mehnert U, Michels L, Zempleni MZ, et al. The supraspinal neural correlate of bladder cold sensation––an fMRI study. Hum Brain Mapp 2011;32:835–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 1997;10:165–70 [DOI] [PubMed] [Google Scholar]
- 16.Cauda F, Costa T, Torta DM, et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage 2012;62:343–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greicius MD, Supekar K, Menon V, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009;19:72–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gusnard DA, Akbudak E, Shulman GL, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001;98:4259–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001;2:685–94 [DOI] [PubMed] [Google Scholar]
- 20.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Théaudin M, Saliou G, Denier C, et al. A correlation between fractional anisotropy variations and clinical recovery in spinal cord infarctions. J Neuroimaging 2013;23:256–8 [DOI] [PubMed] [Google Scholar]
- 23.van der Jagt PK, Dik P, Froeling M, et al. Architectural configuration and microstructural properties of the sacral plexus: a diffusion tensor MRI and fiber tractography study. Neuroimage 2012;62:1792–9 [DOI] [PubMed] [Google Scholar]
- 24.Andrew J, Nathan PW. Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain 1964;87:233–62 [DOI] [PubMed] [Google Scholar]
- 25.Holstege G. Micturition and the soul. J Comp Neurol 2005;493:15–20 [DOI] [PubMed] [Google Scholar]
- 26.de Groat WC. A neurologic basis for the overactive bladder. Urology 1997;50(6A Suppl):36–52; discussion 53–6 [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Voxel-based morphometry––the methods. Neuroimage 2000;11(6 Pt 1):805–21 [DOI] [PubMed] [Google Scholar]
- 28.Caceres A, Hall DL, Zelaya FO, et al. Measuring fMRI reliability with the intra-class correlation coefficient. Neuroimage 2009;45:758–68 [DOI] [PubMed] [Google Scholar]
- 29.Michels L, Mehnert U, Boy S, et al. The somatosensory representation of the human clitoris: an fMRI study. Neuroimage 2010;49:177–84 [DOI] [PubMed] [Google Scholar]
- 30.Jarrahi B, Wanek J, Mehnert U, et al. An fMRI-compatible multi-configurable handheld response system using an intensity-modulated fiber-optic sensor. Conf Proc IEEE Eng Med Biol Soc 2013;2013:6349–52 [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality. Treatment of Overactive Bladder in Women, 2009 [DOI] [PubMed]
- 32.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol 2012;188(6 Suppl):2455–63 [DOI] [PubMed] [Google Scholar]
- 33.International conference on harmonisation. Good clinical practice guideline, 1996
- 34.International organization for standardization. ISO 14155, 2011
- 35.Kucyi A, Moayedi M, Weissman-Fogel I, et al. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS ONE 2012;7:e35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldjian JA, Davenport EM, Whitlow CT. Graph theoretical analysis of resting-state MEG data: identifying interhemispheric connectivity and the default mode. Neuroimage 2014;96:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson JL, Hutton C, Ashburner J, et al. Modeling geometric deformations in EPI time series. Neuroimage 2001;13:903–19 [DOI] [PubMed] [Google Scholar]
- 38.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45–53 [DOI] [PubMed] [Google Scholar]
- 39.World Medical Association. Declaration of Helsinki—Ethical principles for medical research involving human subjects, 1964
- 40.Swiss Academy of Medical Sciences. Guideline—Concerning scientific research involving human beings, 2009 [DOI] [PubMed]
- 41.The Federal Authorities of the Swiss Confederation. Bundesgesetz über den Datenschutz (DSG) vom 19. Juni 1992, Stand. 01.01.2011, 1992
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.