Abstract
The chondroitin sulfate proteoglycan versican is one of the major extracellular components in the developing and adult brain. Here, we show that isoforms of versican play different roles in neuronal differentiation and neurite outgrowth. Expression of versican V1 isoform in PC12 cells induced complete differentiation, whereas expression of V2 induced an aborted differentiation accompanied by apoptosis. V1 promoted neurite outgrowth of hippocampal neurons, but V2 failed to do so. V1 transfection enhanced expression of epidermal growth factor receptor and integrins, and facilitated sustained extracellular signal-regulated kinase/MAPK phosphorylation. Blockade of the epidermal growth factor receptor, β1 integrin, or Src significantly inhibited neuronal differentiation. Finally, we demonstrated that versican V1 isoform also promoted differentiation of neural stem cells into neurons. Our results have implications for understanding how versican regulates neuronal development, function, and repair.
INTRODUCTION
During development, the interactions between cells and the extracellular matrix (ECM) initiate signals to regulate many fundamental processes. The ECM regulates cellular functions such as migration, adhesion, proliferation, differentiation, and morphogenesis, which are mediated by a large diversity of receptors on the cell surface (Juliano and Haskill, 1993). The receptor–matrix interactions regulate gene activities and cell behaviors (Hynes, 1987; Santiago-Garcia et al., 2003). In the brain ECM, proteoglycans are assumed to play a particularly important role in controlling neuronal differentiation and development (Mongiat et al., 2003; Walker et al., 2003). Versican is one of the major extracellular proteoglycans in the developing and mature brain, but how this molecule affects neuronal differentiation, development, and maturation is unknown.
Versican was originally isolated from human fibroblasts and developing limb buds in the chicken (Zimmermann and Ruoslahti, 1989; Shinomura et al., 1993) and was detected in the normal human central nervous system (CNS) and in brain tumors (Paulus et al., 1996). Structurally, versican is made up of an N-terminal globular domain, a chondroitin sulfate (CS) attachment region, and a C terminus containing a selectin-like domain (also named as G3 domain). The latter contains two epidermal growth factor (EGF)-like repeats, a lectin-like motif (also known as carbohydrate recognition domain) and a complement binding protein-like motif (Shinomura et al., 1993). The CS attachment region is encoded by two exons producing CSα and CSβ domains. Due to alternative splicing, four isoforms (V0, V1, V2, and V3) are generated (Ito et al., 1995; Lemire et al., 1999). Versican is known to associate with a number of other molecules in the ECM, including hyaluronan, tenascin, fibulin-1, fibrillin, fibronectin, CD44, and selectins, and link protein (LeBaron et al., 1992; Aspberg et al., 1995; Kawashima et al., 2000; Olin et al., 2001; Isogai et al., 2002; Matsumoto et al., 2003). Studies of brain development and maturation have shown that versican V1/V0 and V2 have complementary expression patterns (Bandtlow and Zimmermann, 2000). Although versican V1/V0 is mainly expressed in the late stage of embryonic development (Landolt et al., 1995), versican V2 becomes a major chondroitin sulfate proteoglycan in the mature brain (Schmalfeldt et al., 1998). These results suggest that versican isoforms may play distinct functions due to the difference in the CS domains.
Our previous studies have demonstrated that different versican domains play different roles in regulating cellular activities (Zhang et al., 1998a,b; Ang et al., 1999; Yang et al., 1999). To investigate the effect of versican on neuronal differentiation and neurite outgrowth, we have cloned and expressed the full-length versican V1 and V2 isoforms in PC12 cells, a model system used extensively in the study of neuronal differentiation (Alema et al., 1985; Vaudry et al., 2002). PC12 cells respond to nerve growth factor (NGF) and other stimuli by shifting from a chromaffin cell-like phenotype to a neurite-bearing sympathetic neuron-like phenotype (Traverse et al., 1994). Here, we show that versican V1 induces NGF-independent neuronal differentiation and promotes neurite outgrowth by enhancing epidermal growth factor receptor (EGFR) and integrin activities. In addition, versican V1 isoform promotes neuronal differentiation of neural stem cells and neurite outgrowth of primary hippocampal neurons.
MATERIALS AND METHODS
Materials
Antibodies against EGFR, Erk, phosphorylated EGFR, phosphorylated Erk, β1 integrin, Trk (B-3), p27, cyclin A, cyclin D, acetylcholine receptor isoforms α3, α4, α7 β2, and Annexin V apoptosis detection kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against fibroblast growth factor (FGF) receptor and integrin α3 and α5 antibodies were purchased from Chemicon International (Temecula, CA). Integrin neutralizing antibody CD29 was from BD Biosciences (San Jose, CA). NGF, rat tail type I collagen, laminin, herbimycin A, MAP-2ab monoclonal antibody (mAb), glial fibrillary acidic protein-Cy3, goat anti-mouse-fluorescein isothiocyanate (FITC), horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody, poly-d-lysine and all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). AG1478 and nerve growth factor receptor (NGFR) inhibitor K252a were from Calbiochem (San Diego, CA). EGF and basic FGF were from Upstate Biotechnology (Lake Placid, NY). Tetramethylrhodamine B isothiocyanate (TRITC)-labeled phalloidin was from Molecular Probes (Eugene, OR). Enhanced chemiluminescence Western blot detection kit was from Amersham Biosciences (Piscataway, NJ). All tissue culture supplies were purchased from Invitrogen (Carlsbad, NY). The PC12 cell line was from the American Type Culture Collection (Manassas, VA).
