Abstract
Objective
The objectives of this study were to develop a coronary heart disease (CHD) risk model among the Korean Heart Study (KHS) population and compare it with the Framingham CHD risk score.
Design
A prospective cohort study within a national insurance system.
Setting
18 health promotion centres nationwide between 1996 and 2001 in Korea.
Participants
268 315 Koreans between the ages of 30 and 74 years without CHD at baseline.
Outcome measure
Non-fatal or fatal CHD events between 1997 and 2011. During an 11.6-year median follow-up, 2596 CHD events (1903 non-fatal and 693 fatal) occurred in the cohort. The optimal CHD model was created by adding high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol and triglycerides to the basic CHD model, evaluating using the area under the receiver operating characteristic curve (ROC) and continuous net reclassification index (NRI).
Results
The optimal CHD models for men and women included HDL-cholesterol (NRI=0.284) and triglycerides (NRI=0.207) from the basic CHD model, respectively. The discrimination using the CHD model in the Korean cohort was high: the areas under ROC were 0.764 (95% CI 0.752 to 0.774) for men and 0.815 (95% CI 0.795 to 0.835) for women. The Framingham risk function predicted 3–6 times as many CHD events than observed. Recalibration of the Framingham function using the mean values of risk factors and mean CHD incidence rates of the KHS cohort substantially improved the performance of the Framingham functions in the KHS cohort.
Conclusions
The present study provides the first evidence that the Framingham risk function overestimates the risk of CHD in the Korean population where CHD incidence is low. The Korean CHD risk model is well-calculated alternations which can be used to predict an individual's risk of CHD and provides a useful guide to identify the groups at high risk for CHD among Koreans.
Keywords: Epidemiology
Strengths and limitations of this study.
Coronary risk scores, which utilise data on multiple risk factors, are ideal for making rational decisions regarding the distribution of scarce health service resources. The Framingham risk score overestimates the coronary risk in other populations, such as contemporary populations in Europe.
There are no data on whether the Framingham risk score overestimates the coronary risk in Korea.
Fatal and non-fatal (hospitalised) events among the study cohort were identified from insurance claims which were reported to the National Health Insurance System between the date of examination and 31 December 2011, giving a median follow-up of 11.6 years.
This is an unusually large study with over 200 000 participants.
The limitations of this study include possible measurement errors and the non-random sample used.
Introduction
In 2008, cardiovascular disease (CVD) accounted for 30% of total global deaths, while 80% of CVD deaths occurred in low-income and middle-income countries. Of these deaths, an estimated 7.3 million were due to coronary heart disease (CHD) and 6.2 million were due to stroke.1 In Korea, CVD is now one of the leading preventable causes of death in Korea, causing 22% of all deaths in 2011.2
CHD risk scores, which utilise data on multiple risk factors, are ideal for making rational decisions regarding the distribution of scarce health service resources. The Framingham CHD Risk Score (FRS), developed in a middle-class population in Massachusetts, is the most commonly used CHD risk score worldwide. However, in recent years, several investigators have demonstrated that the FRS overestimates the coronary risk in other populations, such as contemporary populations in Europe3–5 and Asia,6 including Korea,7 while others have suggested that additional risk factors may add to the predictability of those included in the FRS.8–10 Although some studies have investigated this issue in Asian populations,8 9 none had a follow-up of over 10 years.
To date, no national CHD risk score has been developed for Korea, despite the existence of some very large cohort studies with the potential to produce more reliable estimates of risk associations than have previously been possible.
The objectives of this study were, thus, to evaluate the applicability of the Framingham risk function11 in the Korean Heart Study (KHS) population, and to develop a Korean CHD risk score.
Method
Study population
KHS included 430 920 individuals (266 782 men and 164 138 women) who had voluntarily undergone private health examinations in 18 centres located in the capital and six provinces in South Korea between 1996 and 2004. A full description of KHS has been previously published.12 13
To develop a 10-year CHD risk prediction model, the baseline period used for this study was between 1996 and 2001; however, since we have followed them until December 2011 this gives at least 10 years of potential follow-up for everyone included (figure 1). Sixteen thousand five hundred and seventy-eight participants who reported of having cancer, liver disease, CVD or a respiratory disease at, or prior to, the initial visit were excluded. Also, 5408 participants with missing information on blood pressure, total serum cholesterol, fasting serum glucose, smoking status or body mass index (BMI), or who had an extremely low BMI (<16 kg/m2) or height (≤1.30 m) were excluded. The final study participants were 268 315 individuals.
Figure 1.
