Abstract
This case report describes two cases of dilated cardiomyopathy due to hypocalcaemia as a result of hypoparathyroidism. Patient A suffered from dilated cardiomyopathy due to secondary hypoparathyroidism as a result of previous neck surgery. Patient B suffered from dilated cardiomyopathy with congestive heart failure due to primary hypoparathyroidism. Hypoparathyroidism can exist for years before being recognised, especially after neck surgery. Besides standard treatment of heart failure, restoration of serum calcium levels with calcium and vitamin D supplementation can lead to rapid improvement of cardiac function and should be continued lifelong. Both patients were responding very well to heart failure therapy and calcium supplementation as ejection fraction improved after restoration of plasma calcium levels. This case report emphasises that hypocalcaemia should be in the differential diagnosis of heart failure.
Background
Hypocalcaemia induced by hypoparathyroidism is a rare cause of reversible dilated cardiomyopathy. Extracellular calcium is required for myocardial contraction including in the myocardium. As a result, profound and prolonged hypocalcaemia provokes decreased myocardial contractility and heart failure.
Case presentation
Patient A, a 68-year-old woman was seen at the emergency department with intermittent dyspnoea. She was treated with calcium supplementation after a recent subtotal thyroidectomy. On physical examination, she was tachycardic and tachypnoeic. She did not report muscle spasms. Chest radiography showed normal heart size and bilateral pleural effusion which appeared to be a transudate after aspiration. Blood tests showed high N-terminal probrain natriuretic peptide (NT-proBNP; 7699 ng/L), low sodium (129 mmol/L), slightly low potassium (3.4 mmol/L) and hypocalcaemia (1.15 mmol/L) with normoalbuminaemia (39 g/L). The serum phosphate was high (2.89 mmol/L) and the parathyroid hormone (PTH) level low (<0.5 pmol/L). 25-Mono-hydroxy-vitamin D (52 nmol/L) and thyroid-stimulating hormone (TSH) levels (1.52 mU/L) were normal. CT scan showed lytical lesions of several vertebrae, with absence of M-protein in serum. A bone scan showed some degenerative lesions in the lumbar spine and both hip regions. Transthoracic echocardiography (TTE) showed severe left ventricular dysfunction (ejection fraction (EF) 15%), with moderate mitral and tricuspid regurgitation (figure 1). The estimated pulmonary arterial pressure was 55 mm Hg.
Figure 1.
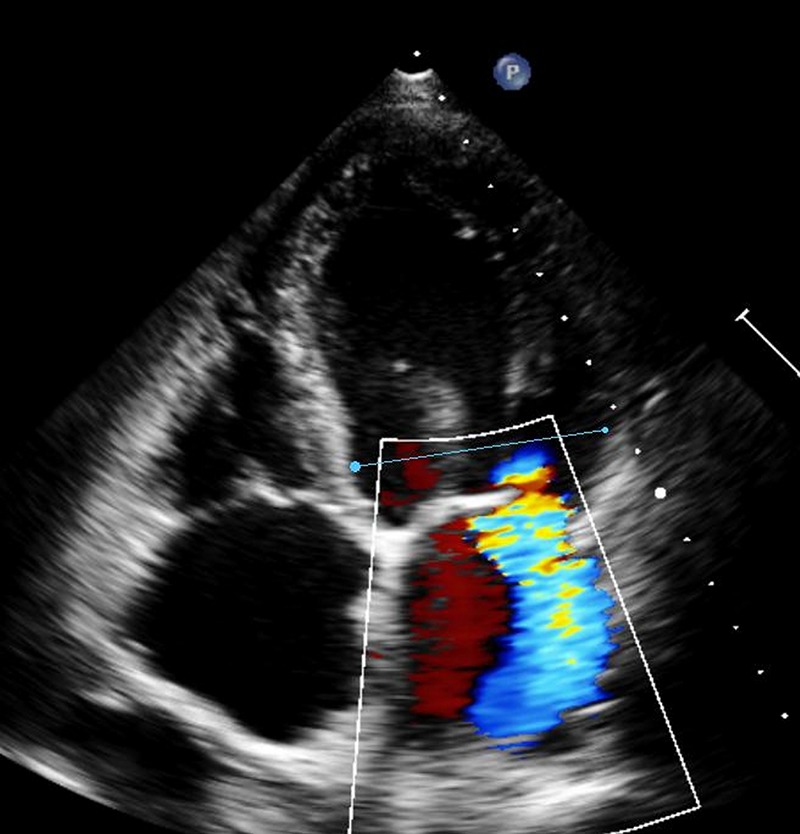
Four-chamber view of echocardiography of patient A showing severely dilated left ventricle with severe mitral insufficiency. Ejection fraction 15%.