Construct Generation and Expression
To study the effect of versican on neuronal differentiation, three constructs were used, including versican V1 (Sheng et al., unpublished data) and V2 isoforms, and a G3 construct described previously (Sheng, Wang, Liang, Zheng, Wu, Lee, Slingerland, Dumont, and Yang; unpublished data). To generate versican V2 construct, the CS fragment in the mini-versican was replaced with CSα domain, which was generated by polymerase chain reaction (PCR) by using two primers pCS1NMluI (aaa acg cgt cgt aaa aaa att gta tca gag cct aca) and pCS1CXhoI (ggg ctc gag tgt atc att gcc tgt gat) and chicken genomic DNA as the template. The PCR product was digested with restriction endonucleases MluI and XhoI. The PCR product, combined with EcoRI-MluI-digested N-terminal domain (from the mini-versican), was inserted into EcoRI-XhoI-digested G3 domain-containing vector (pcDNA3, obtained from the mini-versican construct) to generate the V2 construct. Versican V1, V2, and G3 constructs were stably expressed in PC12 cells by using LipofectAMINE according to the manufacturer's instructions. Cell lines were selected and maintained in 10% fetal bovine serum (FBS)/DMEM containing 1 mg of G418 per milliliter. To analyze gene expression, cell lysate and culture medium were prepared and subjected to Western blotting probed with antibodies indicated in each experiment by using the method described previously (Wu et al., 2001; Chen et al., 2002; Lee et al., 2002).
Cell Differentiation
Confluent PC12 cells were starved in RPMI 1640 medium containing 1% serum for 24 h. The cells were harvested, and 1 × 105 cells were seeded on six-well plates in RPMI 1640 medium containing 1% serum and incubated at 37°C for 14 d. Neurite outgrowth was measured and photographed. As well, 1 × 105 cells were seeded on plastic (uncoated), or collagen type I- (10 μg/ml) or laminin (20 μg/ml)-coated six-well plates in serum-free RPMI 1640 medium in the presence or absence of EGF at 50 ng/ml and incubated at 37°C for 10 d. EGF (50 ng/ml) was added to the cultures every other day to determine its effect on differentiation. Starved PC12 cells (1 × 105 cells) were also seeded on collagen type I-coated six-well plates in RPMI 1640 medium containing 1% serum in the presence or absence of 50 ng/ml NGF and incubated at 37°C for 7 d, to which 50 ng/ml NGF was added every other day. To quantify the extent of neurite outgrowth, we randomly scored 500 cells for each plate and calculated the percentage of cells with neurites longer than two cell bodies in length as described previously (Dikic et al., 1994). All studies were conducted in triplicate by using three different clones.
Immunofluorescence
PC12 cells were grown on type I collagen-coated glass coverslips in RPMI 1640 medium containing 1% serum for 16–48 h, fixed with 4% paraformaldehyde, and blocked with 1% bovine serum albumin. To detect extracellular and cell surface binding protein, the cells were first stained with 4B6 overnight at 4°C, washed with phosphate-buffered saline (PBS), and then permeabilized in PBS containing 0.2% Triton X-100. The cells were then incubated with TRITC-labeled phalloidin for 1.5 h at room temperature, washed, and incubated with FITC-conjugated anti-mouse Ig antibody. To examine intracellular protein expression, the cultures were first permeabilized in PBS containing 0.2% Triton X-100 and then stained with 4B6 overnight at 4°C. After wash, the cells were incubated with TRITC-labeled phalloidin and FITC-conjugated anti-mouse Ig antibody for 1.5 h at room temperature. After the final wash and mounting, the cells were examined using a confocal microscope with a 60× objective.
Hippocampal Neurite Outgrowth
Hippocampal neurons were isolated from rat brain hippocampi and adherent glial cells were removed (Barres et al., 1988). A monolayer of rat astroglial cells was prepared as described previously (Fidler et al., 1999). The glial cells were cultured in 10% FBS/DMEM and treated with glutamate (5 mM) to eliminate any contaminating neurons. Astroglial cells were also cultured in 10% FBS/DMEM on poly-D-lysine–treated glass coverslips overnight and transiently transfected with V1, V2, G3, and a control vector in serum-free DMEM for 6 h. The medium was removed and maintained in neurobasal medium plus B27 supplement and 0.5% FBS for 2 d to obtain monolayer cultures. Primary hippocampal neurons (1000 cells/cm2) were seeded on the top of the astroglial monolayer cultures and incubated in the same medium for 5 d. The cultures were fixed, permeabilized, and stained with neuron-specific MAP-2ab mAb and subsequently goat anti-mouse-FITC for neuron detection. Neuronal neurites were visualized under confocal microscopy. Neurite measurement was adapted from previous studies (Fidler et al., 1999). Briefly, 20 individual neurons from each culture were assessed: in a random sampling of 20 microscopic fields, the cell with the longest processes in each field was used for analysis. The length of a process was measured from the edge of the cell body to the growth tip. Three different experiments were carried out. The average length was derived from the measurements of 60 neurons and was analyzed by t test.
