Abstract
Adoptive immunotherapy of tumors with T cells specific for the cancer-testis antigen NY-ESO-1 has shown great promise in preclinical models and in early stage clinical trials. Tumor persistence or recurrence after NY-ESO-1-specific therapy occurs, however, and the mechanisms of recurrence remain poorly defined. In a murine xenograft model of NY-ESO-1+ multiple myeloma, we observed tumor recurrence after adoptive transfer of CD8+ T cells genetically redirected to the prototypic NY-ESO-1157-165 peptide presented by HLA-A*02:01. Analysis of the myeloma cells that had escaped from T cell control revealed intact expression of NY-ESO-1 and B2M, but selective, complete loss of HLA-A*02:01 expression from the cell surface. Loss of heterozygosity in the Major Histocompatibility Complex (MHC) involving the HLA-A locus was identified in the tumor cells, and further analysis revealed selective loss of the allele encoding HLA-A*02:01. Although loss of heterozygosity involving the MHC has not been described in myeloma patients with persistent or recurrent disease after immune therapies such as allogeneic hematopoietic cell transplantation (HCT), it has been described in patients with acute myelogenous leukemia who relapsed after allogeneic HCT. These results suggest that MHC loss should be evaluated in patients with myeloma and other cancers who relapse after adoptive NY-ESO-1-specific T cell therapy.
Keywords: NY-ESO-1, adoptive immunotherapy, immune escape
Introduction
Adoptive transfer of T cells whose specificity has been genetically redirected to tumor-associated or -specific antigens is an increasingly feasible and effective therapeutic option for several cancers.1-9 Redirection to tumor targets is achieved through transduction with vectors encoding T cell receptors (TCRs) -specific for tumor peptides presented by MHC molecules10-15- or chimeric antigen receptors (CARs).16, 17 This genetic modification can overcome central and peripheral tolerance, and enable the production of autologous products containing large numbers of highly potent tumor-reactive T cells from most patients, even those who harbor few, if any, natively tumor-reactive T cells in their blood.8, 10-15, 18-21
Multiple myeloma (MM) is a suitable malignancy in which to evaluate adoptive therapy with T cells redirected toward cancer-testis (C-T) antigens such as NY-ESO-1. Evidence demonstrates that NY-ESO-1 and other C-T antigens are often expressed in the tumor cells of patients with advanced MM22-26. Primary MM cells from most patients also demonstrate preserved expression of MHC class I molecules, making myeloma particularly suitable for targeting with myelomareactive TCRs27-29. These observations have prompted both preclinical studies21 and clinical trials3, 30 of adoptive therapy for myeloma with autologous T cells transduced with NY-ESO-1-specific TCRs or CARs. Most of these studies targeted the NY-ESO-1157-165 peptide presented by HLA-A*02:01. An affinity-enhanced variant of the 1G4 TCR specific for NY-ESO-1157-165/HLA-A*02:01 demonstrated significant activity in pre-clinical studies,31 and a phase I/II clinical trial in patients with advanced melanoma and synovial sarcoma confirmed the safety and efficacy of adoptive therapy with autologous T cells transduced with this TCR.2
Alternative strategies for targeting the NY-ESO-1157-165/HLA-A*02:01 complex are being explored. A bispecific T cell-engaging molecule that couples a high-affinity, soluble variant of the 1G4 TCR to a single chain, human CD3-specific antibody variable fragment demonstrated in vitro activity against NY-ESO-1+/HLA-A*02:01+ tumor cell lines, but had limited in vivo efficacy in a subcutaneous xenograft model of NY-ESO-1+ bladder carcinoma.32 A CAR recognizing the NY-ESO-1157-165/HLA-A*02:01 peptide complex has also been constructed, and T cells from healthy donors that expressed this CAR showed significant in vivo antitumor activity in a murine xenograft model of human MM.21
Despite encouraging results from studies evaluating NY-ESO-1157-165/HLA-A*02:01-specific therapy, persistence or recurrence of disease has consistently been observed in a subset of subjects. Potential mechanisms of tumor escape include: poor persistence of adoptively transferred T cells; loss of expression of NY-ESO-1, MHC class I, or both in myeloma cells; inability of T cells to penetrate into the tumor micro-environment, and post-infusion inhibition of T cell function by suppressor cells or cytokines in the tumor microenvironment, among others. We observed recurrence of myeloma in a murine xenograft model after adoptive therapy with NY-ESO-1157-165/HLA-A*02:01-specific T cells, and describe our evaluation of the mechanism of tumor escape in this model.
