Abstract
A spectrum of conditions requires sedation and analgesia in pediatric population. Ineffective treatment of pain may result in physiological and behavioral responses that can adversely affect the developing nociceptive system. The recognition of pain in children can be facilitated by different pain scales. This article reviews the procedural sedation and analgesia (PSA) practices in children along with pharmacology of the drugs used for this purpose.
Keywords: Analgesia, children, procedural sedation
Introduction
With the better understanding of the developmental neurobiology, it is now a well recognized fact that nervous system is sufficiently sensitive to nociception even before birth, and thus, children should not be undertreated for pain. This aspect has received considerable attention since the landmark studies of Anand and colleagues.[1,2] They were of the opinion that infants and even preterm babies have the anatomical and functional ability to perceive pain. Infants show greater hemodynamic, immune, hormonal, and metabolic stress responses, which may result in impaired healing of damaged or infected tissue leading to increased morbidity.
The descending inhibitory pathways to the dorsal horn of the spinal cord are not developed at birth, which may lead to accentuation of pain in neonates. Moreover, the dorsal horns have wider receptive fields and lower excitatory thresholds than those in older children.[3,4]
Procedural sedation technique should ideally be individualized as per the requirement. For a child undergoing a procedure, a major deciding factor is whether it is painful or not [Table 1]. Pure sedation is sufficient for the imaging studies, while analgesia is required for all invasive procedures, which inflict pain to the child. Sedation is required during the procedures to allay the anxiety, pain, and movement. Coaxing and physical restraint is not an alternative and this may make the procedure not only difficult but also unsafe for the child. Moreover, the psychological trauma may be severe enough to even lead to stress disorder.[5] If pain is not recognized and adequately treated, the resulting physiological and behavioral responses can lead to long-lasting negative effects on the developing nociception system.[6,7]
Table 1.
Procedures requiring sedation

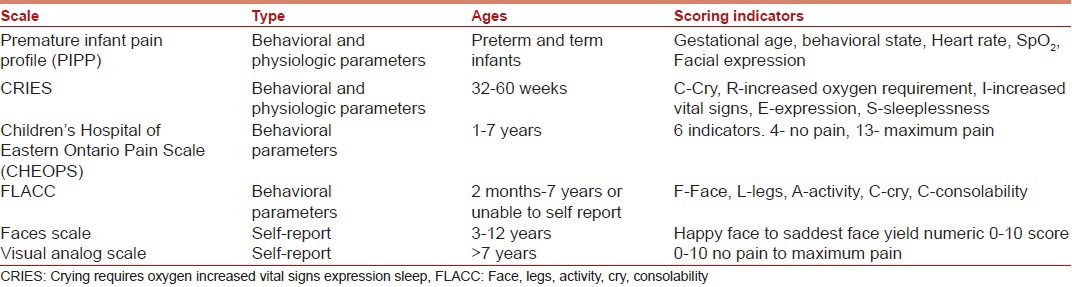
Objective and quantitative measurement of pain in children is a herculean task for most anesthesiologists. Observational pain scales have been validated for neonates and infants to allow pain assessment in those unable to verbalize their pain [Table 2].[8,9,10,11]
Table 2.
Pain scales
Pre-sedation assessment for procedure
Children undergoing sedation should be evaluated beforehand according to the preoperative assessment guidelines of the American Society of Anesthesiologists (ASA). Laboratory workup has no role prior to procedural sedation. Informed consent should be obtained and documented prior to the procedure. ASA fasting guidelines for procedural sedation and analgesia (PSA) may not be followed in every case; the risk in individual patients must be weighed against the risk of delaying an emergent procedure.[11]
Equipment and emergency drugs
It is necessary to have appropriate equipment, monitoring, and reliable back up help before instituting sedation for a procedure in a child. Age and body weight of the child has to be recorded so that appropriate airway and resuscitation equipment be made available. Emergency medications namely atropine, epinephrine, hydrocortisone, flumazenil, naloxone etc., should be available.
Monitoring
Standard non-invasive monitoring should be used in all patients, which includes ECG, BP, pulse oximetry, respiratory rate, and capnography. Sadhasivam et al.[12] concluded that the BIS monitor is a quantitative, non-disruptive, and easy to use depth of sedation monitor in children more than one year.