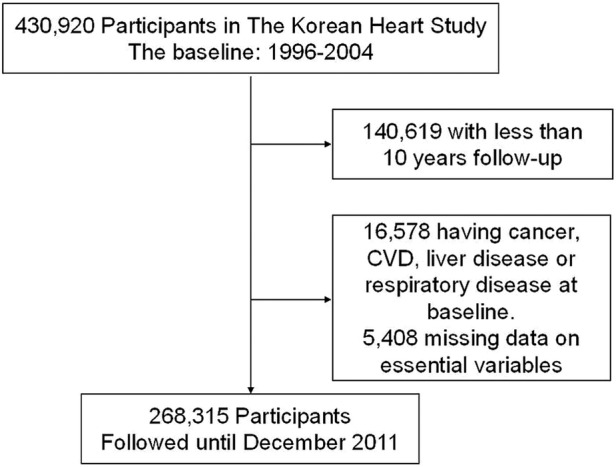
Defining the study population.
Data collection
Data files were collected from centres that maintained electronic databases, and manual data coding was performed by trained personnel for data collected from centres that kept paper records. Smoking habits (current, former or never smoking) was ascertained from the questionnaires. Either registered nurses or blood pressure technicians measured blood pressure using a standard mercury sphygmomanometer. For clinical chemistry assays, serum was separated from peripheral venous blood samples that were obtained from each participant after 12 h of fasting and was stored at −70°C. Biomarkers, such as fasting glucose and lipid profiles, were measured using the Histachi-7600 analyser (Hitachi Ltd, Tokyo, Japan). Details on quality control were published previously.12
In 2006, the Korean Association of Quality Assurance for Clinical Pathology conducted a nationwide study of interlaboratory agreement, and the Department of Clinical Pathology at the Asan Medical Center was in charge of the analysis. After receiving written permission, we reviewed interlaboratory correlations for the participating centres. The interlaboratory correlation coefficients for all measured variables exceeded 0.95.12
Outcome variables
Fatal and non-fatal (hospitalised) events among the study cohort were identified from insurance claims which were reported to the National Health Insurance System (NHIS) between the date of examination and 31 December 2011, giving a median follow-up of 11.6 years. Since NHIS is a national organisation covering all of Korea's population, this should provide a complete event ascertainment.
‘Hard’ CHD events, comprising acute myocardial infarction, sudden death and other coronary deaths, were included in our study outcome variable.11 We ascertained non-fatal CHD events, defined according to the International Classification of Diseases 10th Revision (acute myocardial infarction, code I21), from health insurance claims data from the NHIS. For fatal CHD events, deaths from death records were collected. Death certificates from the National Statistical Office were identified via identification numbers, which are assigned to citizens at birth.
A validation study was conducted by 20 internists from the Korean Society of Cardiology in 2009.14 For the participants who provided written permission for the use of their personal information, 673 CHD events between 1994 and 2007 were confirmed with individual hospital medical records, showing that 73% of designated myocardial infarctions were valid. The validation study was updated in 2013 with a value of 93%.15 The validation study on mortality data has not been conducted.
Statistical analysis
To develop the risk score, an a priori decision was made to emulate the most commonly used version of the FRS11 as far as possible. Thus, age, blood pressure, total and high-density lipoprotein-cholesterol (HDL-C), diabetes and smoking were considered for inclusion in the Korean CHD risk score (KRS). Furthermore, blood pressure, total and HDL-C were analysed in the same ordinal groups as in FRS, except that blood pressure was grouped according to the most recent (7th) guidelines of the Joint National Committee on Hypertension.16 Contemporary guidelines17 were used to define diabetes as self-reported or fasting glucose>=126 mg/dL. In addition, triglycerides and low-density lipoprotein (LDL)-cholesterol were considered for inclusion in the Korean risk score, in place of HDL-C, as was an extension of the definition of smoking status to include former smoking. Another a priori decision was to develop separate risk models for men and women, where, unlike the FRS, the same variables were to be used for each sex. This is thought to be the most sensible for practical applications.
To decide on what factors should enter the risk score, Cox proportional hazards regression models were fitted first to a basic set of classical risk factors: age, blood pressure, total cholesterol (TC), smoking and diabetes. Further models added, one-at-a-time, were HDL-C, LDL-cholesterol and triglycerides to this basic set. The final model was prespecified to be that with the best discrimination and risk classification of the four, averaged across the sexes. Discrimination was assessed by the c-statistic for survival data18 19 and other models were compared, for risk classification, with the basic model using the continuous form of the net reclassification index (NRI) for 10-year risk of CHD.
From the final model, the KRS was derived using standard methods.19 Its calibration was tested by dividing participants, within each sex, into tenths of predicted risk and using the Hosmer-Lemeshow, calibration and reclassification were carried out after splitting the data randomly into 50% development and 50% validation samples.20
Comparison with the Framingham score
We compared the regression coefficients from a Cox proportional hazard model, fitted to the KHS, with the same variables as in the FRS and the regression coefficients in the published account of the FRS11 using Wald tests. We recalibrated Framingham to the KHS population19 and compared this to the KRS. All analyses were conducted using SAS, V.9.1.2 (SAS Institute Inc, Cary, North California, USA).
