Abstract
In asthma, when comorbidities and common causes of poor control have been considered and treated, the clinician may speculate, ‘Is it all asthma?’. In patients with uncontrolled atopic asthma with recurring episodes of symptoms mimicking pneumonia, the suspicion of allergic bronchopulmonary aspergillosis (ABPA) should remain high. ABPA is caused by a complex immunological hypersensitivity reaction to colonisation with Aspergillus fumigatus in the bronchial tree, and is characterised by the presence of atopic asthma, blood eosinophilia, migrating pulmonary opacities and potential bronchiectasis. This case report describes a delay in diagnosing ABPA which was imitating pneumonia. The clinician should pay increased attention to ABPA and test for this in patients with uncontrolled asthma with an ongoing requirement for oral corticosteroids and/or antibiotics and with pulmonary opacities on chest imaging.
Background
Asthma is a common chronic inflammatory disorder of the airways with a wide variability in clinical presentation due to a substantial phenotypic heterogeneity.1 Phenotyping asthma (eg, atopic/non-atopic, eosinophilic/neutrophilic inflammation) has clinical relevance in order to target individual antiasthmatic treatment, but despite the availability of efficient pharmacological treatment, a substantial proportion of patients with asthma remain uncontrolled even on systemic corticosteroids.2 When more typical causes of uncontrolled asthma, for example, non-adherence to antiasthmatic medication and evident comorbidities as well as obvious precipitating factors, have been considered and appropriately treated, the clinician may speculate, ‘Is it all asthma?’.
In patients with uncontrolled atopic asthma with recurring episodes of symptoms mimicking pneumonia, the suspicion of allergic bronchopulmonary aspergillosis (ABPA) should remain high. ABPA may occur as a consequence of a complex immunological hypersensitivity reaction to colonisation with the mould Aspergillus fumigatus (AF) in the bronchial tree. ABPA is characterised by the presence of atopic asthma, blood eosinophilia, migrating pulmonary opacities and potential bronchiectasis.3 4
Case presentation
A 57-year-old non-smoking woman with osteoporosis was admitted to hospital due to nearly 1 month of continuous productive cough with green-brown sputum plugs and a temperature of 39.5°C. Owing to a previously transient dry cough she had been diagnosed with airway hyper-responsiveness, consistent with intermittent asthma on the basis of a positive methacholine bronchoprovocation test with a provocation concentration causing a 20% fall in forced expiratory volume in 1 s (FEV1; PC20) of 4.49 mg/mL. At the time of diagnosis, she was largely asymptomatic, had a normal spirometry with an FEV1 of 2.62 L corresponding to 102% of predicted value, and was thus assessed as having no obvious requirement for controller therapy with inhaled corticosteroids (ICS). Now she described persistent dyspnoea on exertion and night cough between the fever episodes. Neither previous travel activity nor exposure to tuberculosis, air conditioning or birds was identified. However, 1 year earlier she and her colleagues were relocated to new office facilities because of mould findings in the previous workplace. A chest X-ray (CXR) performed on hospital admission showed a pulmonary opacity with subsegmental atelectasis of the middle lobe, which in combination with the clinical presentation and increased inflammatory biochemical markers, was interpreted as pneumonia. Empirical intravenous penicillin was initiated and after 2 days altered to oral treatment, and she was discharged as a consequence of clinical improvement. The sputum and blood cultures subsequently showed no growth of bacteria or fungi.
Investigations
A CXR performed 6 weeks after discharge revealed unchanged middle lobe opacity. Like the CXR, the radiological findings of a supplemental chest CT still suggested ongoing infection, rather than suspicion of malignancy. In spite of antibiotic treatment for 2 weeks the night cough, sputum production and dyspnoea deteriorated. Besides a mild blood eosinophilia of 1.18×109 cells/mL and a total IgE of 892 IU/mL, other biochemical markers were normal. A diagnostic bronchoscopy revealed an inflamed bronchial mucosa in the middle lobe without any other macroscopic pathological findings. Cytological (bronchoalveolar lavage and cytology brush) and microbiological (bacterial culture and PCR tests for Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia psittaci, C. pneumoniae, Pneumocystis jirovicii, respiratory viruses and Aspergillus galactomannan antigen) tests of bronchial lavage were all normal or negative. As the clinical symptoms and radiological findings remitted during a further few weeks, the overall clinical picture was then interpreted as successfully treated pneumonia (figure 1A, B (CXR before/after)), and no additional follow-up was planned in the outpatient clinic. Instead the patient was advocated to consult her general practitioner if symptoms relapsed. The patient stayed stable for 12 months, however, hereafter the patient once again was referred to the outpatient clinic with completely matching symptoms as well as similar CXR findings as earlier, the only exception being a positive growth of AF in the sputum culture. Biochemical tests at this time showed a mild eosinophilia of 0.80×109 cells/mL, positive AF-specific IgE of 49 IU/mL and a total IgE of 1011 IU/mL. AF-specific IgG was normal. ABPA was suspected as a tentative diagnosis, and a high-resolution CT (HRCT) was performed which uncovered bilateral central bronchiectasis and pathognomonic ‘toothpaste shadows’ and ‘finger-in-glove’ opacities in upper lobe segments, representing mucoid impaction in inflamed and thickened bronchi (figure 2A, B).