Results
Transduction of MM patient lymphocytes with 1G4 α95:LY TCR
T cells from G-CSF-mobilized leukapheresis products from HLA-A*02:01+ MM patients were transduced with a retrovirus encoding the affinity-enhanced α95:LY variant of the 1G4 NY-ESO-1157-165-specific, HLA-A*02:01-restricted TCR.31 TCR-transduced cells were identified using a NY-ESO-1157-165/HLA-A*02:01-specific tetramer (Figure 1A). Flow-sorted CD8+tetramer+ and CD8+tetramer− cells were tested for recognition of target cells that expressed the NY-ESO-1157-165 peptide, HLA-A*02:01, both, or neither. Only CD8+tetramer+ cells demonstrated significant cytotoxicity against target cells that expressed both NY-ESO-1157-165 peptide and HLA-A*02:01 (Figure 1B).
Figure 1. CD8+ TCR-transduced cells are specifically cytolytic against NY-ESO-1+, HLA-A*02:01+ target cells.
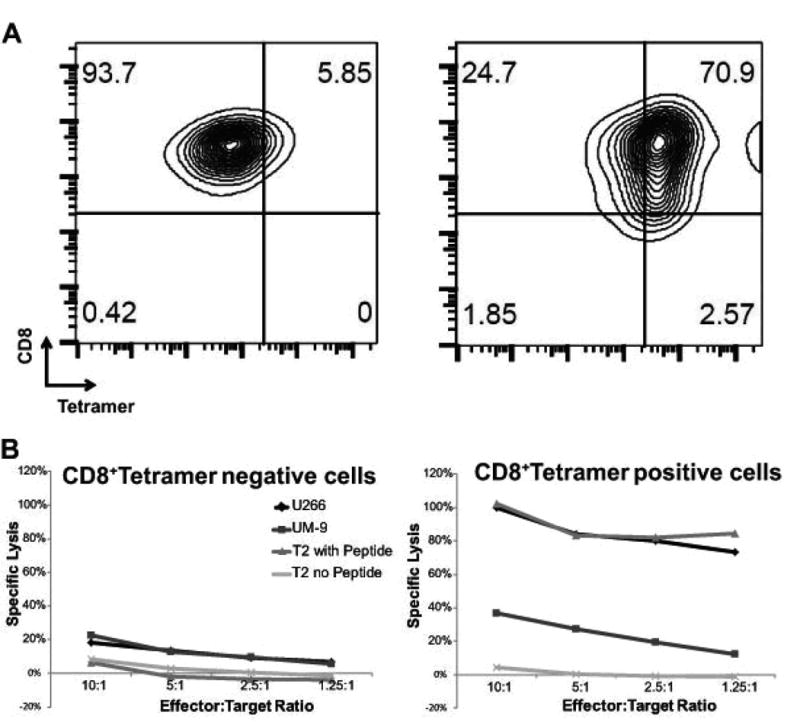
(A) Flow cytometric analysis of CD8+tetramer-negative (left panel) and CD8+tetramer positive (right panel) cells. (B) Cytolytic activity of CD8+tetramer-negative (left) and CD8+tetramer positive (right) T-cells against targets cells that expressed NY-ESO-1 only (UM-9), NY-ESO-1 and HLA-A*02:01 (U266 cells; T2 cells pulsed with NY-ESO-1157-165 peptide), or neither (T2 without peptide).