Special considerations for infants and children
Most analgesics (including opioids and local anesthetics) are conjugated in the liver. Hepatic enzyme systems are not mature in newborns and premature infants. Glomerular filtration rates are diminished in neonates and reach adult levels by one year of age. Newborns have a higher percentage of body weight as water and less fat as compared with older patients. Water-soluble drugs, therefore, often have larger volumes of distribution. Neonates have reduced plasma concentrations of both albumin and alpha-1 acid glycoprotein than older children and adults. This may result in higher concentration of active drug leading to greater drug effect or drug toxicity.[13] Application of eutectic mixture of local anesthetics (EMLA) before venous cannulation can be helpful in reducing pain.
Pharmacology of the drugs
The guiding principle for analgesic administration is the step ladder approach to pain. This is a three-step approach for selecting drugs according to their analgesic potency based on the child's pain level. The non-steroidal anti-inflammatory drugs (NSAIDS) are used for mild to moderate pain by themselves or in combination with other agents for moderate to severe pain. However, opioids are used for moderate to severe pain.
NSAIDS, though used extensively in older children and adults, are not preferred in neonates because of their increased risk of side-effects. Commonly used drugs are acetaminophen and ketorolac. These drugs have to be used along with sedative agents for procedures.
Acetaminophen is the most commonly used NSAID, particularly for mild pain. However, it has limited efficacy for mitigating procedural pain. It is available in oral, rectal, and intravenous (i.v) form. Acetaminophen is administered in dose of 35-45 mg/kg by rectal route. Repeated dosing is 20 mg/kg every 6 hours in infants and children and every 12 hours in newborns. Paracetamol i.v is administrated in a dose of 7.5 mg/kg up to 4 times a day, by infusion over 15 minutes in children weighing less than 10 kg. The minimum interval between each administration must be 4 hours, and the maximum daily dose must not exceed 30 mg/kg. For children weighing more than 10 kg (and less than 33 kg), 15 mg/kg, up to 4 times a day, with maximum daily dose not exceeding 60 mg/kg is recommended (without exceeding 2 g). It has been shown to be effective in treating post-operative pain.[14,15]
Ketorolac is available both as oral tablets (10 mg) or i.v injections (15 mg/ml and 30 mg/ml). It is efficacious as a sole analgesic for minor procedures. However, in acute renal failure,[16] prolonged prothrombin time and hypersensitivity have been reported to occur with its usage. Dose of ketorolac is 0.5 mg/kg i.v, every 6 hours for less than 5 days.
Opioids
With the advent of short-acting opioids, morphine is no longer preferred for short procedures. For procedural sedation, shorter acting opioids like fentanyl are preferable. A single intravenous dose of fentanyl 1-4 μg/kg has rapid onset (<30 s) with a peak at 2-3 min and brief clinical duration (20-60 min). The initial i.v boluses of 0.5-2 μg/kg may be given over 2-5 min titrated to effect, followed by infusion of 0.2-2 μg/kg/h for maintenance of analgesia. As sedation does not occur at low doses (1-2 μg/kg), the concurrent administration of midazolam is required. The combination of fentanyl and midazolam is a safe and popular regimen for procedural sedation and analgesia in children.[17] When given in concert, both should be given in reduced doses to minimize the chance of hemodynamic or respiratory compromise. The effectiveness of oral transmucosal preparation of fentanyl is variable, titration is difficult, and the incidence of emesis is as high as 31-45%[18] Remifentanil is an ultra-short-acting opioid agent that has an onset of action of about 1 min and an elimination half-life of less than 10 min. It has been used successfully as a sedative agent in children.[19,20] It is given as an infusion of 0.05- 0.10 μg/kg/min in combination with midazolam. In case of invasive procedures, before cessation of the remifentanil infusion, a longer acting analgesic may be administered to ensure analgesia when the patient awakens from sedation.
Benzodiazepines
By activating γ-aminobutyric acid, benzodiazepines produce sedation, anxiolysis, amnesia, and anticonvulsant effects.[21] After administration, respiratory depression should be watched for. Time to peak effect for midazolam is brief with i.v administration (2-3 min) and duration is short (45-60 min). Midazolam can also be administered via the intramuscular, oral, intranasal, and rectal routes. The oral route can lead to unreliable concentrations in serum and clinical effect due to first pass hepatic metabolism. The intranasal route irritates the mucosa, which is painful and produces anxiety in the child. It does not have any analgesic properties and requires supplemental analgesia for painful procedures. Paradoxical reactions, characterized by inconsolable crying, disorientation, agitation, and restlessness, have been reported in children receiving midazolam, especially after rapid i.v injection.[22] For upper gastrointestinal endoscopy or bronchoscopy, use of additional topical anesthetic is useful.
