Abstract
A 72-year-old Caucasian woman presented with a 3-week history of confusion, cramping abdominal pain, nausea, vomiting, diarrhoea, fatigue and dehydration. By history, she reported consumption of raw pork and bacon that was salted and cured in brine, but not boiled or cooked. Laboratory testing was significant for an absolute eosinophil count of 3.09×109/L. She was found to have a positive Trichinella serology by ELISA testing. Based on history of raw pork consumption, symptoms, peripheral eosinophilia and positive serology, a diagnosis of trichinosis was made. Treatment was started with oral albendazole. Following initiation of therapy, the patient developed acute kidney injury presumably secondary to albendazole. Therapy was discontinued. Her serum creatinine returned to baseline over the following days. The decision was made to proceed with observation alone, as she did not tolerate treatment. At the 3-week follow-up, her gastrointestinal symptoms had improved and her eosinophilia had resolved.
Background
Trichinosis (or Trichinellosis) is a rarely encountered roundworm infection in the USA. It is caused by nematodes of the genus Trichinella with T. spiralis being the most common species of human trichinosis. Infection is acquired by consuming encysted larvae that are found in muscle tissue of raw or undercooked meat, most commonly from the domestic pig. The minimum infectious dose may be as low as 1–300 organisms.1 Once ingested, the larvae are released from the cysts and develop into adult worms that live in the small intestine. After mating, adult female worms shed newborn larvae that migrate from the intestinal mucosa into the lymphatic system and blood vessels of the host.2 They are then transported to the skeletal musculature where they penetrate and can live within the muscles for up to 40 years.1 3 4 Eventually calcification of the larvae will occur and the larvae will die.
Infection in humans has two phases: the intestinal phase followed by a muscular/systemic phase. Most cases are asymptomatic, especially if they ingest only a small amount of larvae. Generally, the greater the number of encapsulated larvae ingested, the more severe the infection.1 Many patients present with gastrointestinal symptoms including abdominal pain and diarrhoea. These findings typically occur within 2 days of larvae ingestion. During the systemic phase, the larvae migrate, as described above, which triggers immune reactions with activation of mast cells, eosinophils, monocytes and lymphocytes. Peripheral eosinophilia is commonly noted in cases of trichinosis.2 In the systemic phase, patients may develop high fever, periorbital or facial oedema, subungual haemorrhages and myalgias. Other symptoms can include headache, conjunctivitis, anisocoria, urticarial rash, cough, myocarditis, thromboembolic disease and even encephalitis. During the phase of muscular penetration, elevations in lactate dehydrogenase and creatine phosphokinase may be seen. Severe infections can lead to sepsis, compromise of the cardiovascular system and neurological complications including infarcts.1 2 Symptoms generally resolve as the larvae encyst within the skeletal muscles resulting in decreased immunogenicity. However, in some cases, symptoms may persist for years.
An estimated 10 000 cases of trichinosis are reported per year worldwide. However, trichinosis is a relatively uncommon disease in the USA today, due in large part to the implementation of regulations enacted to monitor food safety and meat processing. According to the Centers for Disease Control and Prevention, from 2008–2010, the average number of cases reported was 20/year.5 Symptoms of trichinosis are often non-specific and may mimic other diseases. It is critical for the physician to obtain a detailed history regarding possible exposures, and consider trichinosis in the differential diagnosis. Treatment is recommended with an antihelmintic, such as albendazole. In this case the patient developed acute renal failure after starting therapy with albendazole. Although renal biopsy was not performed in this case, in the absence of an alternative explanation, a hypersensitivity reaction or acute interstitial nephritis secondary to albendazole was considered the likely cause.
Case presentation
A 72-year-old Caucasian woman presented to the hospital with a 3-week history of cramping abdominal pain, nausea, vomiting, diarrhoea, fatigue, weight loss, dehydration, confusion with memory impairment and reported visual hallucinations. Exposure history revealed that the patient had intermittently consumed raw pork products including raw bacon that was salted and cured in brine but not further cooked or boiled. The patient consumed these raw pork products prior to preparing meals and to our knowledge was the only household member exposed.
Pertinent medical history included IgG deficiency, diagnosed in the patient's fifth decade of life, requiring monthly intravenous immunoglobulin infusions, type 2 diabetes mellitus, coronary artery disease, sleep apnoea, chronic obstructive pulmonary disease and stage 2 chronic kidney disease (baseline creatinine 0.8 mg/dL, estimated-glomerular filtration rate 71 mL/min/1.73 m2). Her medications at the time of hospitalisation included: amitriptyline, fluticasone-salmeterol disk, lansoprazole, losartan and pramipexole. Aside from losartan, she was not on any other treatment for her chronic kidney disease.