Adoptive transfer of NY-ESO-1-specific T cells improves survival of myeloma-bearing mice
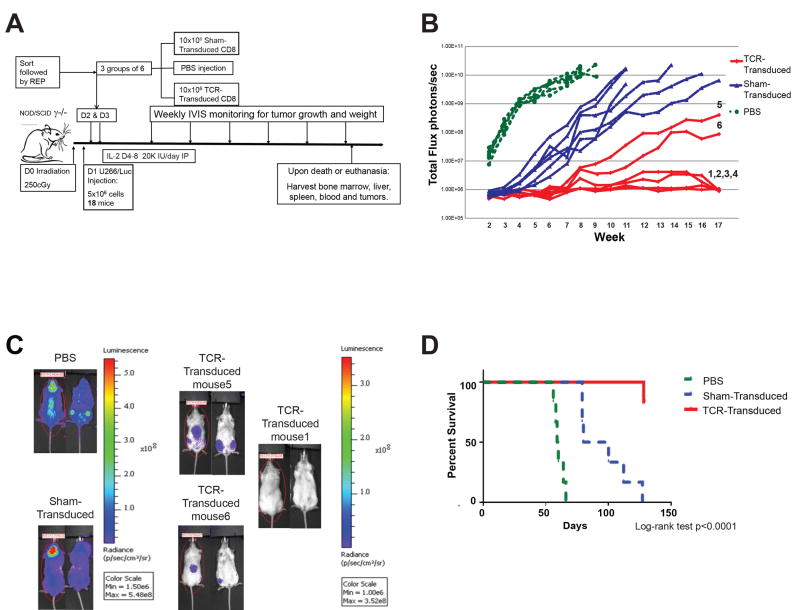
The in vivo activity of sorted CD8+tetramer+ 1G4 α95:LY TCR-transduced T cells (termed TCR-transduced T cells) derived from a HLA-A*02:01+ MM patient was assessed in an immune-deficient mouse xenograft model of disseminated human MM (Figure 2A). Eighteen mice were sub-lethally irradiated one day prior to tail-vein injection of luciferase-transduced U266 (termed U266/Luc) human MM cells, which uniformly express CD138, NY-ESO-1, and HLA-A2 (Figure 3A, B). Subsequently, mice were divided into three cohorts to receive two daily injections of phosphate-buffered saline (PBS), 1×107 sham-transduced T cells or 1×107 TCR-transduced T cells.
Figure 2. Adoptive transfer of CD8+ TCR-transduced T cells can prevent the development of progressive MM.
(A) Experimental schema. (B) Non-invasive bioluminescence monitoring of MM growth in mice that received PBS but no T cells (light gray circles); sham-transduced T cells (triangles), or affinity-enhanced NY-ESO-1-specific TCR-transduced T cells (black rhomboids). (C) Bioluminescence images of selected mice from the PBS, sham-transduced, and TCR-transduced T cell treatment groups, obtained just prior to euthanasia. (D). Kaplan-Meier survival analysis of survival of mice that received PBS, sham-transduced, or TCR-transduced T cells.
Figure 3. Evaluation of mice that escaped NY-ESO-1-specific T cell therapy.
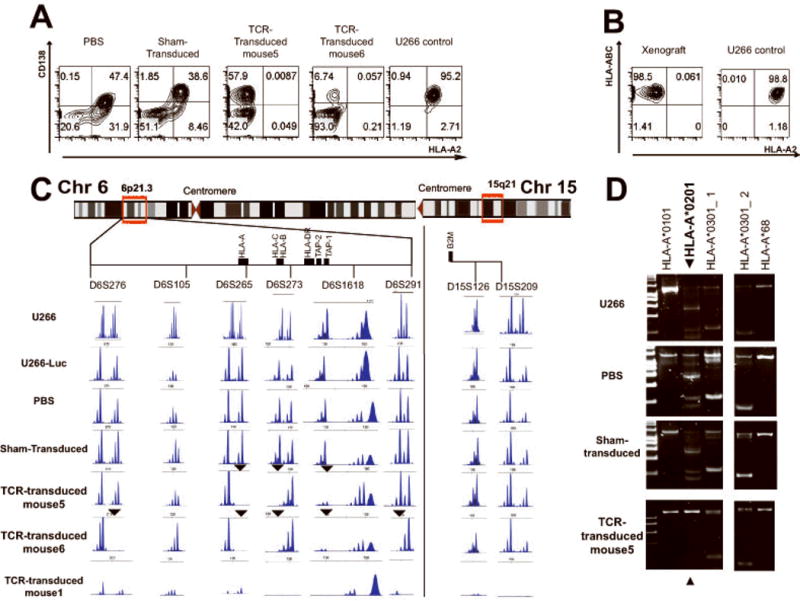
(A) Representative flow cytometric analysis with HLA-A2- and human CD138-specific antibodies of cells harvested from the bone marrow from mice in the indicated treatment groups, compared to control U266 cells maintained in culture. (B) Human MHC class I (HLA-ABC) and HLA-A2 expression in control U266 cells and in CD138+ bone marrow cells from mouse 5 in the TCR-transduced cohort. (C). Loss of heterozygosity analysis with the indicated STR probes mapping to chromosomes 6p (left) or 15q (right). Analysis of genotypes at six STR loci mapping to the region of the MHC on chromosome 6p21.3 and two STR loci on chromosome 15q in CD138+ cells from the indicated sources. The relative location of STRs and genes of interest was constructed based on map locations (human genome assembly hg19) obtained from the UCSC genome browser (genome.ucsc.edu). Arrowheads indicate loci with evidence of LOH. (D) PCR-based HLA-A typing of control U266 and CD138+ cells from representative mice in each treatment group. Selected lanes from the complete 24-lane analysis are shown. The 575, 565, 525, 205, 195 and 155 bp bands in lane 2 define the HLA-A*02:01 genotype (arrowhead), while the 735, 230 and 225 bp bands in lane 3 (labeled HLA-A*03:01_1) and the 180bp band in lane 19 (labeled HLA-A*03:01_2) define the HLA-A*03:01 genotype.