Blocking Assays
Starved PC12 cells (1 × 104) were seeded on 48-well dishes coated with type I collagen in serum-free RPMI 1640 medium in the presence or absence of purified anti-β1-integrin (CD29) mAb at concentrations of 0, 5, 10, 20, and 30 μg/ml and incubated at 4°C for 20 min and maintained at 37°C. Neurite outgrowth was examined under light microscopy and photographed.
Starved PC12 cells (2 × 104) were also seeded on 24-well dishes coated with type I collagen in serum-free RPMI 1640 medium in the presence or absence of Src-selective tyrosine kinase inhibitor herbimycin A at concentrations of 0, 0.2, 0.4, and 0.6 μg/ml, or EGFR-specific inhibitor AG1478 at concentrations of 0, 0.2, 0.4, and 0.6 μM, or NGF inhibitor K252a at concentrations of 0, 10, 20, and 40 nM. The cultures were incubated at 4°C for 20 min and then transferred to an incubator and maintained at 37°C. Neurite outgrowth was examined as above.
Isolation and Culture of Neural Stem Cells
Isolation and culture of neural stem cells were performed as described previously (Kukekov et al., 1999). The cells were seeded onto an ultralow attachment flat bottom of six-well plates at a cell density of 3000 cells/cm2 in DMEM/F12 (3 ml/well) containing 2 mM glutamine, 6% glucose, B27 supplement, 20 ng/ml EGF, and 10 ng/ml basic fibroblast growth factor. The medium was changed every other day. Three days after inoculation, cells formed small neurospheres and 7–10 d later, the neurospheres were collected for immunocytochemistry.
Coculture of Neural Stem Cells and Glial Cells
Neuroglial cells were seeded on poly-D-lysine–coated two-chamber slides at 70% density in DMEM containing 10% FBS and incubated overnight. The cells were transiently transfected with V1 and vector and incubated in DMEM containing 10% FBS for 2 d. The culture medium was replaced with serum-free coculture medium MEM (0.6 ml/chamber) containing N2 supplement, sodium pyruvate (1 mM), glucose (0.2 M), l-glutamine (2 mM), and ovalbumin and incubated overnight. Neurospheres suspended in serum-free coculture medium (0.6 ml) were seeded on top of the monolayer glial cultures (1.2-ml total medium) and incubated for 48 h. The culture medium was changed every 48 h with fresh coculture medium. Neurite outgrowth was monitored and photographed. At day 6, the cultures were fixed and stained with MAP-2ab after permeabilization.
RESULTS
Versican V1 Promotes Neuronal Differentiation and Neurite Outgrowth
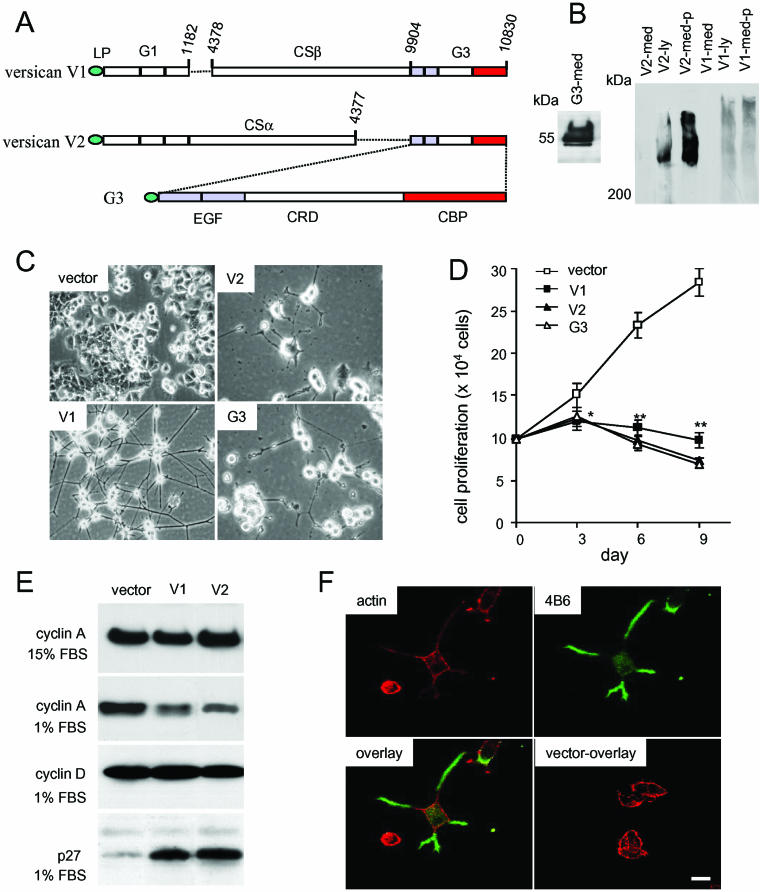
To study the effect of versican on neuronal differentiation, three versican constructs were cloned from chicken, V1, V2, and G3. Versican V1 and V2 constructs contain the full-length coding sequences of versican V1 and V2 isoforms, whereas the G3 construct has been described by us previously (Yang et al., 2000; Chen et al., 2003; Kiani et al., 2003). More than four PC12 clones stably transfected with versican V1, V2, G3, or a control vector (Figure 1A) were selected by Western blot. Typical results are shown (Figure 1B). Four clones were examined for cell differentiation. Cultured in medium containing 1% serum, versican V1-, V2-, and G3-transfected PC12 cells started to differentiate and grew neurites (Figure 1C). V1-transfected cells continued to grow and developed into fully differentiated neuronal morphology with long processes; the cells were viable for 2 wk. The outgrown neurites in V2- and G3-transfected cells were not sustained and gradually disappeared. When the cells were maintained in medium containing 15% serum, V1-, V2-, and G3-transfected cells exhibited slower growth rates and had a high proportion of cells undergoing modest autonomous differentiation with some neurite outgrowth compared with vector controls (our unpublished data).