Barbiturates
Pentobarbital produces a deep sleep with minimal movement and is popular for use during radiological (non-invasive) procedures. It is given in dose of 2-5 mg/kg/dose slow i.v or 2-6 mg/kg/dose intramuscularly not to exceed 100 mg/dose by either route. Oral route has been also used and was found to be better than chloral hydrate.[23] Pentobarbital and chloral hydrate when compared for sedation of infants undergoing MRI and CT scan studies were found to be equally effective, but pentobarbital was better tolerated.[24] Barbiturates have antanalgesic effects and are unsuitable for sedating children for painful procedures.
Chloral hydrate
Chloral hydrate is a pure sedative-hypnotic drug without analgesic properties, once widely used during non-painful diagnostic procedures.[25] It has an aromatic, pungent odor and a slightly bitter, caustic taste, which may not be acceptable to children. Chloral hydrate can cause airway obstruction and respiratory depression at higher doses (75-100 mg/kg).[26] Although restricted in some countries due to risk of potential carcinogenicity, the American Academy of Pediatrics has not found enough evidence to avoid single doses of chloral hydrate for this reason alone.[27] Wheeler and colleagues conducted a randomized, blinded comparison of chloral hydrate and midazolam for procedural sedation and found no difference in mean time for onset of sedation between the two groups, but mean time to recovery was significantly shorter with midazolam.[28] Successful sedation was achieved in 93% of the chloral hydrate patients compared to only 36% of the midazolam group. No adverse events were reported in either group. Its unpredictable onset, long duration, and the lack of a reversal agent make chloral hydrate far from an ideal sedative.
Ketamine
The state of “dissociative sedation” of ketamine is characterized by profound analgesia, sedation, amnesia, and immobilization. Upper airway muscular tone and protective airway reflexes are maintained and spontaneous respiration is preserved[29] although when administered i.v (dose is 1-1.5 mg/kg), it must be given over 1 min to prevent transient respiratory depression. Unpleasant emergence reactions are uncommon in children and teenagers and are typically mild.[30] There is no evidence of any benefit from the prophylactic administration of concurrent benzodiazepines in children,[31] and their role should be confined to treating unpleasant reactions if they arise. Ketamine has been extensively used in pediatric patients in emergency department, radiology, burns dressings, and other painful procedures. It is an ideal sedative in patients with bronchospasm, hypovolemia, and shock.
Propofol
The emergency department, gastroenterology, and critical care literature showed that propofol can be given to children in these settings with good efficacy, apparent safety, and rapid recovery.[32,33] Use of an analgesic is imperative in addition to propofol for painful procedures. Propofol has a wide array of benefits, including anticonvulsant activity, antiemesis, and ability to reduce intracranial hypertension. Propofol infusion syndrome seems an unlikely concern for procedural sedation. The risk factors include poor oxygen delivery, sepsis, serious cerebral injury, and high-dose propofol.[34] Propofol was found to have a safe profile in 17, 066 pediatric patients sedated in a variety of hospital settings.[35] Paspatis et al.[36] compared oral midazolam and propofol versus propofol alone in 54 children undergoing endoscopy. The recovery time for the midazolam group was significantly longer, but the ease of i.v. line placement, parental separation, and patient comfort were significantly better in this group. The rates of respiratory and hemodynamic complications were similar between the two groups, with the midazolam group requiring significantly less additional propofol. Propofol is at least as effective as midazolam in providing desired levels of sedation with an early recovery profile. Ketofol, mixture of ketamine, and propofol is a popular combination used for PSA.
Etomidate
Etomidate has a rapid onset of action, a short duration agent with a relatively mild adverse effect profile. However, it may block cortisol production, and this appears to be its most significant drawback and limits its long-term use. Etomidate has been found to be more effective and efficient than pentobarbital and midazolam and equally safe as propofol.[37,38,39] A study comparing ketamine versus etomidate for procedural sedation in children has been completed and results are awaited.