The patient was a married homemaker, whose husband was a farmer. She was a former tobacco smoker and denied alcohol consumption. She had been independent in her daily activities 1 month prior to presentation. On physical examination, she was afebrile, her heart rate and blood pressure were normal. She was alert and oriented to person, place and time. However, her short-term memory recall was impaired. Her long-term memory was intact. Systemic examination revealed mild right upper quadrant tenderness, pupillary anisocoria and chronic stasis dermatitis on bilateral lower extremities. The remainder of the systemic examination was normal.
Investigations
Her complete blood count showed leukocytosis with a white cell count of 13×109/L (normal 3.5–10.5×109/L) and 30% peripheral eosinophilia with an absolute eosinophil count of 3.1×109/L (normal 0–0.45×109/L). The complete metabolic profile was significant for creatinine 0.8 mg/dL (normal 0.6–1.1 mg/dL), sodium 133 mmol/L (normal 135–145 mmol/L), potassium 3.3 mmol/L (normal 3.6–5.2 mmol/L), chloride 96 mmol/L (normal 100–108 mmol/L) and bicarbonate 20 mmol/L (normal 22–29 mmol/L). Alkaline phosphatase was elevated at 181 units/L (normal 52–144 units/L). Aspartate aminotransferase, alanine aminotransferase and bilirubin were within normal limits. Albumin was 3.2 g/dL (normal 3.5–5 g/dL) and prealbumin was 7 mg/dL (normal 19–38 mg/dL). Creatine kinase was 15 units/L (normal 38–176 units/L). C reactive protein and sedmentation rate were elevated at 37.1 mg/L (normal <8 mg/L) and 33 mm/1 h (normal 0–29 mm/1 h), respectively. Lipase, serum lactate, serum osmolality, glycated haemoglobin and thyroid stimulating hormone were normal. Serology was positive for Trichinella antibody by ELISA (Trichinella Serum Microwell ELISA TN-96 manufactured by SCIMEDX Corporation, Denville, New Jersey, USA). Serology for cysticercosis was negative. Testing for HIV, giardia, strongyloides, toxocara and stool ova and parasites were all negative. CT scan of the head was negative for any acute intracranial findings. Chest X-ray showed calcified and tortuous aorta and mild prominence of the central pulmonary arteries. ECG demonstrated normal sinus rhythm, left atrial enlargement, prolonged QTc interval, right bundle branch block and left anterior fascicular block. The findings on chest X-ray and ECG were attributed to her known history of cardiac disease and were stable when compared with prior examinations.
Differential diagnosis
Infections such as giardiasis, toxocariasis, trichuriasis, strongyloidiasis, HIV infection and cysticercosis were considered but were excluded by the appropriate serological, antigen detection tests and by negative stool examination for ova and parasites. Whipple's disease and inflammatory bowel disease were considered unlikely based on clinical presentation and colonoscopy was not pursued once positive Trichinella serology was reported.
Treatment
Recommended treatment for trichinosis includes anthelmintics, albendazole or mebendazole and often glucocorticoids. The patient was started on treatment with albendazole 400 mg orally twice daily. However, on day 2 of hospital stay, the patient was noted to be febrile and her creatinine was noted to acutely rise to 1.9 mg/dL and blood urea nitrogen elevated to 25 mg/dL. Urinalysis with microscopy showed: pH 5.9 (normal 4.5–8.0), protein 38 mg/dL (normal 0–19 mg/dL), osmolality 288 mOsm/kg (normal 150–1150 mOsm/kg), protein/osmolality ratio 1.32 (normal <0.12), red blood cells <3/hpf (normal <3/hpf), white cell count 11–20/hpf (normal for females 1–10/hpf), 1–3 transitional epithelial cells/hpf (normal 0/hpf), 4–10 squamous epithelial cells/hpf (normal 0/hpf) and urine eosinophils 1–5% (normal 0%). A renal ultrasound was performed and showed no evidence of hydronephrosis or dilation of the collecting system for either kidney. The left and right kidneys had normal cortical thickness and parenchymal echogenicity. These findings were consistent with stage 2 acute kidney injury likely secondary to albendazole therapy. Albendazole was discontinued and oral prednisone therapy was initiated for treatment of possible hypersensitivity reaction or acute interstitial nephritis. Alternative treatment for trichinosis with pyrantel was considered. However, pyrantel is active only against worms in the gut and has no efficacy once the larvae have migrated outside of the intestine. As the patient's symptoms had been present for several weeks and her symptoms were most consistent with the systemic phase of disease, pyrantel was unlikely to be of benefit. The decision was made to proceed with observation as the patient did not tolerate the treatment.