Mice in the PBS cohort developed detectable MM within two weeks, which thereafter progressed steadily (Figure 2B). All such mice met criteria for euthanasia by week 9. Mice receiving sham-transduced T cells exhibited slower development of myeloma compared with those that received PBS (Figure 2B, and supplemental Figure 1), but nonetheless uniformly developed progressive myeloma and met criteria for euthanasia by day +127 (18 weeks). Of the six mice in the TCR-transduced cohort, four (mice 1-4) had no evidence of MM by either bioluminescence or necropsy evaluation at the end of study (day +128). Two mice in this group (mice 5 and 6) however, had a low burden of MM detected by bioluminescence at the time of their sacrifice on day +128 (Figure 2B, C). Flow cytometric examination of tissues harvested from mice of the PBS and sham-transduced cohorts demonstrated evidence of myeloma cells in both bone marrow and blood, but no significant engraftment in either liver or spleen (data not shown). There was no evidence of U266/Luc cells in mice 1-4 of the TCR-transduced cohort. U266/Luc cells were present in the bone marrow of mice 5 and 6, the two mice that developed myeloma by bioluminescence (Figure 3A).
Kaplan-Meier analysis demonstrated significant improvement of overall survival in the groups that received T cells, with the best survival noted in the cohort that received TCR-transduced T cells (Log-rank test p<0.001; Figure 2D). One of the mice that received TCR-transduced T cells died unexpectedly on day +127, but no evidence of MM was detected by either bioluminescence or post-mortem exam.
Escape from NY-ESO-1-specific T cell therapy via selective loss of HLA-A2 expression
Flow cytometric analysis of the human CD138+ cells recovered from all of the mice in the cohorts that received PBS or sham-transduced T cells demonstrated no significant change in the aggregate level of expression of MHC class I molecules (Figure 3A) compared with the parental U266/Luc cells. In contrast, mice 5 and 6 of the TCR-transduced cohort with evidence of MM, showed complete loss of surface expression of HLA-A2 (Figure 3A, B), which would prevent their recognition by TCR-transduced T cells. Real-time PCR analysis of human CD138+ cells obtained from the bone marrow of all of the mice that developed MM demonstrated comparable expression of NY-ESO-1 and beta-2-microglobulin (B2M) transcripts compared with the parental U266Luc cells (data not shown).
Loss of heterozygosity in the MHC underlying selective loss of HLA-A*02:01 expression
To evaluate whether selective loss of HLA-A2 expression in the myeloma xenografts resistant to NY-ESO-1-specific T cells was associated with changes in the genomic locus containing the HLA-A*02:01 allele, loss of heterozygosity (LOH) analysis was performed. The genotypes of the parental U266 and U266/Luc cells, and the human CD138+ cells recovered from mice in the PBS, sham-transduced, and TCR-transduced cohorts were determined at six short tandem repeat (STR) loci spanning the MHC in the chromosome 6p21.3 region and two loci on chromosome 15 (Figure 3C). The parental U266 and U266/Luc cells, and the myeloma cells recovered from the mice that received either PBS or sham-transduced T cells, have identical genotypes at these six loci, and are heterozygous at five of the six STR loci on chromosome 6p21.3 and one of two loci on chromosome 15. Bone marrow cells from mice without evidence of disease had minimal to no signal (mouse 1 on Figure 3C). In contrast, myeloma cells isolated from mice 5 and 6 of the TCR-transduced cohort had evidence for LOH at several STR loci on chromosome 6p21.3, involving the MHC and the HLA-A locus, but not at the loci on chromosome 15 (Figure 3C). LOH was observed at three loci in myeloma cells from mouse 5 and at five in mouse 6, suggesting that the genomic events occurring in the myeloma cells from the two mice were not identical. Analysis of the STR fragment sizes at the affected loci, however, suggested involvement of the same HLA-A allele in the two cases (Figure 3C). To confirm this conclusion, HLA-A typing by PCR was performed. Although the parental U266 and U266/Luc cells were typed as HLA-A*02/HLA-A*03, the myeloma cells recovered from the two mice were typed as HLA-A*03, demonstrating that the LOH events involved the HLA-A*02 allele in both cases (Figure 3D).