Dexmedetomidine
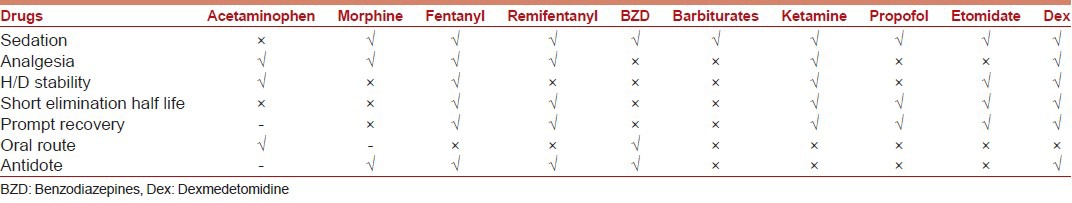
Dexmedetomidine is a highly selective alpha-2 adrenoreceptor agonist with sedative, anxiolytic, and mild analgesic properties with no depressant effect on respiratory drive. In 2008, approval was granted for its use in non-intubated patients requiring sedation. Although it does not have US FDA approval for use in children, its use has been well described in multiple settings.[40] It is administered as a loading dose of 0.5-1 μg/kg over 10 minutes followed by an infusion of 0.2-1 μg/kg/hr. In a study by Koroglu et al., propofol when compared with dexmedetomidine provided a faster onset of action and time to recovery than dexmedetomidine but also resulted in more episodes of hypotension and oxygen desaturation.[41] Dexmedetomidine as sole agent for invasive procedures has not always been successful. Combination of ketamine and dexmedetomidine speeds up the onset of sedation process while neutralizing the side effects of each other.[42] Though no single agent fulfils the criteria of an ideal agent for PSA [Table 3], dexmedetomidine approaches close to it [Table 4]. Over the last decade, it is being widely used for the purpose of sedation all over the world.
Table 3.
Ideal agent for procedural sedation and analgesia

Table 4.
Comparative properties of different drugs
Indian scenario
In Indian context, use of agents like remifentanil, dexmedetomidine, and etomidate is restricted due to their non-availability or limited supply. Remifentanil is still not available but will soon be marketed in India. Use of dexmedetomidine is continually increasing as more and more centers acquire it. Till then, midazolam and ketamine are the most commonly used drugs used for PSA in India.[43] The monitoring practice varies from one center to another depending on facilities available. There is a need for further research regarding sedation and analgesia practices in India. The ASA guidelines recommended that only personnel with training in the delivery of general anesthesia should administer sedation as it is likely that children may pass into a deeper level of sedation than originally intended.[44] Level III evidence suggests that PSA with propofol, including deep sedation, is equally safe in the hands of anesthesiologists and non-anesthesiologists provided they are well-trained and are part of a dedicated sedation team.[45]
Conclusion
Though professional skill and knowledge remain the determining factors, the goal should be to practice and promote safe sedation and analgesia. The introduction of newer pharmacologic agents and better monitoring devices helped us to achieve this. With an ever increasing load of out of operating room procedures, non-anesthesiologists should be formally trained to deliver PSA and manage complications should they occur.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Anand KJ, Hickey PR. Pain and its effects on the human neonate and fetus. N Engl J Med. 1987;317:1321–9. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: Effects on stress response. Lancet. 1987;1:62–6. doi: 10.1016/s0140-6736(87)91907-6. [DOI] [PubMed] [Google Scholar]
- 3.Pattinson D, Fitzgerald M. The neurobiology of infant pain: Development of excitatory and inhibitory neurotransmission in the spinal dorsal horn. Reg Anesth Pain Med. 2004;29:36–44. doi: 10.1016/j.rapm.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerals M, Howard R. The neurobiologic basis of pediatric pain. N.L.C.B.M. In: Schechter NL, Berde CB, Yaster M, editors. Pain in Infants, Children, and Adolescents. 2nd ed. Baltimore: Lippincott Williams and Wilkins; 2001. pp. 19–42. [Google Scholar]
- 5.Daviss WB, Racusin R, Fleischer A, Mooney D, Ford JD, McHugo GJ. Acute stress disorder symptomatology during hospitalization for pediatric injury. J Am Acad Child Adolesc Psychiatry. 2000;39:569–75. doi: 10.1097/00004583-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99:377–83. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- 7.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Grunau RV, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 9.Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: Development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Merkle SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioural scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 11.Smally AJ, Nowicki TA, Simelton BH. Procedural sedation and analgesia in the emergency department. Curr Opin Crit Care. 2011;17:317–22. doi: 10.1097/MCC.0b013e328348bf43. [DOI] [PubMed] [Google Scholar]