Results
Baseline risk factors and CHD incidence rates
After excluding participants with missing values for any of the FRS risk factors, 268 315 individuals (164 005 men and 104 310 women) were included in these analyses (table 1). These were largely middle aged at enrolment (mean age 45.8 years in men and 47.6 years in women). Those excluded due to missing values did not differ from those included, by age and sex. The mean levels of BMI and LDL-cholesterol were similar in both sexes. However, smoking was prevalent in men but uncommon in women. Similar prevalence rates for high TC and LDL-cholesterol were observed in men and women. The prevalence of reduced HDL-C and diabetes was higher in men than in women (table 2).
Table 1.
Baseline characteristic of study participants, 1996–2001, the Korean Heart Study
| Men | Women | |
|---|---|---|
| N | 164 005 | 104 310 |
| Mean±SD | Mean±SD | |
| Age, years | 45.8±9.4 | 47.6±10.0 |
| Body mass index, kg/m2 | 23.8±2.8 | 23.2± 3.1 |
| Systolic blood pressure, mm Hg | 123.3±16.7 | 121.0±19.6 |
| Diastolic blood pressure, mm Hg | 78.3±11.6 | 75.3±11.8 |
| Total cholesterol, mg/dL | 194.5±34.8 | 195.1±37.4 |
| HDL-cholesterol, mg/dL | 47.9±10.6 | 54.5±12.7 |
| LDL-cholesterol, mg/dL | 118.4±32.2 | 118.9±33.6 |
| Triglyceride, mg/dL | 148.2±87.1 | 113.3±67.5 |
| Fasting glucose, mg/dL | 97.1±24.2 | 93.6±20.6 |
| % | % | |
| Smoking status | ||
| Ex-smoker | 23.0 | 4.0 |
| Current smoker | 53.0 | 5.1 |
| Diabetes* | 8.4 | 6.0 |
| Medication | 1.9 | 1.6 |
| Hypertension† | 28.6 | 24.7 |
| Medication | 4.4 | 5.3 |
*Diabetes was defined as fasting serum glucose greater than 126 mg/dL or diabetic treatment history.
†Hypertension was defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg or medication.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Baseline risk factors, person-years of follow-up and CHD events in men and women in the Korean Heart Study, aged 30–74 years, 1996–2011*
| Men (n=164 005) |
Women (n=104 310) |
|||||
|---|---|---|---|---|---|---|
| Person-years of follow-up | Hard CHD events† | Incidence per 100 000 PY¶ |
Person-years of follow-up | Hard CHD events† | Incidence per 100 000 PY¶ |
|
| Total† | 1 961 999 | 2086 | 121.1 | 1 281 102 | 510 | 52.1 |
| Blood pressure | ||||||
| Normal | 683 826 | 468 | 92.4 | 602 146 | 91 | 25.9 |
| Prehypertension | 798 672 | 774 | 116.7 | 415 032 | 167 | 48.5 |
| Stage-1 hypertension | 347 579 | 539 | 149.0 | 178 672 | 133 | 61.8 |
| Stage-2 hypertension | 131 922 | 305 | 194.8 | 85 253 | 119 | 94.2 |
| Total cholesterol, mg/dL | ||||||
| <160 | 293 873 | 173 | 81.4 | 213 533 | 32 | 17.1 |
| 160–199 | 854 406 | 698 | 99.8 | 533 694 | 145 | 45.7 |
| 200–239 | 619 971 | 810 | 139.3 | 382 484 | 200 | 53.4 |
| 240–279 | 166 830 | 316 | 185.2 | 123 315 | 93 | 64.1 |
| ≥280 | 26 918 | 89 | 355.8 | 28 076 | 40 | 118.7 |
| HDL-cholesterol, mg/dL | ||||||
| <35 | 157 245 | 292 | 193.7 | 42 652 | 28 | 59.6 |
| 35–44 | 618 462 | 768 | 140.5 | 242 097 | 128 | 63.5 |
| 45–49 | 438 772 | 427 | 116.5 | 205 596 | 107 | 71.2 |
| 50–59 | 523 426 | 429 | 99.0 | 390 158 | 144 | 45.0 |
| ≥60 | 224 094 | 170 | 80.7 | 400 598 | 103 | 36.6 |
| LDL-cholesterol, mg/dL | ||||||
| <100 | 562 674 | 372 | 82.7 | 385 816 | 75 | 42.2 |
| 100–129 | 733 988 | 661 | 106.6 | 463 200 | 158 | 46.2 |
| 130–149 | 353 968 | 447 | 138.9 | 217 960 | 113 | 54.7 |
| ≥150 | 311 369 | 606 | 195.2 | 214 126 | 164 | 66.5 |
| TG, mg/dL | ||||||
| <100 | 621 804 | 441 | 87.5 | 685 429 | 134 | 35.9 |
| 100–149 | 583 391 | 609 | 115.7 | 332 866 | 142 | 47.6 |
| 150–199 | 357 517 | 464 | 149.0 | 143 896 | 110 | 65.7 |
| 200–249 | 189 163 | 269 | 148.5 | 62 412 | 70 | 99.6 |
| ≥250 | 210 123 | 303 | 169.0 | 56 499 | 54 | 83.0 |
| Smoking status | ||||||
| Ex-smoker | 454 230 | 448 | 94.2 | 51 928 | 24 | 53.8 |
| Current smoker | 1 038 830 | 1271 | 155.0 | 64 612 | 38 | 88.0 |
| Diabetes | 162 348 | 399 | 217.7 | 75 840 | 98 | 96.1 |
SI conversions: to convert HDL-C and TG to mmol/L, multiply by 0.0259.
