Abstract
Bilateral thalamic tumors are rare. Pediatric bilateral thalamic glioblastomas are even rarer, only five cases reported in the English literature till date. The clinical presentation, natural history, and prognosis of pediatric thalamic tumors are still relatively obscure. In this article, we report an 8-year-old patient with large bilateral thalamic glioblastomas and briefly discuss its clinical presentation, possible modalities of management, and prognosis, in the light of available literature.
Keywords: Bilateral thalamus, encephalitis, glioblastoma, outcome, pediatric
Introduction
Bilateral primary thalamic tumors are rare and bilateral thalamic glioblastomas are even rarer.[1,2,3,4] The clinical presentation of these patients is varied, ranging from headache and focal neurological deficits to personality changes and/or mental deterioration, including memory loss, inattention, confusion, hallucination, and hyperphagia.[5] As a result, these patients are often confused with encephalitis and its sequel, as happened in our patient. The diagnostic modality in bilateral thalamic gliomas is stereotactic biopsy. Radiotherapy is usually planned after establishing the diagnosis. The role of surgery is limited, owing to the architectural complexity and depth of location of the lesion.
Case Report
An 8-year-old patient was referred to the pediatric emergency services with complaints of moderate- to high-grade fever for 10 days, 2 months back. On 5th day of fever, the patient developed choreoathetoid movements on right side of the body, which was more severe in the upper limb. Few days later, he developed progressive loss of ambulation, cognition, and speech.
On examination, he was in a state of altered consciousness (E2V2M5). However, his vitals were stable. The neurological assessment revealed, increased tone in all four limbs, with exaggerated deep tendon reflexes and bilateral extensor plantar reflex. Horizontal nystagmus with alternating convergent squint was also present. Fundoscopy revealed mild papilledema, pupils were of normal size and reacting to light. His complete hematological profile and serum chemistries were unremarkable.
Noncontrast computed tomography (CT) of the head revealed a bilateral thalamic iso- to hyperdense lesion with moderate hydrocephalus [Figure 1]. The MR examination of the brain showed significant swelling of thalamus with diffuse hypointense signal on T1-weighted (T1W) imaging and hyperintense signal on T2 and fluid-attenuated inversion recovery (FLAIR) sequences [Figure 2]. The contrast images showed mild peripheral enhancement [Figure 3]. There was evidence of moderate hydrocephalus with periventricular ooze. Hypointense signals on T1 and hyperintense signal changes on T2W and FLAIR were also present in the basal ganglia, head of the caudate nuclei, and upper brainstem. Findings were considered nonspecific and diagnosis of encephalitis was considered, however, bilateral thalamic glioma was the second possibility.
Figure 1.
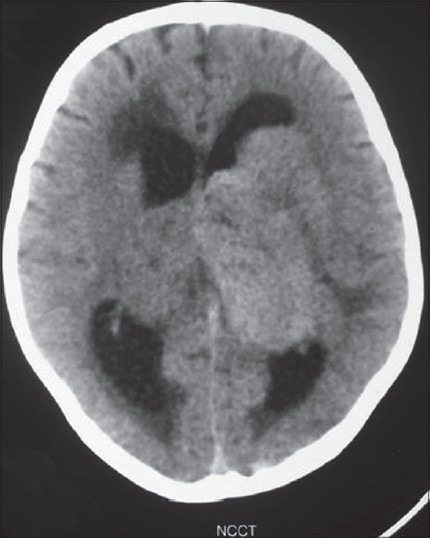
Noncontrast computed tomography of head reveals bilateral iso- to hyperdense thalamic masses with hydrocephalus and periventricular ooze
Figure 2.
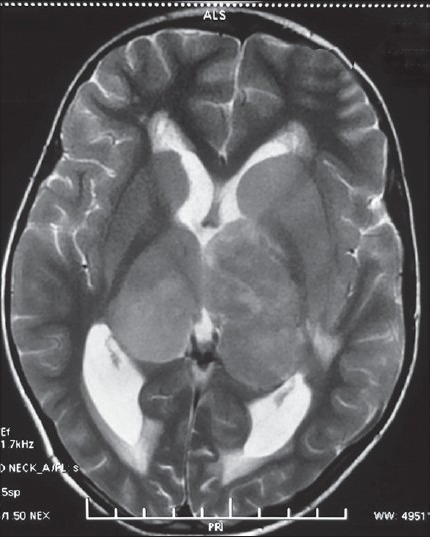
T2-weighted magnetic resonance imaging reveals bilateral hyperintense thalamic masses with focal hypointensities
Figure 3.