- 12.Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383–8. doi: 10.1213/01.ANE.0000184115.57837.30. [DOI] [PubMed] [Google Scholar]
- 13.Rose JB. Pediatric analgesic pharmacology. In: Litmann RS, editor. Pediatric Anesthesia: The Requisites in Anesthesiology. Philadelphia: Mosby Inc, Elsevier Mosby; 2004. pp. 196–205. [Google Scholar]
- 14.Murat I, Baujard C, Foussat C, Guyot E, Petel H, Rod B, et al. Tolerance and analgesic efficacy of a new i.v. paracetamol solution in children after inguinal hernia repair. Paediatr Anaesth. 2005;15:663–70. doi: 10.1111/j.1460-9592.2004.01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Capici F, Ingelmo PM, Davidson A, Sacchi CA, Milan B, Sperti LR, et al. Randomized controlled trial of duration of analgesia following intravenous or rectal acetaminophen after adenotonsillectomy in children. Br J Anaesth. 2008;100:251–5. doi: 10.1093/bja/aem377. [DOI] [PubMed] [Google Scholar]
- 16.Buck ML, Norwood VF. Ketorolac-induced acute renal failure in a previously healthy adolescent. Pediatrics. 1996;98:294–6. [PubMed] [Google Scholar]
- 17.Kennedy RM, Porter FL, Miller JP, Jaffe DM. Comparison of fentanyl/midazolam with ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics. 1998;102:956–63. doi: 10.1542/peds.102.4.956. [DOI] [PubMed] [Google Scholar]
- 18.Klein EJ, Diekema DS, Paris CA, Quan L, Cohen M, Seidel KD. A randomized, clinical trial of oral midazolam plus placebo versus oral midazolam plus oral transmucosal fentanyl for sedation during laceration repair. Pediatrics. 2002;109:894–7. doi: 10.1542/peds.109.5.894. [DOI] [PubMed] [Google Scholar]
- 19.Kaynar A, Kelsaka E, Karakaya D, Sungur M, Baris S, Demirkaya M, et al. Effects of different doses of remifentanil infusion on hemodynamics and recovery in children undergoing pediatric diagnostic cardiac catheterization. J Cardiothorac Vasc Anesth. 2011;25:660–4. doi: 10.1053/j.jvca.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Antmen B, Saşmaz I, Birbiçer H, Ozbek H, Burgut R, Işik G, et al. Safe and effective sedation and analgesia for bone marrow aspiration procedures in children with alfentanil, remifentanil and combinations with midazolam. Paediatr Anaesth. 2005;15:214–9. doi: 10.1111/j.1460-9592.2004.01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Blumer JL. Clinical pharmacology of midazolam in infants and children. Clin Pharmacokinet. 1998;35:37–47. doi: 10.2165/00003088-199835010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Massanari M, Novitsky J, Reinstein LJ. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr (Phila) 1997;36:681–4. doi: 10.1177/000992289703601202. [DOI] [PubMed] [Google Scholar]
- 23.Rooks VJ, Chung T, Connor L, Zurakowski D, Hoffer FA, Mason KP, et al. Comparison of oral pentobarbital sodium (nembutal) and oral chloral hydrate for sedation of infants during radiologic imaging: Preliminary results. AJR Am J Roentgenol. 2003;180:1125–8. doi: 10.2214/ajr.180.4.1801125. [DOI] [PubMed] [Google Scholar]
- 24.Mason KP, Sanborn P, Zurakowski D, Karian VE, Connor L, Fontaine PJ, et al. Superiority of pentobarbital versus chloral hydrate for sedation in infants during imaging. Radiology. 2004;230:537–42. doi: 10.1148/radiol.2302030107. [DOI] [PubMed] [Google Scholar]
- 25.Olson DM, Sheehan MG, Thompson W, Hall PT, Hahn J. Sedation of children for electroencephalograms. Pediatrics. 2001;108:163–5. doi: 10.1542/peds.108.1.163. [DOI] [PubMed] [Google Scholar]
- 26.Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: Analysis of medications used for sedation. Pediatrics. 2000;106:633–44. doi: 10.1542/peds.106.4.633. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Pediatrics Committee on Drugs. Use of chloral hydrate for sedation in children. Pediatrics. 1993;92:471–3. [PubMed] [Google Scholar]
- 28.Wheeler DS, Jensen RA, Poss WB. A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Clin Pediatr (Phila) 2001;40:381–8. doi: 10.1177/000992280104000704. [DOI] [PubMed] [Google Scholar]