*Participants with risk factor at baseline (1996–2001).
†Mean ages of Korean were 45.8 years in men and 47.6 years in women, respectively.
¶Incidences were standardized to the age distribution in the 2005 Korean population.
CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PY, person-years; TG, triglycerides.
During an 11.6-year median follow-up, 2596 CHD events (1903 non-fatal and 693 fatal) occurred in the cohort. The average 10-year risk for CHD was 1.03% for men and 0.40% for women. Summary statistics of person-years of follow-up and CHD events by baseline risk factor level, and overall, within sex, are shown in table 2.
Tables 3 (men) and 4 (women) show the HR for each level of each prognostic factor, compared to its reference. As expected, in the 10-year predictions of CHD, all five predictors in the basic model (age, blood pressure, TC, smoking and diabetes) were statistically significant in both men and women. Models 1, 2 and 3 added HDL-C, LDL-cholesterol and triglycerides, respectively, to the basic model. Among these models, model 1 was the best discriminating model (area under the curve (AUC)=0.764) for men and also the model with best reclassification of risk (NRI=0.284) compared with the basic model (to which it was superior). For women, model 3 was the best model on both criteria (AUC=0.815 and NRI=0.207). The second best model that reflects the women is model 1 (AUC=0.812 and NRI=0.177). The KRS was adopted from model 1 for both the sexes. The KRS algorithm is given in online supplementary appendix A.
Table 3.
HRs for CHD risk factors in men in the Korean Heart Study, aged 30–74, 1996–2011
| Basic model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.13 (1.09 to 1.18) | 1.13 (1.08 to 1.18) | 1.13 (1.08 to 1.18) | 1.13 (1.08 to 1.18) |
| Blood pressure | ||||
| Normal | 1.00 | 1.00 | 1.00 | 1.00 |
| Prehypertension | 1.30 (1.16 to 1.46) | 1.29 (1.15 to 1.45) | 1.32 (1.17 to 1.48) | 1.28 (1.14 to 1.43) |
| Stage-1 hypertension | 1.74 (1.53 to 1.97) | 1.72 (1.52 to 1.96) | 1.78 (1.57 to 2.02) | 1.68 (1.48 to 1.91) |
| Stage-2 hypertension | 2.22 (1.91 to 2.57) | 2.20 (1.90 to 2.56) | 2.28 (1.97 to 2.65) | 2.13 (1.84 to 2.48) |
| Total cholesterol, mg/dL | ||||
| <160 | 1.00 | 1.00 | 1.00 | 1.00 |
| 160–199 | 1.26 (1.07 to 1.49) | 1.34 (1.14 to 1.59) | 1.09 (0.90 to 1.32) | 1.21 (1.02 to 1.43) |
| 200–239 | 1.81 (1.53 to 2.13) | 2.02 (1.71 to 2.38) | 1.23 (0.99 to 1.53) | 1.67 (1.42 to 1.98) |
| 240–279 | 2.42 (2.01 to 2.92) | 2.77 (2.30 to 3.34) | 1.34 (1.04 to 1.73) | 2.19 (1.81 to 2.65) |
| ≥280 | 3.79 (2.93 to 4.91) | 4.45 (3.44 to 5.76) | 2.02 (1.47 to 2.77) | 3.37 (2.59 to 4.38) |
| Smoking | ||||
| Never | 1.00 | 1.00 | 1.00 | 1.00 |
| Former | 1.01 (0.88 to 1.16) | 1.02 (0.89 to 1.17) | 1.02 (0.89 to 1.17) | 1.00 (0.87 to 1.15) |
| Current | 1.93 (1.72 to 2.17) | 1.86 (1.65 to 2.09) | 1.96 (1.75 to 2.21) | 1.87 (1.66 to 2.11) |
| Diabetes | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.69 (1.51 to 1.89) | 1.63 (1.46 to 1.82) | 1.72 (1.53 to 1.92) | 1.65 (1.48 to 1.85) |
| HDL-cholesterol, mg/dL | ||||
| <35 | 1.00 | |||
| 35–44 | 0.66 (0.57 to 0.75) | |||
| 45–49 | 0.56 (0.48 to 0.65) | |||
| 50–59 | 0.45 (0.39 to 0.52) | |||