Discussion
Current techniques for redirecting the antigenic specificity of T cells to tumor-associated or -specific antigens via TCR or CAR gene transfer allows for reliable generation of T cell products with potent antitumor activity. Using a vector that encodes an affinity-enhanced TCR specific for NY-ESO-1, we redirected CD8+ T cells from MM patients to recognize the NY-ESO-1157-165 peptide presented by HLA-A*02:01. TCR-transduced cells were specifically cytolytic against NY-ESO-1+HLA-A*02:01+ MM cells, and adoptive transfer of TCR-transduced cells was protective against an otherwise lethal challenge of MM cells in four of six mice. Two mice developed MM despite adoptive therapy with TCR-transduced T cells, and analysis of the tumor cells in these mice revealed selective loss of expression of HLA-A*02:01, associated with LOH in the MHC, as the likely mechanism of immune escape. An intriguing observation is the lag in disease development in mice that received sham-transduced T cells. We hypothesize that this lag was due to weak alloreactivity of the polyclonal CD8+ sham-transduced T cells against U266/Luc.
Other studies of adoptive T cell therapy targeting NY-ESO-1 have likewise demonstrated potent antitumor activity but occasional therapeutic failures.2, 3, 21, 30 A recent preclinical study of adoptive therapy with T cells expressing a CAR specific for the NY-ESO-1157-165/HLA-A*02:01 complex showed in vivo activity against the human U266 MM line, but resistance was observed in some animals.21 The mechanism underlying resistance in this study was not investigated. Adoptive therapy with autologous T cells transduced with the α95:LY variant of the 1G4 TCR used in our study was evaluated in a clinical trial in melanoma and sarcoma patients. Clear antitumor activity was observed in some but not all patients.2 An ongoing trial of a very similar approach in patients with advanced MM has similarly shown objective clinical responses in many patients, but lack of response in others.3, 30
Given abundant evidence that in vivo persistence of adoptively transferred T cells correlates with response, much attention has been focused on the lack of T cell persistence as the main mechanism for treatment failure. However, other potential mechanisms such as antigen loss, inability of T cells to migrate within the tumor microenvironment, and post-infusion inhibition of T cell function are also possible. For example, antigen loss has recently been described in a patient with acute lymphocytic leukemia who received autologous CD19-CAR-transduced T cells.18
MHC class I loss, as demonstrated in our study, is a potential mechanism by which tumors can escape adoptive therapy with CD8+ T cells expressing tumor-reactive TCRs. Loss of MHC class I expression has been described extensively in solid tumors (reviewed in 33) and has been associated with LOH in patients with acute leukemia or high risk myelodysplastic syndrome who relapsed after haploidentical HCT.34, 35 In the latter setting, it is likely that mitotic recombination in the tumor cells led to acquired uniparental disomy for the short arm of chromosome 6 and resultant loss of the non-shared MHC haplotype, thereby allowing the tumor cells to escape from recognition by donor T cells specific for MHC molecules encoded on that haplotype. Published reports on the outcome of haploidentical transplantation in patients with MM are limited,36-38 however, and it is unknown if the same phenomenon can occur in MM cells after haploidentical transplantation. More generally, the extent to which MHC loss can contribute to recurrence of MM after T cell-based immunotherapy – such as donor lymphocyte infusion after allogeneic HCT 39-44 – is also unknown. The observation in our study of escape from NY-ESO-1-directed adoptive T cell therapy via specific loss of the MHC allele recognized by the TCR-transduced cells, however, suggests that immune escape due to MHC loss could also occur in the clinical setting. Although our study is based on a genetically unstable human MM cell line, relapsed MM patients exhibit genetic instability and clonal evolution. Given that adoptive T cell therapy targeting NY-ESO-1 is currently being evaluated in clinical trials,3 close monitoring for MHC class I loss and related mechanisms of immune escape is warranted.