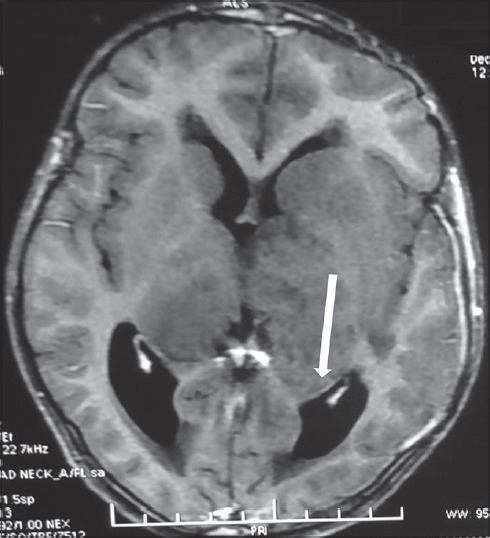
T1-weighted axial post-contrast image reveals mild peripheral enhancement
Patient was managed on the lines of encephalitis and its sequelae. He was started on antiepileptic drugs and decongestants to decrease raised intracranial pressure and a low pressure ventricular shunt was inserted. The patient underwent MR-guided biopsy of the left thalamic mass.
Histopathological examination of the biopsy specimen revealed a highly cellular tumor with foci of palisading necrosis [Figure 4a] and microvascular proliferation [Figure 4b]. The tumor cells showed marked nuclear pleomorphism and frequent mitotic figures [Figure 4b], including atypical forms. Bizarre multinucleated tumor giant cells were identified. On immunohistochemistry, the tumor cells were immunopositive for p53 [Figure 4c] and were negative for epidermal growth factor receptor (EGFR). MIB-I labeling index was approximately 60% in the highest proliferating areas [Figure 4d]. Based on these features, a diagnosis of glioblastoma multiforme, World Health Organization (WHO) grade IV, was arrived at.
Figure 4.
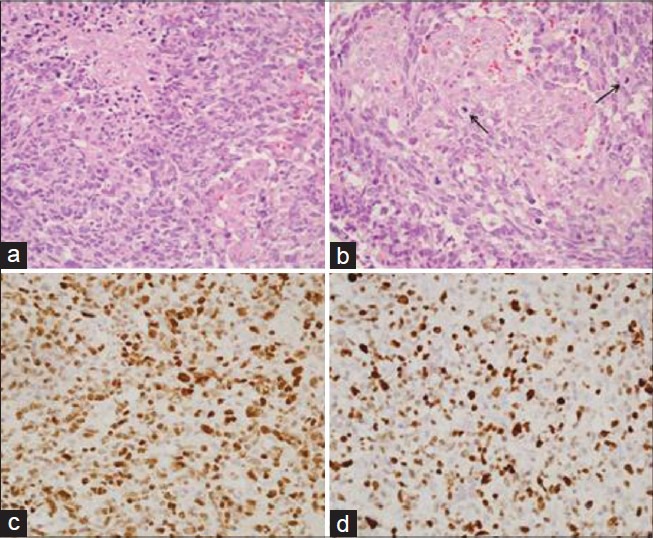
Photomicrographs showing (a) a highly cellular tumor with foci of palisading necrosis (hematoxylin and eosin (H and E), original magnification ×400) and (b) microvascular proliferation (H and E, original magnification ×400). (b) The tumor cells display marked nuclear pleomorphism and frequent mitotic figures (H and E, original magnification ×400). (c) Immunohistochemistry for p53 showed nuclear positivity (immunohistochemistry (IHC), original magnification ×400). (d) MIB-I labeling index is 60% (IHC, original magnification ×400)
Tracheostomy was done in view of poor Glasgow Coma Scale (GCS) considering the need of prolonged ventilatory support. He was gradually weaned off the ventilator over a 2 weeks period. The patient was subsequently discharged and advised to have chemoradiotherapy for further management.
Discussion
Although thalamic tumors constitute between 2 and 5% of all the pediatric intracranial tumors, it is only 1% in adult series.[1,3,6] The pediatric literature remains relatively rare. Bilateral pediatric thalamic glioblastomas are extremely rare and only five cases are published in the English literature.[3] The largest series on pure thalamic tumors to date is from the Necker Enfants Malades Hospital, Paris,[7] which reported 69 children treated over a 14-year period. Nine tumors were bilateral, and three of these were high-grade gliomas. Fernandez and colleagues reported on 14 thalamic tumors, two of which were glioblastomas.[4]
Lesions in the ventrolateral thalamus typically manifest with motor deficits, given their close proximity to the internal capsule.[7,8] The visual signs and symptoms are present in as many as 50% of these patients[9] and are due to involvement of the optic tracts and the lateral geniculate body. The oculomotor deficits are due to involvement of the retrolenticular segment of the internal capsule or the midbrain tegmentum. Involvement of the mammillothalamic tracts or the fornices can lead to cognitive dysfunction and memory problems.[9,10] Bilateral thalamic lesions are associated with changes in personality, memory deficits, confusion, hallucinations, hyperphagia, and bradyphrenia.[5]
Diffuse and bilateral involvement of thalamic nuclei by these tumors makes the surgical therapy very difficult. No case of radical removal is so far described in the literature. The role of surgery, hence, is limited and is performed for histological diagnosis as was done in our patient. The role of chemotherapy and radiotherapy is of questionable significance and are utilized as adjuvant therapies. Bilateral thalamic tumors are typically treated with radiation therapy, regardless of whether they are histologically malignant.[7] For bilateral thalamic tumors, the prognosis remains relatively poor regardless of tumor histology and treatment.[3,7,9,10,11]
In our patient, left thalamic tumor was biopsied under magnetic resonance imaging (MRI) guidance. Histopathology of the sample revealed glioblastoma multiforme (GBM). The molecular genetics of pediatric GBMs has been intensely studied over the past years. Cheng et al.,[12] documented p53 protein accumulation in 75% of high-grade childhood gliomas, and p53 mutation in 38%. Pollack et al.,[13] documented increased expression of p53 in about 35% of high-grade gliomas in children, which increased to 58% when only glioblastomas were considered. The authors determined that this feature was an adverse prognostic factor in a cohort of children who were treated uniformly with surgery, radiotherapy, and chemotherapy.[14,15] On the other hand, EGFR amplification is much less common in pediatric glioblastomas compared to adult primary glioblastomas and the reports on EGFR protein expression have been quite varied. Sure et al.,[16] reported EGFR overexpression in only two of 20 cases (10%). Similar observations were made by Cheng et al.,[12] who found only very small foci of tumor cells showing EGFR immunopositivity in four of 13 glioblastomas.