| ≥60 | 0.34 (0.28 to 0.41) | |||
| LDL-cholesterol, mg/dL | ||||
| <100 | 1.00 | |||
| 100–129 | 1.23 (1.06 to 1.43) | |||
| 130–149 | 1.50 (1.25 to 1.80) | |||
| ≥150 | 1.97 (1.61 to 2.40) | |||
| Triglycerides, mg/dL | ||||
| <100 | 1.00 | |||
| 100–149 | 1.21 (1.07 to 1.37) | |||
| 150–199 | 1.35 (1.18 to 1.54) | |||
| 200–249 | 1.39 (1.19 to 1.63) | |||
| ≥250 | 1.30 (1.11 to 1.52) | |||
| ROC (95% CI) | 0.756 (0.745 to 0.766) | 0.764 (0.752 to 0.774) | 0.758 (0.747 to 0.769) | 0.757 (0.746 to 0.768) |
| Continuous NRI (95% CI) | Referent model | 0.284 (0.231 to 0.339) | 0.185 (0.124 to 0.246) | 0.109 (0.051 to 0.162) |
SI conversions: to convert HDL-C and TC to mmol/L, multiply by 0.0259.
CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NRI, net reclassification index; ROC, receiver operating characteristic; TC, total cholesterol.
Table 4.
HRs for CHD risk factors in women in the Korean Heart Study, aged 30–74, 1996–2011
| Basic model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.14 (1.03 to 1.26) | 1.13 (1.02 to 1.24) | 1.14 (1.03 to 1.26) | 1.12 (1.01 to 1.24) |
| Blood pressure | ||||
| Normal | 1.00 | 1.00 | 1.00 | 1.00 |
| Prehypertension | 1.61 (1.24 to 2.09) | 1.57 (1.21 to 2.04) | 1.61 (1.32 to 2.09) | 1.54 (1.19 to 2.01) |
| Stage-1 hypertension | 2.01 (1.52 to 2.66) | 1.93 (1.46 to 2.56) | 2.01 (1.52 to 2.66) | 1.88 (1.42 to 2.50) |
| Stage-2 hypertension | 3.15 (2.35 to 4.21) | 3.02 (2.26 to 4.05) | 3.15 (2.36 to 4.22) | 2.90 (2.17 to 3.89) |
| Total cholesterol, mg/dL | ||||
| <160 | 1.00 | 1.00 | 1.00 | 1.00 |
| 160–199 | 1.17 (0.80 to 1.72) | 1.26 (0.86 to 1.86) | 1.10 (0.71 to 1.69) | 1.12 (0.76 to 1.65) |
| 200–239 | 1.45 (0.99 to 2.12) | 1.62 (1.10 to 2.38) | 1.29 (0.79 to 2.10) | 1.31 (0.87 to 2.00) |
| 240–279 | 1.53 (1.01 to 2.31) | 1.77 (1.16 to 2.68) | 1.36 (0.78 to 2.38) | 1.32 (0.87 to 2.00) |
| ≥280 | 2.38 (1.48 to 3.82) | 2.78 (1.72 to 4.50) | 2.12 (1.14 to 3.95) | 1.96 (1.21 to 3.18) |
| Smoking | ||||
| Never | 1.00 | 1.00 | 1.00 | 1.00 |
| Former | 1.17 (0.78 to 1.77) | 1.20 (0.79 to 1.80) | 1.17 (0.78 to 1.77) | 1.19 (0.79 to 1.80) |
| Current | 2.06 (1.47 to 2.87) | 2.00 (1.43 to 2.79) | 2.06 (1.48 to 2.88) | 1.95 (1.39 to 2.72) |
| Diabetes | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.96 (1.57 to 2.45) | 1.89 (1.51 to 2.37) | 1.96 (1.57 to 2.46) | 1.84 (1.47 to 2.30) |
| HDL-cholesterol, mg/dL | ||||
| <35 | 1.00 | |||
| 35–44 | 0.79 (0.52 to 1.19) | |||
| 45–49 | 0.86 (0.56 to 1.31) | |||
| 50–59 | 0.66 (0.44 to 1.00) | |||
| ≥60 | 0.51 (0.33 to 0.78) | |||
| LDL-cholesterol, mg/dL | ||||
| <100 | 1.00 | |||
| 100–129 | 1.11 (0.80 to 1.54) | |||
| 130–149 | 1.16 (0.79 to 1.72) | |||
| ≥150 | 1.13 (0.74 to 1.74) | |||
| Triglyceride, mg/dL | ||||
| <100 | 1.00 | |||
| 100–149 | 1.19 (0.93 to 1.51) | |||
| 150–199 | 1.61 (1.23 to 2.09) | |||
| 200–249 | 2.13 (1.58 to 2.89) | |||
| ≥250 | 1.63 (1.17 to 2.27) | |||
| ROC (95% CI) | 0.809 (0.789 to 0.829) | 0.812 (0.792 to 0.833) | 0.809 (0.789 to 0.829) | 0.815 (0.795 to 0.835) |
| Continuous NRI (95% CI) | Referent model | 0.177 (0.043 to 0.313) | 0.160 (0.019 to 0.295) | 0.207 (0.087 to 0.336) |
SI conversions: to convert HDL-C and TC to mmol/L, multiply by 0.0259.
CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NRI, net reclassification index; ROC, receiver operating characteristic; TC, total cholesterol.
Comparison of the Framingham and the Korean risk equations
Compared with the HRs from the KHS using the same variables, there were no big differences with the published HRs for Framingham, with the exception of LDL-cholesterol (table 5). The FRS overestimated, overall by a factor of six, the number of CHD events actually observed in the KHS. Figure 2 shows the agreement between the original and recalibrated Framingham function and the KRS in the KHS population. The KRS and recalibrated FRS are clearly in close agreement, as would be anticipated from table 5.
Table 5.
HRs of coronary events in Framingham and Korean studies in men and women
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Framingham Heart Study | Korean Heart Study | p Value | Framingham Heart Study | Korean Heart Study | p Value | |
| Age (year) | 1.05 (1.04–1.06) | 1.07 (1.06–1.07) | 0.0040 | 1.04 (1.03–1.06) | 1,09 (1.08–1.11) | <0.0001 |
| Blood pressure (mm Hg) | ||||||
| Optimal+normal | 1.00 | 1.00 | 1.00 | 1.00 | ||
| High normal | 1.32 (0.98–1.78) | 1.28 (1.13–1.45) | 0.8352 | 1.34 (0.88–2.05) | 1.40 (1.07–1.83) | 0.8659 |
| Stage 1 | 1.73 (1.32–2.26) | 1.62 (1.46–1.81) | 0.6668 | 1.75 (1.21–2.54) | 1.65 (1.31–2.09) | 0.7907 |
| Stage 2–4 | 1.92 (1.36–2.24) | 2.10 (1.83–2.40) | 0.6409 | 2.19 (1.46–3.27) | 2.61 (2.04–3.34) | 0.4701 |
| LDL-cholesterol (mg/dL) | ||||||
| <130 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 130–159 | 1.19 (0.91–1.54) | 1.56 (1.42–1.73) | 0.0611 | 1.24 (0.84–1.81) | 1.24 (1.01–1.53) | 0.9931 |
| ≥160 | 1.74 (1.36–2.34) | 2.34 (2.09–2.62) | 0.0326 | 1.68 (1.17–2.40) | 1.43 (1.14–1.79) | 0.4602 |
| HDL-cholesterol (mg/dL) | ||||||
| <35 | 1.46 (1.15–1.85) | 1.68 (1.48–1.90) | 0.3092 | 2.08 (1.33–3.25) | 1.25 (0.85–1.84) | 0.0911 |
| 35–59 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| ≥60 | 0.61 (0.41–0.91) | 0.66 (0.57–0.78) | 0.7080 | 0.64 (0.47–0.87) | 0.72 (0.58–0.89) | 0.5517 |
| Smoking (yes/no) | 1.71 (1.39–2.10) | 1.89 (1.72–2.07) | 0.3917 | 1.49 (1.13–1.97) | 1.95 (1.40–2.72) | 0.2230 |
| Diabetes (yes/no) | 1.47 (1.04–2.08) | 1.71 (1.53–1.91) | 0.4182 | 1.80 (1.18–2.74) | 1.96 (1.57–2.45) | 0.7276 |
The regression coefficients for the Framingham and Korean cohorts were compared using a two-tailed z statistic, where z=(b[F]−b[C])/SE.
The SE is the standard error of the difference in coefficients, and SE=(SE[F]2+SE[K]2)1/2.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 2.