Figure 1.
Expression of versican V1 isoform induces PC12 cell differentiation and process outgrowth. (A) Diagram of the structures of versican V1, V2, and G3 constructs. (B) Stable expression of V1, V2, or G3 in PC12 cells was confirmed on Western blot by using culture medium (med) and cell lysate (ly), with or without protein precipitation (p) to increase the loading capacity, probed with 4B6. (C) Versican V1-, V2-, and G3-transfected cells were cultured in 1% serum/RPMI 1640 medium for 2 wk. Some cells exhibited neurite outgrowth, an indication of cell differentiation. Typical fields are shown. Only versican V1-transfected cells exhibited sustained neurite outgrowth. (D) Counting of cell number indicated that V1-, V2-, or G3-transfected cells exhibited a significantly reduced rate of proliferation in 1% serum compared with the vector-transfected cells (n = 3, *p < 0.05, **p < 0.01). (E) Expression of cyclin A, cyclin D, and p27 was analyzed by Western blotting. Transfection of V1 and V2 isoforms reduced cyclin A but increased p27 expression in medium containing 1% serum. (F) PC12 cells stably expressing versican G3 were cultured on coverslips and incubated in medium containing 1% serum for 48 h. The cells were probed with antibody against an epitope in the G3 construct (4B6) before permeabilization and with TRITC-labeled phalloidin (binding to actin) after permeabilization. Vector-transfected cells were cultured and stained with the same antibodies as a control. G3 was stained on the cell surface of the neurites (in green). No staining was detected in the vector-transfected cells. Scale bar, 10 μm.
To examine whether these cells were undergoing apoptosis, we conducted Annexin V apoptosis assay by using method described previously (Wu et al., 2002) and found that V2-transfected PC12 cells had a higher proportion of cells in apoptosis (61.8 ± 4.9) compared with the vector- (43.2 ± 3.7) and V1 (40.7 ± 3.9)-transfected cells (n = 3, p < 0.01) when cultured in a medium containing 1% FBS for 10 d. In this culture condition, the numbers of versican V1-, V2-, and G3-expressing cells declined gradually due to differentiation and apoptosis, whereas the vector-transfected cells did not differentiate and continued to proliferate (Figure 1D). We did not detect obvious deviation among different clones. Consistent with cell differentiation and proliferation, cell cycle analysis indicated that V1- and V2-transfected PC12 cells had a higher proportion of cells detected in the G0/G1 phase (Table 1). Cell cycle-regulating protein cyclin A was down-regulated, whereas cyclin kinase inhibitor p27 was up-regulated in V1- and V2-transfected cells (Figure 1E). The differentiated cells were immunostained with a mAb 4B6 (which recognizes an epitope engineered in all constructs). We found that all expressed products tended to be localized around the neurites. Although versican is known to bind to the cell surface through interaction with hyaluronan by its N-terminal domain, we found large amounts of G3 product interacting with the neurites (Figure 1F).
Table 1.
Effect of versican on cell cycle regulation in PC12 cells
| 15% serum culture
|
1% serum culture
|
|||
|---|---|---|---|---|
| G0/G1 | S | G0/G1 | S | |
| Vector | 48.6 ± 2.3 | 37.3 ± 1.7 | 59.7 ± 2.6 | 28.4 ± 1.7 |
| V1 | 71.3 ± 3.6* | 21.4 ± 1.3* | 81.5 ± 3.1* | 10.2 ± 1.4* |
| V2 | 67.5 ± 3.1* | 22.8 ± 1.5* | 77.6 ± 2.9* | 11.4 ± 1.5* |
Cells expressing versican V1 and V2 had reduced S-phase entry but increased in G0/G1 phases compared with vector-transfected cells (n = 3, p < 0.001).