Materials and Methods
Cell culture
The MM cell lines U266 (ATCC, Manassas, VA) and UM-9 (kind gift from Drs. Tuna Mutis and Henk Lokhorst, University of Utrecht, The Netherlands) were cultured in complete medium comprising RPMI-HEPES, 1% penicillin-streptomycin, 1% L-glutamine, and 20% FBS. T2 cells were cultured in complete medium with 10% FBS. U266 was transduced with a retrovirus (kind gift from Dr. Elizabeth Budde, FHCRC), comprising a MMLV backbone with firefly luciferase, a neomycin resistance gene, and THY1.1 as a reporter. The retrovirus was produced in the packaging cell line Phoenix G. Transduced cells were subsequently selected in 800 μg/mL geneticin (Sigma-Aldrich, St. Louis, MO) to produce the U266-Luc cell line. All PBMC and T cells were cultured in RPMI 1640 medium supplemented with 1% penicillin-streptomycin, 10 mM L-glutamine, 50 μM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), and 10% heat-inactivated human serum. T cells were expanded with 30ng/ml of anti-CD3 antibody (Centocor Ortho Biotech, Horsham, PA) and 50IU/ml of interleukin-2 (IL-2, Novartis, Basel, Switzerland) as previously described45 for 13-15 days prior to their use in functional assays.
Generation and functional evaluation of NY-ESO-1157-165/HLA-A*02:01-specific T cells
MM patients undergoing autologous stem cell collection via leukapheresis were enrolled on an IRB-approved protocol to provide up to 10 mL of leukapheresis product after the targeted CD34+ cell count was collected. The products were washed with PBS/EDTA, counted, and cryopreserved. HLA-A typing of patient samples was performed using the HLA-A locus Allset Gold SSP Low Resolution (Invitrogen, Carlsbad, CA). Mononuclear cells from leukapheresis products were transduced with a retrovirus containing the MSGV1 backbone and encoding the α and β chains of a variant of the 1G4 TCR specific for NY-ESO-1157-165/HLA-A*02:01 with dual amino acid substitutions at positions 95-96 in the α chain (α95:LY), packaged in the Phoenix Ampho cell line.31, 46 After activation with anti-CD3 antibody (Centocor Ortho) at 300 ng/mL for 24 hours, the cells were spinoculated in presence of protamine (Sigma-Aldrich) and IL-2 on each of two subsequent days. Sham-transduced T cells were obtained in an analogous fashion, but in absence of retroviral vector. Cytotoxicity was determined by 51Cr release assay, as previously described.46
Human myeloma xenografts
Eighteen NOD/SCID/IL-2Rγ -/- (NSG) mice were irradiated with 250 cGy from a 137Cs source (JL Shepherd Mark I) to allow for reproducible xenografting47. One day after irradiation, three cohorts of six mice each received 5×106 U266/Luc cells via tail vein injection. The mice subsequently received tail vein injections on day +2 and day +3 of either PBS, 1×107 sham-transduced T cells in PBS, or 1×107 TCR-transduced T cells in PBS. Starting two weeks after U266/Luc injection, mice were injected intraperitoneally with 40 mg/kg D-luciferin (Caliper Life Sciences, Hopkinton, MA) and imaged on a Xenogen in vivo imaging system (Caliper Life Sciences). Mice were euthanized when they had lost 20% of baseline weight and/or had other signs of suffering such as lethargy, hind limb paralysis, and/or hunching. Bioluminescence images were analyzed using Living Image 3.2 software (Caliper Life Sciences).
Flow cytometry
Transduced cells were evaluated for expression of the 1G4/α95:LY TCR using an APC-labeled NY-ESO-1157-165/HLA-A*02:01 tetramer (Immune Monitoring Facility, FHCRC) and anti-CD8-FITC (BD Biosciences). CD8+/tetramer+ population was isolated by fluorescence-activated cell sorting (FACS). Engraftment of U266/Luc in the blood, bone marrow, and selected organs of xenografted mice was assessed using anti-CD138-APC, anti-HLA-A2-FITC and -APC, and anti-human MHC class I-FITC antibodies (BD Biosciences).
Loss of heterozygosity analysis
Integrity of the MHC in genomic DNA (gDNA) from MM cells recovered from the xenografted mice in all treatment groups was assessed by LOH analysis with previously defined STR markers mapping to chromosomes 6 and 15.48 Multiplex PCR amplification was performed using Multiplex PCR kit (QIAGEN). Each 20 μL reaction contained 1.2 μL of gDNA, 2 μL of Primer Mix (final concentration 5 mM each primer), 10 μL of 2× Multiplex master mix, and 2 μL of 5× Q solution. Tagged primer combinations included: Mix 1: D6S105 [6-FAM]; D6S276 [TET]; Mix 2: D6S291 [6-FAM]-D6S273 [TET]; Mix 3: D15S209 [6-FAM] D6S311 [6-FAM]; Mix 4: D6S126 [6-FAM] D6S275 [6-FAM]. The D6S1618 [TET] marker was evaluated alone. Amplification of STR loci was performed on the Mastercycler ProS (Eppendorf, Hamburg, Germany) using a thermal cycling profile of 95°C for 15 min; 40 cycles of 94°C for 30 sec, 55°C for 40 sec, 72°C for 45 sec; and 72°C for 10 minutes. Aliquots of each PCR reaction were diluted 1:100 and the products were separated on an ABI-3730xl Genetic Analyzer and quantitated using GeneMapper® v4 software.