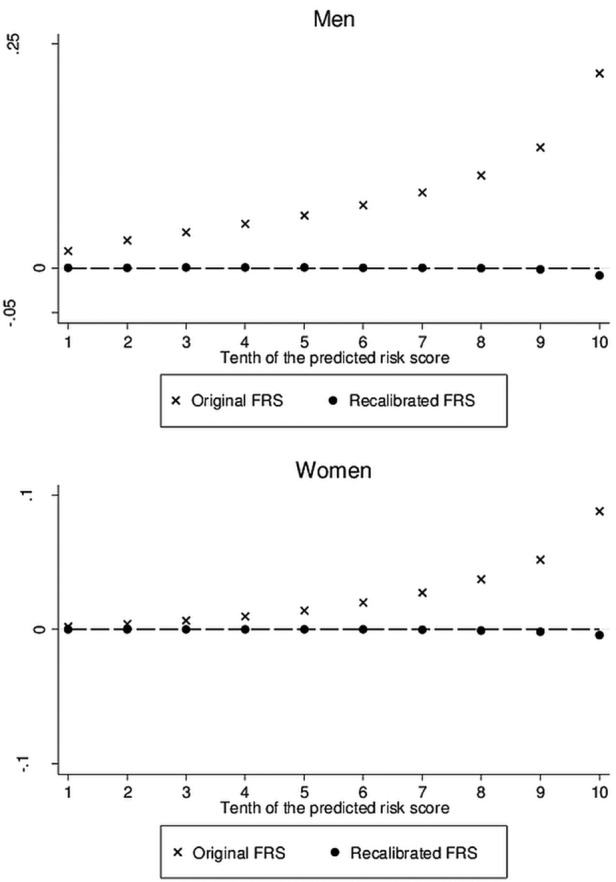
Agreement between both the original and recalibrated Framingham risk scores and the Korean risk score for coronary heart disease, by 10th of the predicted risks from the Korean risk score.
Discussion
The objectives of this study were (1) to develop a Korean CHD model; (2) to evaluate the ability of the FRS to predict the 10-year CHD risk in the Korean population. Over 2700 first CHD events occurred in this cohort of over 268 000 Koreans in 11.6 years. The KRS was developed based on these data. When compared with the actual CHD cases, the KRS performed well, but not substantially better than a recalibrated version of the FRS.
Estimation of absolute risk required for treatment and for prevention of CHD commonly relies on prediction models developed from the experience of prospective cohort studies.8–10 21 Although prediction algorithms developed by the Framingham investigators have been widely adopted to formulate clinical guidelines in the USA and elsewhere,21 the Framingham functions have overestimated the CHD risk in some populations, leading to a concern that it may not be appropriate to other populations.3–6 8 In our study, the FRS also overestimated the risk of CHD in the Korean population, where CHD incidence is relatively low. Another factor of relevance is the inclusion of softer CHD end points in the derivation of the FRS than we used in deriving the KHS.
Several studies3 6 have examined CHD events in the Framingham Heart Study (FHS). Due to problems anticipated risk Framingham, Ahn et al7 previously suggested the need for Korean guidelines for the management of CHD to avoid artificial inflation of costs in primary prevention.
To compare the CHD risk factors in men and women in the KHS to those in the FHS, the same six variables that were examined in the FHS paper were analysed—age (year), blood pressure (mm Hg), TC (mg/dL), HDL-C (mg/dL), diabetes and smoking. The FHS did not yield significant HDL-C ≥60 mg/dL results for men (RR, 0.63, 95% CI 0.34 to 1.18) and women (RR, 0.58, 95% CI 0.33 to 1.02).6 21 In Korean men, however, 11% had HDL-C ≥60 mg/dL (RR, 0.33, 95% CI 0.28 to 0.41), a 66% (RR, 0.34) decrease in risk compared with KHS men with HDL-C <35 mg/dL. The considerable reduction of CHD risk in participants with higher HDL-C levels reinforces the need for Korean populations to monitor their cholesterol levels closely.
Hippisley-Cox et al22 also developed, validated and evaluated a new QRISK model to estimate lifetime risk of CVD in the UK. This model was made in light of the emergence of a new cardiovascular risk prediction tool, which was shown to have greater predictive ability than the Framingham risk equation.23 Without recalibration they urge caution in using the FRS to identify high risk patients in the UK. Recently, the key issue is the extent of reallocation. Allocation is critically dependent on the cardiovascular risk score used and its performance in contemporaneous, ethnically diverse UK populations.24
The strengths of this cohort study include a large sample size, a wide age range and a nationally representative sample. The KHS cohort consisted of 268 315 participants aged 30–74 years, which was compared to 5251 participants aged 30–74 in the FHS. Examination of alternative lipid variables, triglycerides and LDL-cholesterol, and finer classification of smoking habits to include former smokers, strengthens this study. Moreover, discrimination, calibration and reclassification are all considered here, using contemporary methods.19 The limitations of this study include possible measurement errors. Clinical data from the health promotion centres were one-time measurements of blood pressure and medical examinations. Also, participants might have under-reported the smoking status and alcohol consumption in the self-report questionnaires. In addition, the validation study on mortality data has not been conducted.