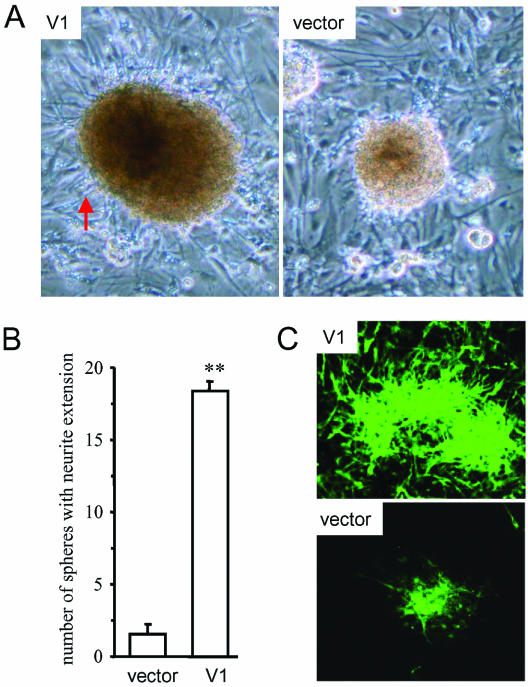
To corroborate the role of versican V1 in neuronal differentiation, rat neural stem cells were isolated and cocultured with rat astroglial cells that had been transiently transfected with versican V1 construct or a control vector. V1 expression was confirmed by Western blot (our unpublished data). The neural stem cells in both neurospheres and individual cells were seeded on the top of the astroglial cells and incubated for 6 d. The neurospheres cocultured with V1-transfected astroglial cells exhibited increased numbers of processes compared with these cocultured with vector-transfected cells (Figure 2, A and B). Stained with anti-MAP-2ab antibody for neuronal differentiation (Song et al., 2002), the neurospheres cocultured with V1-transfected astroglial cells exhibited significantly increased positively stained cells than those cocultured with vector-transfected cells (Figure 2C).
Figure 2.
Versican V1 promotes neuronal differentiation in neural stem cells. The neurospheres were cocultured with V1- or vector-transfected astroglial cells for 6 d. (A) Compared with vector-transfected cells, the V1-transfected glial cells facilitated cell survival of the neurospheres, which were larger and bore more outgrown neurites (arrow). (B) Effect of V1 and vector transfection on neurite outgrowth was quantified by counting the neurospheres bearing neurites. Twenty of the largest spheres per well were counted and the spheres with >10 extended neurites per neurosphere were considered as spheres with neurite outgrowth. More neurospheres with outgrown neurites were found in V1-transfected coculture (n = 3, error bar, SD; t test, **p < 0.001). (C) Cultures were stained with anti-MAP-2ab antibody, and more cells in V1-transfected coculture were positively stained (in green).
Versican V1-induced PC12 Differentiation Is EGF and NGF Independent
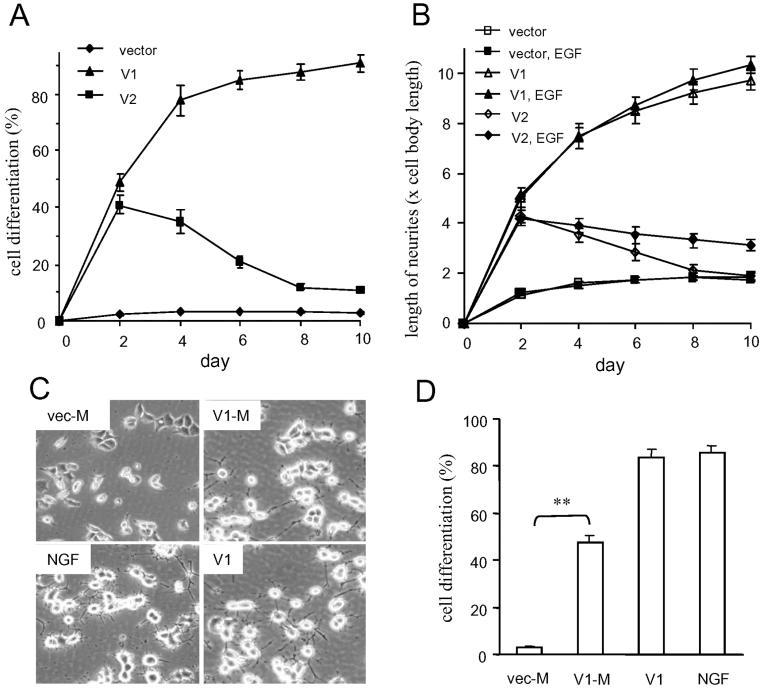
To identify the factors that contributed to V1-induced PC12 cell differentiation, PC12 cells stably expressing V1 were cultured in different conditions (Table 2). The cells did not develop neurites in serum-free medium on tissue culture plates. However, when the cells were seeded on collagen type I- or laminin-coated plates in serum-free medium, the cells differentiated (Figure 3A) and produced extensive neurites (Figure 3B). Although both V1 and V2 expression initiated cell differentiation, V1-transfected cells showed sustained and enhanced neurite growth, whereas V2-transfected cells ceased neurite expression and the cells gradually died. The addition of EGF to the culture only slightly increased neurite outgrowth in both V1- and V2-transfected cells (Table 2 and Figure 3B). When culture medium collected from vector-transfected (vec-M) or V1-transfected (V1-M) PC12 cells was introduced into parental PC12 cell cultures, the V1-conditioned medium induced PC12 cell differentiation (Figure 3, C and D). However, the differentiation was not as strong as that in cells stably expressing V1, which had equal activity as NGF in the induction of differentiation (Figure 3, C and D).
Table 2.