Statistical Analysis
Survival differences were analyzed using the Kaplan-Meier method with correction for multiple comparisons. Serial bioluminescence levels in the three groups of xenografted mice were analyzed by one-way ANOVA. A p value of <0.05 was considered significant, and a Bonferroni adjustment was applied to correct for multiple comparisons.
Supplementary Material
Acknowledgments
The authors thank Melissa Comstock and LaKeisha Perkins of the FHCRC NOD/Scid Core Facility for their assistance with the murine xenograft studies. The authors also thank the patients who have donated their blood and tissues for our work. These studies were supported by the J. Orin Edson Fund for Immunotherapy, a Senior Research Award from the Multiple Myeloma Research Foundation (to EHW), and NIH grants P30 CA015704-34, 5T32HL007093-39, and P30 DK056465.
Footnotes
Conflict of interest: All authors declared no conflicts of interest.
Supplementary information is available at Gene Therapy's website
References
- 1.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport AP, Stadtmauer EA, Vogl DT, Weiss BM, Binder-Scholl GK, Brewer JE, et al. Adoptive Transfer of Gene-Modified T-Cells Engineered to Express High-Affinity TCRs for Cancer-Testis Antigens (CTAs) NY-ESO-1 or Lage-1, in MM Patients Post Auto-SCT. ASH Annual Meeting Abstracts. 2012;120(21):472. [Google Scholar]
- 4.Porter DL, Kalos M, Zheng Z, Levine B, June C. Chimeric Antigen Receptor Therapy for B-cell Malignancies. J Cancer. 2011;2:331–2. doi: 10.7150/jca.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163(1):507–13. [PubMed] [Google Scholar]
- 11.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74(17):8207–12. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujio K, Misaki Y, Setoguchi K, Morita S, Kawahata K, Kato I, et al. Functional reconstitution of class II MHC-restricted T cell immunity mediated by retroviral transfer of the alpha beta TCR complex. J Immunol. 2000;165(1):528–32. doi: 10.4049/jimmunol.165.1.528. [DOI] [PubMed] [Google Scholar]
- 13.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–59. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss PA. Redirecting T cell specificity by TCR gene transfer. Nat Immunol. 2001;2(10):900–1. doi: 10.1038/ni1001-900. [DOI] [PubMed] [Google Scholar]
- 15.Schaft N, Willemsen RA, de Vries J, Lankiewicz B, Essers BW, Gratama JW, et al. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170(4):2186–94. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Chopra RK, Royal RE, Yang JC, Rosenberg SA, Hwu P. A T cell-independent antitumor response in mice with bone marrow cells retrovirally transduced with an antibody/Fc-gamma chain chimeric receptor gene recognizing a human ovarian cancer antigen. Nat Med. 1998;4(2):168–72. doi: 10.1038/nm0298-168. [DOI] [PubMed] [Google Scholar]
- 17.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Nishimura MI, et al. Transfer of TCR genes into mature T cells is accompanied by the maintenance of parental T cell avidity. J Immunol. 2003;170(3):1209–17. doi: 10.4049/jimmunol.170.3.1209. [DOI] [PubMed] [Google Scholar]
- 21.Schuberth PC, Jakka G, Jensen SM, Wadle A, Gautschi F, Haley D, et al. Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther. 2013;20(4):386–95. doi: 10.1038/gt.2012.48. [DOI] [PubMed] [Google Scholar]
- 22.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–12. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 23.Condomines M, Hose D, Raynaud P, Hundemer M, De Vos J, Baudard M, et al. Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol. 2007;178(5):3307–15. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 24.Dhodapkar MV, Osman K, Teruya-Feldstein J, Filippa D, Hedvat CV, Iversen K, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003;3:9. [PubMed] [Google Scholar]
- 25.Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106(1):167–74. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 26.van Duin M, Broyl A, de Knegt Y, Goldschmidt H, Richardson PG, Hop WC, et al. Cancer testis antigens in newly diagnosed and relapse multiple myeloma: prognostic markers and potential targets for immunotherapy. Haematologica. 2011;96(11):1662–9. doi: 10.3324/haematol.2010.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105(1):251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 28.Crucian BE, Moscinski LC, Androlewicz M, Ballester OF, Widen RH, Yu H. Assessment of intracellular TAP-1 and TAP-2 in conjunction with surface MHC class I in plasma cells from patients with multiple myeloma. Br J Haematol. 1997;98(2):426–32. doi: 10.1046/j.1365-2141.1997.2173032.x. [DOI] [PubMed] [Google Scholar]