Outcome and follow-up
The patient received a total of 30 days of prednisone. Her serum creatinine slowly decreased after discontinuation of albendazole. The trend of her creatinine levels is shown in (figure 1).
Figure 1.
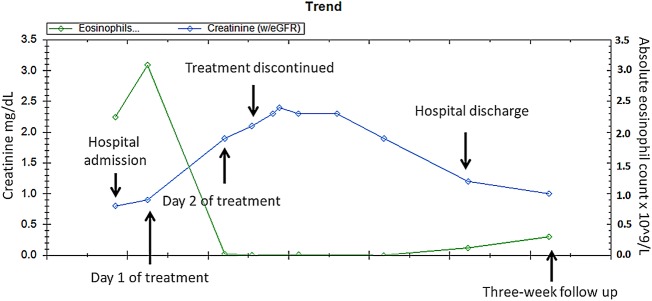
Creatinine level and absolute eosinophil count at admission, throughout hospitalisation and at a follow-up visit.
She was seen in the outpatient clinic 3 weeks after discharge from the hospital for a follow-up visit. Her creatinine had improved to 1 mg/dL. Her absolute eosinophil count had normalised to 0.30×109/L (normal 0.05–0.50×109/L). Her symptoms of confusion, memory impairment and visual hallucinations had completely resolved. She and her husband felt that she had recovered to her baseline cognitive functions.
Discussion
Trichinosis is a rare diagnosis in the USA today. In this case, the diagnosis was made based on a qualitative ELISA assay for IgG. This particular assay has a sensitivity of 94.4% when compared with positive muscle biopsy and a specificity of 93.8% in normal hosts. The cross sensitivity for other parasitic infections is as follows: ascaris 100%, hookworm 83.3%, strongyloides 83.3%, toxocara 66.6% and trichuris 83.3%. Antibody titres were not available as a qualitative assay was utilised. Our reference laboratory does not perform a western blot to verify the results. However, all positive results are repeated three times and are reported as true positives if two of the three samples return positive. In order to account for cross reactivity with other pathogens, stool microscopy for ova and parasites, strongyloides cultures of faecal material and serological testing for toxocara and strongyloides were performed and all results returned negative, supporting our impression that ELISA testing for Trichinella was true positive. Testing of the raw pork that the patient had consumed was not feasible as suspected meat was not available to us for confirmation. However, our patient was a homemaker without other risk factors for exposure to trichinosis. Specifically the patient had not travelled, did not hunt and was not exposed to horses, wild boars or other wildlife that can transmit Trichinella.
In the absence of an alternative explanation, albendazole was considered the likely cause of nephrotoxicity in our patient. The mechanisms of drug-induced nephrotoxicity are generally categorised based on the anatomical involvement of renal histology. Drugs can affect and cause inflammatory changes in the glomerulus, renal tubular cells or interstitium, leading to a decline in renal function. Some drugs exert their nephrotoxic effects by more than one pathogenic mechanism. In general, nephrotoxicity is an adverse reaction that has been observed to be a class effect. However, we were unable to find any reported cases of acute renal failure from all members of the benzimidazoles, which include albendazole, mebendazole, thiabendazole and triclabendazole. To the best of our knowledge, this is the first reported case of albendazole-associated acute renal failure.
The precise aetiology of renal failure could not be established in this patient's case as renal biopsy was not performed. However, it is likely that the acute renal failure was due to a hypersensitivity reaction or acute interstitial nephritis (AIN) secondary to albendazole. The patient's urinary findings did not show red cell casts, abnormal/dysmorphic red cells or nephrotic range proteinuria, which are the typical features of acute glomerulonephritis. There were minimal free tubular epithelial cells and no epithelial cell casts on urinalysis to suggest acute tubular necrosis. The gold standard for diagnosis of the pathogenic mechanism of acute renal failure is renal biopsy, which was not performed in our patient. Although she did not have the classic triad of AIN, which includes fever, rash and eosinophilia, this classic triad is present in less than 15% of patients.6 Our patient had sterile pyuria with eosinophiluria on urinalysis. She was continued on her home medications in the hospital and albendazole was the only new medication she received during this admission. Her serum creatinine started to rise after only one day of exposure to albendazole. Her renal function started to improve within 48 h after the discontinuation of albendazole and the initiation of prednisone therapy and returned to her baseline before discharge from the hospital. In the absence of an alternative explanation for eosinophiluria and temporal correlation with starting albendazole therapy, a hypersensitivity reaction or acute interstitial nephritis secondary to albendazole was considered the most likely cause of acute renal failure in our patient. Although definitive diagnosis of the pathogenic mechanism of drug-induced nephrotoxicity is made by renal biopsy, it is considered unnecessary for patients who have clearly documented onset of decline in renal function after initiation of the offending agent, and in those whose renal function improves promptly on discontinuation of the said agent.