Patient B, a 59-year-old woman was seen at the cardiology outpatient clinic because of progressive exertional dyspnoea and ankle oedema. On her first visit, the only abnormal finding on physical examination was a systolic murmur. Her blood tests showed no abnormalities except a severely elevated NT-proBNP level of 16 046 ng/L. The ECG was normal. TTE showed ventricular dilation with an estimated EF of 39%, moderate mitral and severe tricuspid regurgitation (figure 2). On her second visit to the outpatient clinic she developed a seizure. CT of the brain showed extensive calcifications in the basal ganglia (figure 3). There were no signs of cerebral bleeding or ischaemia. The ECG showed sinus rhythm with leftward axis, prolonged QTc interval of 533 ms and some ST depression in V4–V6. Laboratory tests showed low plasma calcium (1.24 mmol/L) with normoalbuminaemia (35.9 g/L). The magnesium level was decreased (0.62 mmol/L) and the phosphate level was high (2.5 mmol/L). The PTH level was undetectable (<0.6 pmol/L) with normal 25-mono-hydroxy-vitamin D status (75 nmol/L). Further history taking revealed that the patient suffered from progressive muscle spasms and constipation since a few weeks. She was diagnosed with hypocalcaemia resulting from primary hypoparathyroidism. The hypocalcaemia was considered to be the cause of the seizure, since other causes were excluded. Her dual-energy X-ray absorptiometry scan showed osteopenia.
Figure 2.
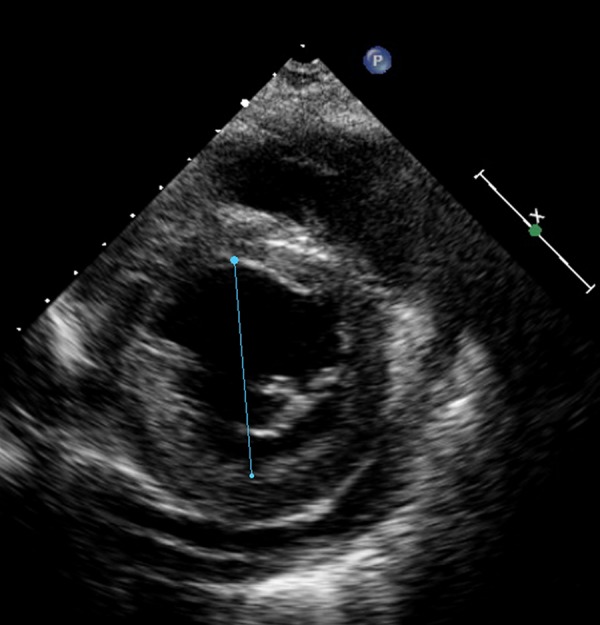
Short axis view of echocardiography of patient B showing minor dilated hypertrophic left ventricle with moderate pericardial effusion. Ejection fraction 39%.
Figure 3.
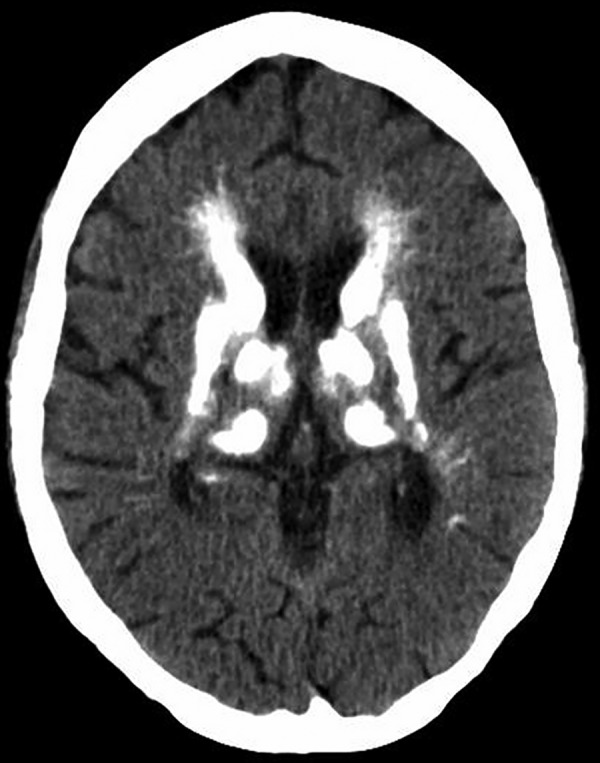
CT of the brain of patient B: calcifications in the basal ganglia.