Versican induces neurite outgrowth of differentiated PC12 cells
| Vector | V1 | V2 | G3 | |
|---|---|---|---|---|
| No coating | - | - | - | - |
| EGF | - | - | - | - |
| Coll | - | ++++ | + | + |
| Coll + EGF | ± | +++++ | ++ | ++ |
| LM | - | +++++ | + | + |
| LM + EGF | ± | +++++ | ++ | ++ |
| NGF | - | - | - | - |
| Coll + NGF | ++++ | ++++ | ++++ | ++++ |
Vector-, V1-, and V2-transfected PC12 cells were seeded on tissue culture plates coated with or without type I collagen (Coll) or laminin (LM) in serum-free RPMI 1640 medium in the presence or absence of EGF or NGF and incubated for 7 d. Percentage of differentiated cells bearing at least one neurite of length ≥2 times its cell body diameter was calculated from counting 300 individual cells from randomly selected fields. -, <10%; ±, 11-15%; +, 16-25%; ++, 25-45%; +++, 46-65%; ++++, 65-85%; and +++++, 86-100%. The results were the means of six separate experiments by using three different clones.
Figure 3.
Effects of matrix molecules on V1-induced neuronal differentiation. (A) In collagen-coated serum-free culture, the effects of V1 and V2 on neuronal differentiation differed significantly in terms of the percentage of cells bearing neurites. (B) PC12 cell cultures were incubated in the presence or absence of EGF. After differentiation, the length of neurite was assessed. One hundred individual cells bearing the longest neurites from 10 light microscopic fields (10 cells from each field) were counted. The addition of EGF to serum-free cultures had little effect on neurite outgrowth in both V1- and V2-transfeced cells. The results were the means of three separate experiments. (C and D) Culture medium from V1-transfected PC12 cells (V1-M) or vector-transfected cells (vec-M) was added to parental PC12 cell cultures. V1-transfected cells alone (V1) or vector-transfected cells containing NGF (50 ng/ml) were used as controls. After 4 d of treatment, photographs of differentiation (C) and the percentage of cells bearing neurites (D) were acquired. The percentage of cells bearing neurites was counted using the same method as described in Table 2 (n = 3; for V1-M vs. vec-M, p < 0.001; V1 vs. NGF, p > 0.05). Each data point represented the mean ± SEM.
Versican V1 Promotes Hippocampal Neurite Outgrowth
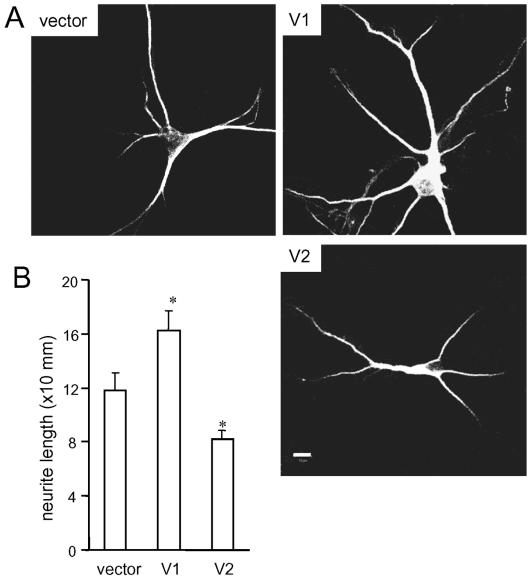
We then examined whether versican V1 enhanced neurite growth in primary neurons cocultured with astroglial cells. Rat hippocampal neurons were seeded on top of monolayer astroglial cultures transfected with versican V1, V2, or a control vector. After 5 d of coculture, the neurons were stained with anti-MAP-2ab antibody. V1-expressing astroglial cells promoted hippocampal neurite outgrowth, whereas V2 expression failed to support neurite outgrowth (Figure 4A). Measurement of neurite length indicated that these effects were statistically significant (Figure 4B). These results further dissected different effects of versican V1 and V2 isoforms on neurite outgrowth.
Figure 4.
Versican V1 promotes hippocampal neurite outgrowth. (A) Rat hippocampal neurons were seeded on astroglial monolayer cultures transfected with versican V1, V2, or a control vector. Five days later, the neurons were stained with MAP-2ab and anti-mouse IgG-FITC and examined under a fluorescence microscope. Representative images of the neuron(s) in the cultures were shown. Scale bar, 10 μm. (B) Mean of the longest neurites in each culture was derived from measurements of 60 neurons of three different experiments. Each data point represented the mean ± SEM (*p < 0.001).