The first and most important step in the management of drug-induced nephrotoxicity is immediate discontinuation of the offending agent. There is no specific therapy for acute kidney injury aside from supportive care, which includes adequate hydration, adjusting dosages of medications for declined renal function and avoiding further nephrotoxins. Corticosteroids have been used in treating AIN but their benefit is controversial. It would be difficult to determine if prednisone therapy offered additional benefit to our patient. The most recent multicenter retrospective study published by González et al7 in 2008 showed significant benefits of corticosteroids in AIN. However, an earlier smaller study in 2004 was unable to demonstrate the benefit of steroid therapy in this scenario.8
Interestingly, our patient had known IgG deficiency requiring monthly intravenous immunoglobulin infusions. This immunodeficiency may have predisposed her to infection with trichinosis as it has been shown that certain subtypes of IgG may play a role in immunity against parasitic disease.9 Patients with IgG deficiency are typically prone to recurrent upper and lower respiratory tract infections caused by the common respiratory pathogens including Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.10 The patient received no further treatment for trichinosis. Antiparasitic therapy is most effective during the earlier stages of this disease. Most patients, like ours, are diagnosed several weeks after infection occurs. Treatment in the late stage of the disease may be ineffective against those larvae that have encysted in the muscle. Ivermectin has been shown to have some effects against systemic phase of trichinosis in mice11 but there have been no clinical studies or published case reports of ivermectin use for trichinosis in humans. However, treatment is recommended in patients with severe and/or potential life-threatening systemic symptoms such as cardiac, pulmonary or central nervous system involvement. The effect of anthelmintic therapy on an advanced stage of trichinosis after the encystment of larvae is unclear.1
Early recognition of albendazole-associated acute renal failure is critical. Immediate discontinuation of this agent in patients with acute renal failure is vital for halting further deterioration of renal function. Treatment is generally supportive. However, steroid therapy can be considered in severe cases if the pathogenic mechanism is likely due to AIN.
Learning points.
Consumption of raw or undercooked meat is a risk factor for trichinosis.
Infection in humans can be categorised into two phases: the intestinal phase followed by a muscular/systemic phase.
Mebendazole or albendazole are recommended for the treatment of acute, symptomatic infection with this parasite.
Albendazole should be immediately discontinued if acute renal failure due to hypersensitivity reaction is suspected.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 2009;22:127–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupouy-Camet J, Murrell KD. eds. FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis. 2007. http://www.fao.org/ [Google Scholar]
- 3.Bruschi F. Trichinellosis in developing countries: is it neglected? J Infect Dev Ctries 2012;6:216–22 [DOI] [PubMed] [Google Scholar]
- 4.Gamble HR, Bessonov AS, Cuperlovic K, et al. International Commission on trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol 2000;93:393–408 [DOI] [PubMed] [Google Scholar]
- 5.2012. Trichinellosis—Epidemiology and Risk Factors. http://www.cdc.gov/parasites/trichinellosis/epi.html.
- 6.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int 2010;77:956–61 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez E, Gutierrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008;73:940–6 [DOI] [PubMed] [Google Scholar]
- 8.Clarkson MR, Giblin L, O'Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004;19:2778–83 [DOI] [PubMed] [Google Scholar]
- 9.Ottesen EA, Skvaril F, Tripathy SP, et al. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol 1985;134:2707–12 [PubMed] [Google Scholar]
- 10.Herrod HG. Clinical significance of IgG subclasses. Curr Opin Pediatr [Rev] 1993;5:696–9 [DOI] [PubMed] [Google Scholar]
- 11.Soliman GA, Taher ES, Mahmoud MA. Therapeutic efficacy of dormectin, ivermectin and levamisole against different stages of Trichinella spiralis in rats. Turkiye Parazitol Derg 2011;35:86–91 [DOI] [PubMed] [Google Scholar]