In conclusion, we developed a CHD prediction model for Koreans and compared the estimates from the model with the actual CHD cases. This new model provides an accurate prediction of the risk of CHD among Korean. With the gradual rise in CHD events among Koreans, the prevention and treatment of CHD risk factors such as hypertension, hypercholesterolaemia and diabetes are important public health concerns.
Supplementary Material
Acknowledgments
The authors thank the staff of the Korean National Health Insurance Service.
Footnotes
Contributors: Data analysis was undertaken by SHJ, YM and KJJ. The article was drafted by SHJ, YJ, MW and YM. SHJ, YJ, DJO, B-HO, SHL, S-WP, K-BS, YM, KJJ, HK, YDY, SJB, DCL, SHC, MJK, JS, BLC, ESK, B-YY, T-YL, JSK, Y-JL, J-KO, SHK, J-KP, SBK, SBP, SYL, C-IY, MCK, H-KK, J-sP, HCK, GJL and MW substantially contributed to the conception or design of the work, revising the work, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This study was funded by Seoul City R&BD programme (10526) and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0715).
Competing interests: None.
Ethics approval: The Institutional Review Board of Human Research of Yonsei University and all the health promotion centres that participated in the KHS approved the study. We used the physical examination data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs) [Data file]. Retrieved from http://www.who.int/mediacentre/factsheets/fs317/en/index.html [Google Scholar]
- 2.National Bureau of Statistics, Republic of Korea. Annual report on the cause of death statistics. Seoul, Republic of Korea, 2011 [Google Scholar]
- 3.Hense H, Schulte H, Lowel H, et al. Framingham risk function overestimates risk of coronary heart disease in men and women from Germany—results from the MONICA Augsburg and the PROCAM cohorts. Eur Heart J 2003;24:937–45 [DOI] [PubMed] [Google Scholar]
- 4.Empana JP, Tafflet M, Escolano S, et al. Predicting CHD risk in France: a pooled analysis of the D.E.S.I.R., three city, PRIME, and SU.VI.MAX studies. Eur J Cardiol Prev Rehabil 2011;1:175–85 [DOI] [PubMed] [Google Scholar]
- 5.Vergnaud AC, Bertrais S, Galan P, et al. Ten-year risk prediction in French men using the Framingham coronary score: results from the national SU.VI.MAX cohort. Prev Med 2008;47:61–5 [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Hong Y, D'Agostino Ret al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese multi-provincial cohort study. JAMA 2004;291:2591–9 [DOI] [PubMed] [Google Scholar]
- 7.Ahn KA, Yun JE, Cho ER, et al. Framingham equation model overestimates risk of ischemic heart disease in Korean men and women. Korean J Epidemiol 2006;28:162–70 [Google Scholar]
- 8.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward M, Tunstall-Pedoe H, Rumley A, et al. Does fibrinogen add to prediction of cardiovascular disease? Results from the Scottish Heart Health Extended Cohort Study. Br J Haematol 2009;146:442–6 [DOI] [PubMed] [Google Scholar]
- 10.Tzoulaki I, Liberopoulos G, Ioannidis JPA. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA 2009;302:2345–52 [DOI] [PubMed] [Google Scholar]
- 11.Wilson P, D'Agostino R, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47 [DOI] [PubMed] [Google Scholar]
- 12.Jee SH, Batty GB, Jang Y, et al. The Korean heart study: rationale, objectives, protocol, and preliminary results for a new prospective cohort study of 430,920 men and women. Eur J Prev Cardiol 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Kim CH, Kim EH, et al. Impaired fasting glucose and risk of cardiovascular disease in Korean men and women: the Korean Heart Study. Diabetes Care 2013;36:328–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimm H, Yun JE, Lee SH, et al. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean Heart Study (1). Korean Circ J 2012;42:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean Heart Study [Master thesis]. Yonsei University, Seoul, Korea, June 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Diabetes Mellitus: Report of a WHO Study Group. Geneva, Switzerland: World Health Organization, 1985; Technical report series 727 [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–23 [DOI] [PubMed] [Google Scholar]
- 19.Woodward M. Epidemiology: study design and data analysis. 3rd edn Boca Raton: CRC Press, 2014 [Google Scholar]
- 20.Nam B-H. Discrimination and calibration in survival analysis [dissertation]. Boston, MA, Boston University, 2000 [Google Scholar]
- 21.D'Agostino R, Grundy S, Sullivan L, et al. Validation of the Framingham coronary heart disease prediction scores results of a multiple ethnic groups investigation. JAMA 2001;286: 180–7 [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins GS, Altman DG. An independent and external validation of QRISK2 cardiovascular disease risk score: a prospective open cohort study. BMJ 2010;340:c2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hippisley-Cox J, Coupland C, Robson J, et al. Advantages of QRISK2 (2010): the key issue is ethnicity and extent of reallocation. Heart 2011;97:515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