Differential diagnosis
Summarised, patient A suffered from dilated cardiomyopathy due to secondary hypoparathyroidism as a result of previous neck surgery. Additional laboratory tests in patient B showed a negative HIV test, negative antinuclear antibody, normal TSH and no antibodies against calcium-sensing receptor (6.81, reference ratio <10). Therefore patient B suffered from dilated cardiomyopathy with congestive heart failure due to primary hypoparathyroidism.
Treatment
Treatment of hypoparathyroidism consists of supplementation of calcium and 1-hydroxylated-vitamin D3 (calcitriol), as PTH deficiency and hyperphosphatemia cause decreased 1-α-hydroxylase activity in the kidney.
In patient A, treatment was initiated with a β-blocker, an ACE inhibitor, spironolactone and supplementation of calcium and vitamin D. The plasma calcium and other electrolyte levels returned to normal values within 2 weeks of initiation of therapy. Chest radiography showed complete resolution of pleural effusion. EF improved to 35% after 3 weeks of treatment and up to 50% after 1 year (figure 4).
Figure 4.
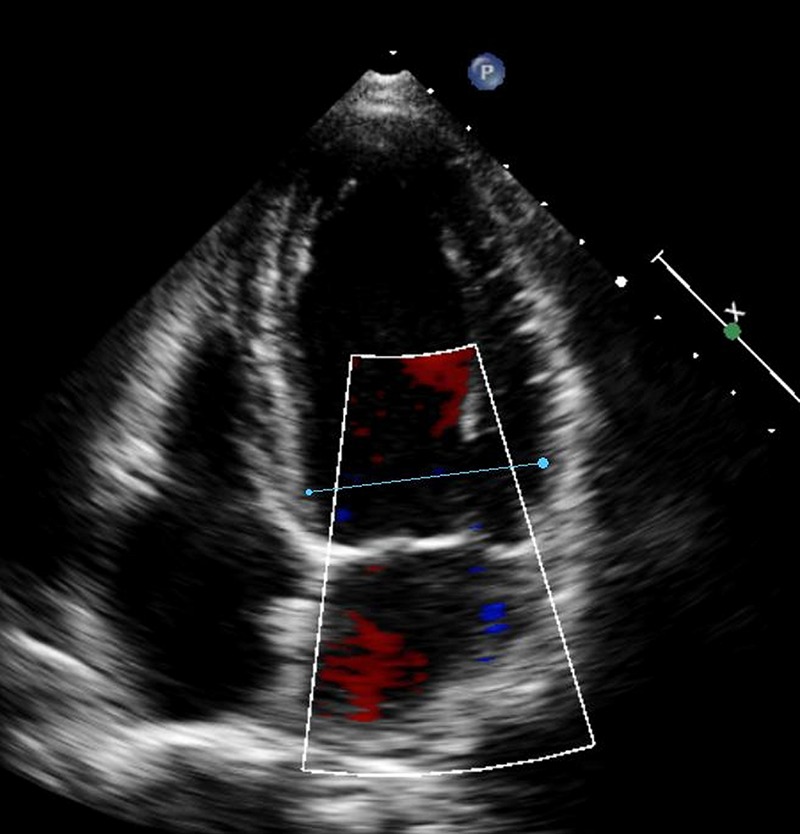
After treatment, reduction in left ventricle end diastolic diameter and absence of mitral regurgitation in patient A. Ejection fraction 50%.
Patient B was treated with vitamin D and intravenous and oral calcium supplementation, resulting in normalisation of the plasma calcium level, and with β-blocker (metoprolol) and ACE inhibitor. A week after normalisation of the calcium level, TTE showed significant improvement of the left ventricular function (figure 5).
Figure 5.