- 29.Kim G, Green SM, Denmark TK, Krauss B. Ventilatory response during dissociative sedation in children-a pilot study. Acad Emerg Med. 2003;10:140–5. doi: 10.1197/aemj.10.2.140. [DOI] [PubMed] [Google Scholar]
- 30.Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med. 2004;44:460–71. doi: 10.1016/S0196064404006365. [DOI] [PubMed] [Google Scholar]
- 31.Wathen JE, Roback MG, Mackenzie T, Bothner JP. Does midazolam alter the clinical effects of intravenous ketamine sedation in children. A double-blind, randomized, controlled emergency department trial? Ann Emerg Med. 2000;36:579–88. doi: 10.1067/mem.2000.111131. [DOI] [PubMed] [Google Scholar]
- 32.Green SM, Krauss B. Propofol in emergency medicine: Pushing the sedation frontier. Ann Emerg Med. 2003;42:792–7. doi: 10.1016/s0196-0644(03)00746-7. [DOI] [PubMed] [Google Scholar]
- 33.Barbi E, Gerarduzzi T, Marchetti F, Neri E, Verucci E, Bruno I, et al. Deep sedation with propofol by nonanesthesiologists: A prospective pediatric experience. Arch Pediatr Adolesc Med. 2003;157:1097–103. doi: 10.1001/archpedi.157.11.1097. [DOI] [PubMed] [Google Scholar]
- 34.Ahlen K, Buckley CJ, Goodale DB, Pulsford AH. The ‘propofol infusion syndrome’: The facts, their interpretation and implications for patient care. Eur J Anaesthesiol. 2006;23:990–8. doi: 10.1017/S0265021506001281. [DOI] [PubMed] [Google Scholar]
- 35.Lamond DW. Review article: Safety profile of propofol for paediatric procedural sedation in the emergency department. Emerg Med Australas. 2010;22:265–86. doi: 10.1111/j.1742-6723.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- 36.Paspatis G, Charoniti I, Manolaraki M, Vardas E, Papanikolaou N, Anastasiadou A, et al. Synergistic sedation with oral midazolam as a premedication and intravenous propofol versus intravenous propofol alone in upper gastrointestinal endoscopies in children: A prospective, randomized study. J Pediatr Gastroenterol Nutr. 2006;43:195–9. doi: 10.1097/01.mpg.0000228099.04702.39. [DOI] [PubMed] [Google Scholar]
- 37.Baxter AL, Mallory MD, Spandorfer PR, Sharma S, Freilich SH, Cravero J Pediatric Sedation Research Consortium. Etomidate versus pentobarbital for computed tomography sedations: Report from the Pediatric Sedation Research Consortium. Pediatr Emerg Care. 2007;23:690–5. doi: 10.1097/PEC.0b013e3181558d5c. [DOI] [PubMed] [Google Scholar]
- 38.Di Liddo L, D’ Angelo A, Nguyen B, Bailey B, Amre D, Stanciu C. Etomidate versus midazolam for procedural sedation in pediatric outpatients: A randomized controlled trial. Ann Emerg Med. 2006;48:433–40.e1. doi: 10.1016/j.annemergmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Denny MA, Manson R, Della-Giustina D. Propofol and etomidate are safe for deep sedation in the emergency department. West J Emerg Med. 2011;12:399–403. doi: 10.5811/westjem.2011.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMorrow SP, Abramo TJ. Dexmedetomidine sedation: Uses in pediatric procedural sedation outside the operating room. Pediatr Emerg Care. 2012;28:292–6. doi: 10.1097/PEC.0b013e3182495e1b. [DOI] [PubMed] [Google Scholar]
- 41.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63–7. doi: 10.1213/01.ANE.0000219592.82598.AA. [DOI] [PubMed] [Google Scholar]
- 42.Tobias JD. Dexmedetomidine and ketamine: An effective alternative for procedural sedation? Pediatr Crit Care Med. 2012;13:423–7. doi: 10.1097/PCC.0b013e318238b81c. [DOI] [PubMed] [Google Scholar]
- 43.Arora RS, Kulkarni KP, Alston RD. A survey of procedural sedation and analgesia practices in pediatric oncology centres in India. Indian J Pediatr. 2012;79:1610–6. doi: 10.1007/s12098-012-0724-x. [DOI] [PubMed] [Google Scholar]
- 44.Advisory on granting privileges for deep sedation to non anesthesiologist sedation practitioners. Committee of Origin: Ad Hoc on Non-Anesthesiologist Privileging (Approved by the ASA House of Delegates on October 20, 2010) [Google Scholar]
- 45.Leroy PL, Schipper DM, Knape HJ. Professional skills and competence for safe and effective procedural sedation in children: Recommendations based on a systematic review of the literature. Int J Pediatr 2010. 2010 doi: 10.1155/2010/934298. 934298. [DOI] [PMC free article] [PubMed] [Google Scholar]