- 29.Yi Q, Dabadghao S, Osterborg A, Bergenbrant S, Holm G. Myeloma bone marrow plasma cells: evidence for their capacity as antigen-presenting cells. Blood. 1997;90(5):1960–7. [PubMed] [Google Scholar]
- 30.Levine BL, Rapoport AP, Stadtmauer EA, Vogl DT, Weiss B, Binder-Scholl GK, et al. Adoptive Transfer of Gene-Modified T-cells Engineered to Express High-affinity TCR's for Cancer-testis Antigens NY-ESO-1 or LAGE-1, in Multiple Myeloma (MM) Patients Post-Autologous Hematopoietic Stem Cell Transplant (ASCT) Cytotherapy. 2013;15(4S):S13. [Google Scholar]
- 31.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–31. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack E, Adams KJ, Hassan NJ, Kotian A, Lissin NM, Sami M, et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother. 2013;62(4):773–85. doi: 10.1007/s00262-012-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195(3):346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 34.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 35.Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115(15):3158–61. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 36.Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol. 2006;34(12):1746–52. doi: 10.1016/j.exphem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nonami A, Miyamoto T, Kuroiwa M, Kunisaki Y, Kamezaki K, Takenaka K, et al. Successful treatment of primary plasma cell leukaemia by allogeneic stem cell transplantation from haploidentical sibling. Jpn J Clin Oncol. 2007;37(12):969–72. doi: 10.1093/jjco/hym130. [DOI] [PubMed] [Google Scholar]
- 39.Zomas A, Stefanoudaki K, Fisfis M, Papadaki T, Mehta J. Graft-versus-myeloma after donor leukocyte infusion: maintenance of marrow remission but extramedullary relapse with plasmacytomas. Bone Marrow Transplant. 1998;21(11):1163–5. doi: 10.1038/sj.bmt.1701236. [DOI] [PubMed] [Google Scholar]
- 40.Alyea E, Weller E, Schlossman R, Canning C, Webb I, Doss D, et al. T-cell--depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98(4):934–9. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 41.Bellucci R, Alyea EP, Weller E, Chillemi A, Hochberg E, Wu CJ, et al. Immunologic effects of prophylactic donor lymphocyte infusion after allogeneic marrow transplantation for multiple myeloma. Blood. 2002;99(12):4610–7. doi: 10.1182/blood.v99.12.4610. [DOI] [PubMed] [Google Scholar]
- 42.El-Cheikh J, Crocchiolo R, Furst S, Ladaique P, Castagna L, Faucher C, et al. Lenalidomide plus donor-lymphocytes infusion after allogeneic stem-cell transplantation with reduced-intensity conditioning in patients with high-risk multiple myeloma. Exp Hematol. 2012;40(7):521–7. doi: 10.1016/j.exphem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18(16):3031–7. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 44.Orsini E, Alyea EP, Chillemi A, Schlossman R, McLaughlin S, Canning C, et al. Conversion to full donor chimerism following donor lymphocyte infusion is associated with disease response in patients with multiple myeloma. Biol Blood Marrow Transplant. 2000;6(4):375–86. doi: 10.1016/s1083-8791(00)70014-0. [DOI] [PubMed] [Google Scholar]
- 45.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 46.Chou J, Voong LN, Mortales CL, Towlerton AM, Pollack SM, Chen X, et al. Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother. 2012;35(2):131–41. doi: 10.1097/CJI.0b013e31824300c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brehm MA, Racki WJ, Leif J, Burzenski L, Hosur V, Wetmore A, et al. Engraftment of human HSCs in nonirradiated newborn NOD-scid IL2rgamma null mice is enhanced by transgenic expression of membrane-bound human SCF. Blood. 2012;119(12):2778–88. doi: 10.1182/blood-2011-05-353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramal LM, Maleno I, Cabrera T, Collado A, Ferron A, Lopez-Nevot MA, et al. Molecular strategies to define HLA haplotype loss in microdissected tumor cells. Hum Immunol. 2000;61(10):1001–12. doi: 10.1016/s0198-8859(00)00171-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.