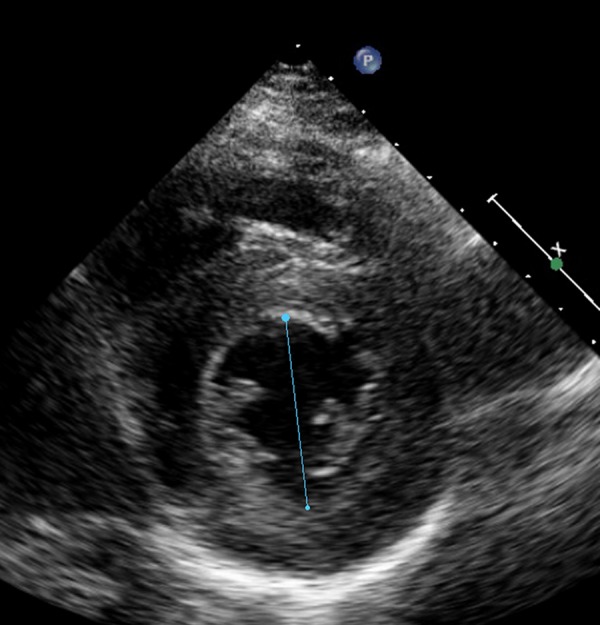
After treatment, reduction in left ventricular end-diastolic dimension with no pericardial effusion in patient B. Ejection fraction 70%.
Outcome and follow-up
Both patients were responding very well to heart failure therapy and calcium supplementation as EF improved after restoration of plasma calcium levels.
In patient A EF improved to 35% after 3 weeks of treatment and up to 50% after 1 year (figure 4).
In patient B on follow-up 5 months later, TTE showed normalisation of the EF and no tricuspid regurgitation. The cerebral calcifications had not changed on a repeat CT scan 6 months later.
Treatment should be continued lifelong.
Discussion
Hypocalcaemia induced by hypoparathyroidism is a rare cause of reversible dilated cardiomyopathy. It has only been described in very few case reports.1–13
Cardiac contractility is mainly influenced by calcium levels in extracellular fluid to initiate excitation and contraction of cardiac muscle fibres.2 11 Calcium plays a central role as initiator of electric activation and ion channel gating since increase of free calcium level in the cytoplasm is the trigger for myocardial contraction.2 10 The sarcoplasmic reticulum (SR) is an intracellular storage pool of calcium ions, but it cannot store enough calcium ions to start the contraction itself. Therefore, extracellular influx of calcium ions is essential.2 8 Influx of calcium from the SR starts muscle contraction as it binds to the troponin C-tropomyosin complex allowing actin and myosin to cross-link.8 The contraction ends when calcium runs back into the SR and a small amount flows back to the interstitium. Calcium ions are also important in regulation of the magnitude of the contraction. This process is dependent on the maximum contractility of the myofilaments and this in turn depends on the available amount of extracellular calcium ions.1 Furthermore, calcium is involved in cardiac epinephrine-induced glycogenolysis and calcium can increase the expression of some excitation/contraction coupling proteins.10 Hypocalcaemia increases renal sodium reabsorption in the tubular cells as well, which may result in fluid retention as a contributing factor to the development of pleural effusion.12 Finally, hypocalcaemia can result in arrhythmias, a prolonged QT interval and even torsades de pointes leading to ventricle tachycardia and death.1 3–4 6 9–10
PTH is the main regulator of calcium homoeostasis. PTH activates osteoclasts in the bone and stimulates calcium uptake in the intestine. Furthermore, calcium is reabsorbed and active vitamin D formed under influence of PTH in the kidney.5 Moreover, PTH itself has positive inotropic effects on the heart. PTH releases endogenous myocardial norepinephrine which has a positive inotropic effect through β-adrenergic stimulation increasing myocardial contractility.7 It is not likely that PTH stimulates β-adrenergic receptors directly, as PTH does not stimulate myocardial adenylate cyclase activity. PTH action on the myocardium is also influenced by promoting the calcium influx across the plasma membranes into myocardial cells.14 Hypocalcaemia resulting from hypoparathyroidism may be caused by neck surgery, radiation therapy or malignancy.5 After surgery of the neck, symptomatic hypoparathyroidism occurs mainly within the first weeks, but has been described to evolve even after several years.4 Other known causes of hypoparathyroidism are autoimmune conditions and HIV infection among others.10 The primary symptoms of hypoparathyroidism are tetany, epileptic seizures, cognitive and psychiatric disorders such as hallucinations, delirium, anxiety disorder, paranoidism, apathy, dementia and mental retardation.4 Bilateral calcifications of the brain mainly the basal ganglia, may be seen on CT scan.3 15 These calcifications in the basal ganglia, as seen in patient B, are thought to be caused by complexes of calcium and acidic mucopolysaccharides in the small blood vessels.16 Most times, these deposits are asymptomatic, but may lead to (extra) pyramidal or cerebellar symptoms. Treatment of hypoparathyroidism consists of supplementation of calcium and 1-hydroxylated-vitamin D3 (calcitriol) as PTH deficiency and hyperphosphatemia cause a decreased 1-α-hydroxylase activity in the kidney.