Our patient was planned for radiotherapy at the time of writing this paper. However, the patient died before the initiation of radiotherapy.
Conclusion
Thalamic lesions continue to be a challenging entity to the neurosurgeons, particularly owing to their deep location and complex circuitry. Unilateral tumors are increasingly being tackled by the neurosurgeons with an improved patient outcome. However, bilateral thalamic glioblastomas have a very poor prognosis, irrespective of the modality of treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Beks JW, Bouma GJ, Journee HL. Tumours of the thalamic region. A retrospective study of 27 cases. Acta Neurochir (Wien) 1987;85:125–7. doi: 10.1007/BF01456108. [DOI] [PubMed] [Google Scholar]
- 2.Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997;13:514–20. doi: 10.1007/s003810050128. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Gajjar A, Sanford RA, Heideman RL, Walter AW, Thompson SJ, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29:29–35. doi: 10.1159/000028681. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez C, Maues de Paula A, Colin C, Quilichini B, Bouvier-Labit C, Girard N, et al. Thalamic gliomas in children: An extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006;22:1603–10. doi: 10.1007/s00381-006-0184-6. [DOI] [PubMed] [Google Scholar]
- 5.Partlow GD, del Carpio-O’Donovan R, Melanson D, Peters TM. Bilateral thalamic glioma: Review of eight cases with personality change and mental deterioration. AJNR Am J Neuroradiol. 1992;13:1225–30. [PMC free article] [PubMed] [Google Scholar]
- 6.McKissock W, Paine KW. Primary tumours of the thalamus. Brain. 1958;81:41–63. doi: 10.1093/brain/81.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Puget S, Crimmins DW, Garnett MR, Grill J, Oliveira R, Boddaert N, et al. Thalamic tumors in children: A reappraisal. J Neurosurg. 2007;106:354–62. doi: 10.3171/ped.2007.106.5.354. [DOI] [PubMed] [Google Scholar]
- 8.Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: A report of the Children's Cancer Group trial No. CCG-945. J Neurosurg. 1998;89:52–9. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 9.Souweidane MM, Hoffman HJ. Current treatment of thalamic gliomas in children. J Neurooncol. 1996;28:157–66. doi: 10.1007/BF00250196. [DOI] [PubMed] [Google Scholar]
- 10.Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, et al. Low-grade astrocytoma: A decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792–9. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 11.Di Rocco C, Iannelli A. Bilateral thalamic tumors in children. Childs Nerv Syst. 2002;18:440–4. doi: 10.1007/s00381-002-0609-9. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Ng HK, Zhang SF, Ding M, Pang JC, Zheng J, et al. Genetic alterations in pediatric high-grade astrocytomas. Hum Pathol. 1999;30:1284–90. doi: 10.1016/s0046-8177(99)90057-6. [DOI] [PubMed] [Google Scholar]
- 13.Pollack IF, Hamilton RL, Burnham J, Holmes EJ, Finkelstein SD, Sposto R, et al. Impact of proliferation index on outcome in childhood malignant gliomas: Results in a multi-institutional cohort. Neurosurgery. 2002;50:1238–45. doi: 10.1097/00006123-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Reifenberger G, Collins VP. Pathology and molecular genetics of astrocytic gliomas. J Mol Med (Berl) 2004;82:656–70. doi: 10.1007/s00109-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 15.Yoon KS, Lee MC, Kang SS, Kim JH, Jung S, Kim YJ, et al. P53 mutation and epidermal growth factor receptor overexpression in glioblastoma. J Korean Med Sci. 2001;16:481–8. doi: 10.3346/jkms.2001.16.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sure U, Ruedi D, Tachibana O, Yonekawa Y, Ohgaki H, Kleihues P, et al. Determination of p53 mutations, EGFR overexpression, and loss of p16 expression in pediatric glioblastomas. J Neuropathol Exp Neurol. 1997;56:782–9. [PubMed] [Google Scholar]