Versican V1 Enhances and Sustains EGFR and Erk Phosphorylation
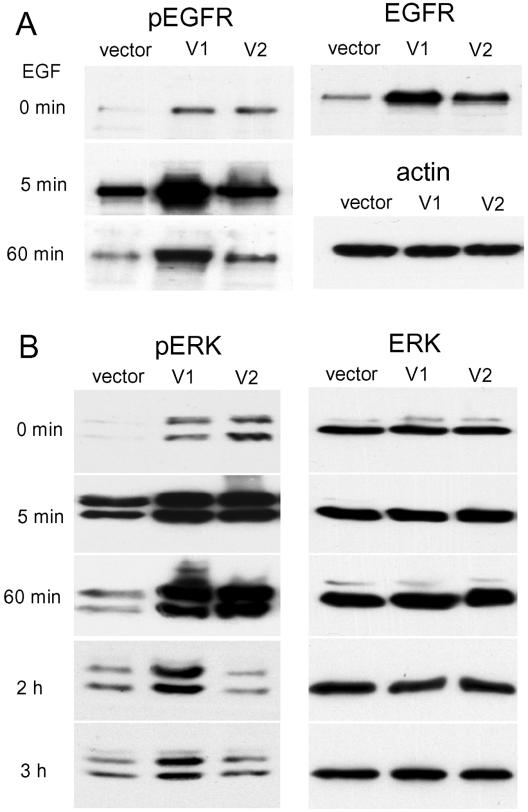
To investigate how versican induced PC12 cell differentiation, we analyzed expression of EGFR. EGFR expression was greatly up-regulated in versican V1- and V2-transfected cells; up-regulation was most evident in V1-transfected cells (Figure 5A). Versican V1 also induced EGFR phosphorylation. Furthermore, a sustained Erk phosphorylation (for up to 3 h), which is the downstream signal of EGFR, was observed in V1-transfected cells upon EGF stimulation, whereas the phosphorylation in the vector-transfected cells only lasted for 60 min (Figure 5B). This is in agreement with previous reports that a sustained Erk phosphorylation is required for PC12 cell differentiation (Vaudry et al., 2002). Although V2-transfected cells had enhanced Erk phosphorylation, it was not sustained. This might explain, at least in part, why V2-transfected PC12 cells did not undergo complete differentiation.
Figure 5.
Versican expression stimulates EGFR and Erk phosphorylation. (A) Phosphorylation of EGFR was analyzed in PC12 cells stably transfected with V1, V2, or a control vector. V1 and V2, particularly V1, up-regulated EGFR expression. On EGF stimulation, V1- and V2-transfected cells exhibited higher levels of EGFR phosphorylation. The phosphorylation was more sustained in V1-transfected cells than in V2-transfected cell. (B) Phosphorylation of Erk was enhanced and sustained in V1-transfected cells. In the serum deprivation-starved PC12 cells, V1 and V2 expression stimulated relatively elevated levels of Erk phosphorylation. Addition of EGF enhanced Erk phosphorylation in both V1- and V2-transfected cells. However, phosphorylation was sustained longer in V1-transfected cells compared with V2-transfected cells. The levels of Erk protein were similar in all cells transfected with V1, V2, or a control vector.
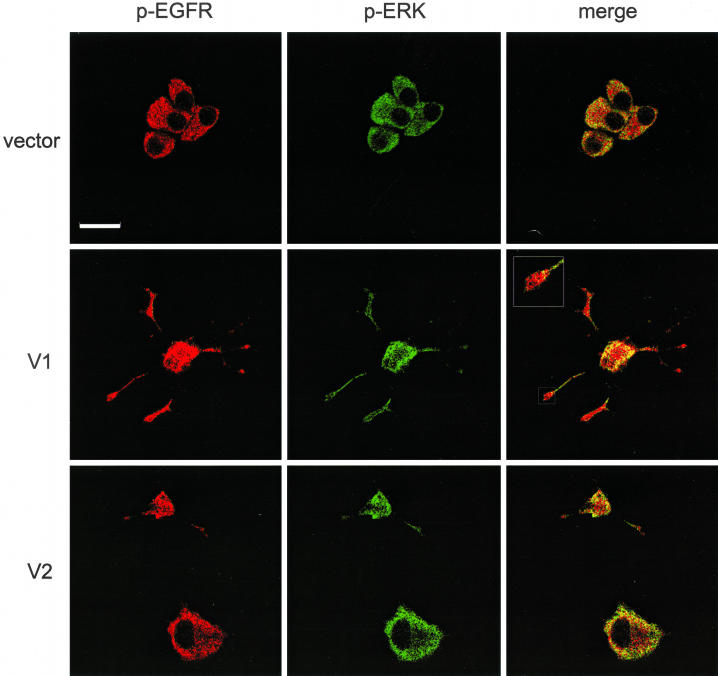
The effect of versican on EGFR expression and distribution on the cell surface was examined. PC12 cells transfected with V1 and vector were treated with or without EGF. After EGF treatment, cells were stained with antiphosphorylated EGFR (pEGFR) and antiphosphorylated Erk (pErk) antibodies (Figure 6). V1-transfected cells exhibited increased levels of EGFR clusters on the cell surface compared with the vector-transfected cells. The receptors were densely distributed on the neurites, particularly on the ends of the neurites and axons. Normally, upon EGFR tyrosine phosphorylation, EGFR undergoes rapid degradation. One hour after EGF stimulation, EGFR phosphorylation was still evident in V1-transfected cells, whereas the phosphorylation was barely detected in the vector-transfected cells.
Figure 6.
Distribution of EGFR and Erk in versican-transfected cells. V1-, V2-, or vector-transfected PC12 cells were cultured in 1% serum/RPMI 1640 medium for 72 h, followed by stimulation with EGF for 1 h and doubly stained with anti-pEGFR (TRITC) and pErk (FITC) antibodies after permeabilization. V1-transfected cells exhibited increased EGFR phosphorylation on the cell surface, particularly on the ends of neurites, and more intensive pErk staining in the cytoplasm. Scale bar, 10 μm.