Learning points.
Hypoparathyroidism-induced hypocalcaemia is a rare cause of severe but reversible dilated cardiomyopathy.
Hypoparathyroidism can exist for years before being recognised, especially after neck surgery.
Restoration of serum calcium levels besides standard treatment of heart failure can lead to rapid improvement of cardiac function, therefore hypocalcaemia should be in the differential diagnosis of heart failure.
Supplementation of calcium and vitamin D should be continued lifelong in case of hypocalcaemia due to hypoparathyroidism.
Footnotes
Contributors: MV carried out the data collection and drafted the manuscript. MdJ coordinated and carried out the data collection and drafted the manuscript. PdR helped to draft the manuscript. RT carried out data collection and helped to draft the manuscript. All the authors have read and approved the final manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Connor TB, Rosen BL, Blaustein MP, et al. Hypocalcemia precipitating congestive heart failure. N Engl J Med 1982;307:869–72 [DOI] [PubMed] [Google Scholar]
- 2.Rimailho A, Bouchard P, Schaison G, et al. Improvement of hypocalcemic cardiomyopathy by correction of serum calcium level. Am Heart J 1985;109:611–13 [DOI] [PubMed] [Google Scholar]
- 3.Bruyn RP, Hazenberg GJ, Lips PT. Seizure or tetany? Ned Tijdschr Geneeskd 1987;131:1249–51 [PubMed] [Google Scholar]
- 4.Bolk J, Ruiter JH, van Geelen JA. Hypocalcemia as a cause of reversible heart failure. Ned Tijdschr Geneeskd 2000;144:900–3 [PubMed] [Google Scholar]
- 5.Stevens M, Deinum J, Willems MH. Clinical thinking and decision making in practice. A young women with muscle cramps. Ned Tijdschr Geneeskd 2001;145:818–21 [PubMed] [Google Scholar]
- 6.Fisher NG, Armitage A, McGonigle RJ, et al. Hypocalcaemic cardiomyopathy; the relationship between myocardial damage, left ventricular function, calcium and ECG changes in a patient with idiopathic hypocalcemia. Eur J Heart Fail 2001;3:373–6 [DOI] [PubMed] [Google Scholar]
- 7.Avsar A, Dogan A, Tavli T. A rare cause of reversible dilated cardiomyopathy: hypocalcemia. Echocardiography 2004;21:609–12 [DOI] [PubMed] [Google Scholar]
- 8.Tziomalos K, Kakavas N, Kountana E, et al. Reversible dilated hypocalcaemic cardiomyopathy in a patient with primary hypoparathyroidism. Clin Endocrinol 2006;64:717–18 [DOI] [PubMed] [Google Scholar]
- 9.Chavan CB, Sharada K, Rao HB, et al. Hypocalcemia as a cause of reversible cardiomyopathy with ventricular tachycardia. Ann Intern Med 2007;146:541–2 [DOI] [PubMed] [Google Scholar]
- 10.Mavroudis K, Aloumanis K, Stamatis P, et al. Irreversible end-stage heart failure in a young patient due to severe chronic hypocalcemia associated with primary hypoparathyroidism and celiac disease. Clin Cardiol 2010;33:72–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jariwala PV, Sudarshan B, Aditya MS, et al. Hypoparathyroidism, a cause of reversible dilated cardiomyopathy . J Assoc Physicians India 2010;58:500–2 [PubMed] [Google Scholar]
- 12.Lekas P, Goldenstein PT, Bargman JM. Myocardial dysfunction and pulmonary edema post parathyroidectomy: the role of hypocalcemia. Adv Perit Dial 2010;26:125–9 [PubMed] [Google Scholar]
- 13.de Jong MFC, Soetekouw R, de Ronde W, et al. Hypoparathyroidism: a rare cause of severe but reversible cardiomyopathy [abstract]. Abstractbook Internistendagen 2012;2012;59–60 [Google Scholar]
- 14.Katoh Y, Klein KL, Kaplan RA, et al. Parathyroid hormone has a positive inotropic action in the rat. Endocrinology 1981;109:2252–4 [DOI] [PubMed] [Google Scholar]
- 15.Sachs C, Sjöberg HE, Ericson K. Basal ganglia calcifications on CT: relation to hypoparathyroidism. Neurology 1982;32:779–82 [DOI] [PubMed] [Google Scholar]
- 16.Goswami R, Sharma R, Sreenivas V, et al. Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol 2012;77:200–6 [DOI] [PubMed] [Google Scholar]