Figure 1.
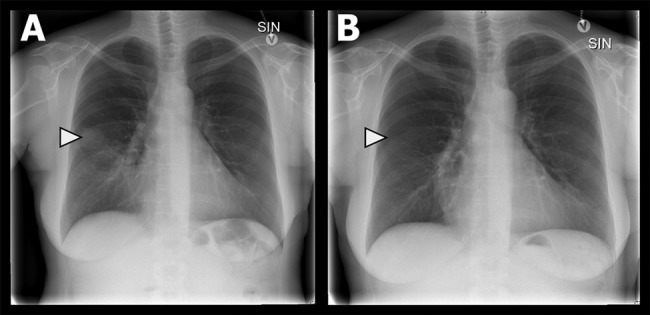
(A and B) Chest X-ray on admission in posteroanterior projection with a pulmonary opacity and subsegmental atelectasis in the apical part of the middle lobe (A) with remission 2 months later (B). Courtesy of Department of Radiology, Odense University Hospital.
Figure 2.
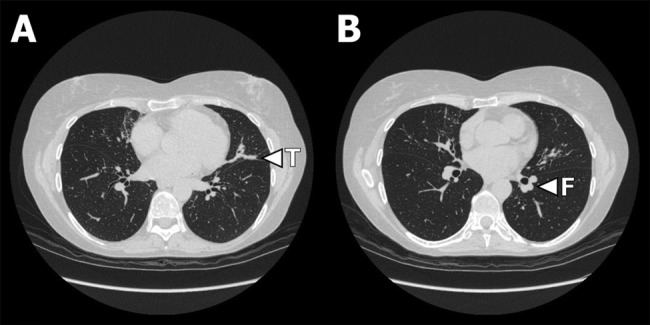
(A and B) High-resolution CT showing bilateral central bronchiectasis and patonogmonic ‘toothpate shadows’ (T in picture A) and ‘finger-in-glove’ (F in picture B) opacities in middle lobe and lingual segments representing mucus impaction in inflamed and thickened bronchi. Courtesy of Department of Radiology, Odense University Hospital.
Differential diagnosis
During the course of the disease differential diagnoses such as infectious and eosinophilic pneumonia, allergic asthma and pulmonary aspergillosis were considered. However, the composite findings of asthma, migrating pulmonary opacities on imaging studies, blood eosinophilia, elevated serum total IgE above 1000 IU/mL, positive sputum culture for AF, central bronchiectasis on HRCT and positive AF-specific IgE fulfilled the conditions for diagnosing the patient with classic ABPA according to the Rosenberg-Patterson criteria.5 6
Treatment
When the ABPA diagnosis was considered likely, treatment with prednisolone and itraconazole was initiated, with a treatment duration of 4 months. In addition, low-dose ICS was prescribed in order to obtain full asthma control.
Outcome and follow-up
At clinical follow-up after fulfilling the treatment period the patient stated full remission of cough and sputum production. She still had a normal spirometry, but with an observed decline in FEV1 to 2.33 L (92% of predicted) during nearly 18 months. Consistent with the improved clinical presentation, the total IgE was reduced by almost 70% to 331 IU/mL (figure 3). Follow-up was performed every second month during the first year after treatment cessation with no relapse of ABPA or asthma symptoms and with total IgE being stable at around 300 IU/mL.
Figure 3.
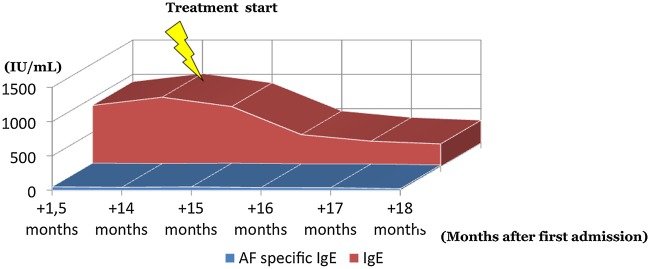
Course of total IgE and Aspergillus fumigatus-specific IgE after initiation of treatment with prednisolone and itraconazole.
Discussion
The ubiquitous AF is one of the several human pathogen moulds, mainly occurring in decomposing organic material, and giving rise to different pulmonary manifestations including allergic hypersensitivity reactions (ABPA, hypersensitivity pneumonitis), colonisation in structural damaged lung tissue (aspergilloma, chronic necrotising/cavitating aspergillosis) and potential invasion in immunoincompetent patients (invasive pulmonary aspergillosis).3
The prevalence of ABPA is estimated to be 2%, but higher and around 7–15% among patients with asthma with severe uncontrolled disease with a daily requirement of systemic corticosteroid, and in AF colonised patients with cystic fibrosis (CF).3 4
In healthy individuals inhalation of conidia, the non-motile spores of a fungus, from AF is eliminated by secretory IgA in bronchial fluid or IgG in the blood. Among patients with asthma and CF, inhaled AF conidia can induce colonisation with AF in the bronchial system, resulting in an immunological and inflammatory reaction of bronchi and surrounding lung parenchyma. The immunological reaction is suitable to types I and III hypersensitivity reactions inducing IgE, IgG and IgA production, while the inflammatory reaction is due to Th2 CD4 T-cell mediated activation of primarily eosinophils. This bronchial eosinophilic inflammation can initiate the clinical, radiological and biochemical characteristics of ABPA: consolidation and mucus plugging, intrabronchial obstruction with potential asthmatic symptoms, bronchiectasis, thickening of the bronchial wall and pulmonary fibrosis. ABPA can be classified into five stages ((1) acute ABPA, (2) remission of ABPA, (3) exacerbation in ABPA, (4) corticosteroid-dependent ABPA and (5) end-stage/fibrotic ABPA according to its clinical and radiological presentation, which, however, do not necessarily occur in a continuum. Knowledge of these disease stages is important in order to decide the future treatment strategy.3 5 6
Treatment of ABPA aims to avoid development of bronchiectasis and fibrosis and comprises a combination of systemic corticosteroids and antifungal agents to suppress immunological activity and to reduce persistent colonisation. First-choice combination therapy is prednisolone and itraconazole. Two randomised clinical trials have shown beneficial effects of combining 400 mg of itraconazole with prednisolone daily compared with placebo and prednisolone with respect to fewer exacerbations, reduced sputum production, reduced total IgE, improved FEV1 and partial or total remission of radiological findings.7 8 Generally, prednisolone is administered in a dose of 0.5–1 mg/kg daily for 2 weeks with slow phasing out and possibly adjusted according to ABPA stage. The exact duration of treatment for ABPA has not been established with certainty, but most trials have been treated for 4–6 months.
As serum IgE acts as a surrogate marker of disease activity, IgE should be frequently monitored after treatment initiation to evaluate treatment response. The overall objective of therapy is achievement of a stable total IgE matching a value corresponding to 35–50% of the value at the time of diagnosis, remission of radiological findings and clinical improvement.3
In conclusion, our case report illustrates a typical case of ABPA including the common delay in diagnosis, in which a preceding mould exposure with AF presumably induced a hypersensitivity reaction aggravating an existing, but otherwise well controlled, intermittent asthma, including findings imitating pneumonia. As the prevalence of ABPA increases with the severity of asthma, the clinician should pay increased attention to ABPA and test for this in patients with uncontrolled asthma with an ongoing requirement of systemic corticosteroids and/or antibiotics and with pulmonary opacities on chest imaging. Although ABPA can be challenging to diagnose, it is of major importance, as early initiated antiasthmatic as well as immunosuppressive and antifungal treatment is required to achieve asthma control, and to prevent development of permanent lung parenchymal damage.
Learning points.
Allergic bronchopulmonary aspergillosis (ABPA) should be suspected in patients with uncontrolled asthma and pulmonary opacities.
ABPA is the result of a complex hypersensitivity reaction to exposure and colonisation in the bronchial tree with conidia from the mould Aspergillus fumigatus.
ABPA treatment consists of immunosuppression in combination with antifungal treatment for a period of around 4–6 months with first choice being prednisolone and itraconazole.
ABPA treatment response should be frequently monitored by total serum IgE which acts as a surrogate marker of ABPA disease activity, and should be reduced by 35–50% compared with the index value at the time of diagnosis.
ABPA clinical and biochemical follow-up is advocated every 2–3 months until 2 years after diagnosis and for treatment initiation.
Footnotes
Contributors: JRD, PHM and CBL contributed to the conception and design, acquisition of the data, analysis and interpretation of the data. They drafted the submitted article and approved the final version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med 2004;10:44–50 [DOI] [PubMed] [Google Scholar]
- 2.Cazzoletti L, Marcon A, Janson C, et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol 2007;120:1360–7 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest 2009;135:805–26 [DOI] [PubMed] [Google Scholar]
- 4.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011;20:156–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977;86:405–14 [DOI] [PubMed] [Google Scholar]
- 6.Greenberger PA, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy 1986;56:444–8 [PubMed] [Google Scholar]
- 7.Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000;342:756–62 [DOI] [PubMed] [Google Scholar]
- 8.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol 2003;111:952–7 [DOI] [PubMed] [Google Scholar]