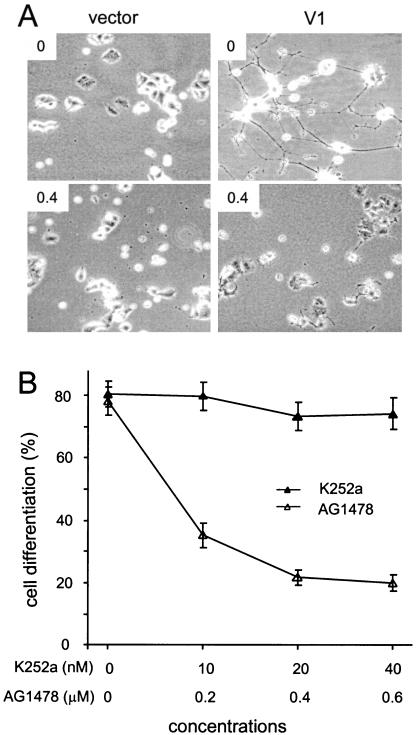
To confirm the role of the EGFR signal in V1-induced PC12 cell differentiation, a specific EGFR tyrosine phosphorylation inhibitor AG1478 was added to the cultures. This inhibitor significantly blocked V1-transfected PC12 cell differentiation (Figure 7, A and B). In contrast, addition of the NGFR inhibitor K252a had little effect on V1-induced differentiation (Figure 7B).
Figure 7.
Involvement of EGFR and NGFR in neurite outgrowth. (A) Cells were cultured in the presence or absence of EGFR inhibitor AG1478 (0 and 0.4 μM) for 5 d. Differentiation of V1-transfected PC12 cells was greatly inhibited. (B) Cells were also treated with different concentrations of AG1478 or NGFR inhibitor K252a for 5 d. Cell differentiation was indicated by the percentages of cells bearing neurites. Differentiation of V1-transfected PC12 cells was significantly inhibited by AG1478 (n = 3, **p < 0.001), but not by K252a (n = 3, p > 0.05).
Versican V1-induced PC12 Differentiation Is Integrin Dependent
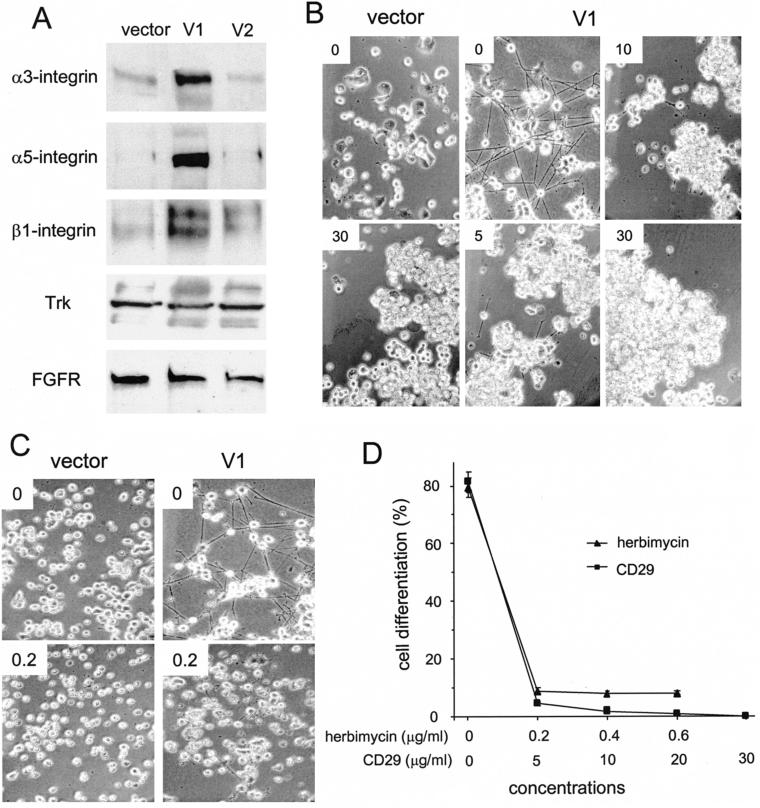
In serum-free medium, vector-transfected PC12 cells seeded on collagen-coated plates did not differentiate, whereas the V1-transfected cells did. This suggested that other factors such as integrins might be involved in V1-induced differentiation. We hypothesized that the cells might require anchorage to a matrix molecule through integrins before the initiation of differentiation. Thus, integrin expression was examined in PC12 cells stably transfected with V1, V2, or vector, and it was observed that integrin β1, α3, and α5 were up-regulated in V1-transfected cells (Figure 8A). To confirm whether these up-regulated integrins were responsible for V1-induced differentiation, we performed an integrin blocking assay by using the β1-integrin blocking mAb (CD29). At a concentration as low as 5 μg/ml, CD29 antibody inhibited >90% of neurite outgrowth (Figure 8B). At increased concentrations, CD29 antibody completely abolished V1's effect on neurite outgrowth (Figure 8D).
Figure 8.
Involvement of integrins in V1-induced neuronal differentiation. (A) An equal number of transfected PC12 cells were grown in 1% serum/RPMI 1640 medium for 24 h and lysed. Equal amounts of proteins were analyzed on Western blot probed with anti-integrin (β1, α3, and α5) antibodies, or anti-Trk and FGFR antibodies. V1 transfection enhanced integrin expression. (B and C) PC12 cells were cultured in the presence or absence of anti-CD29 antibody at concentrations of 0, 5, 10, and 30 μg/ml (B) or herbimycin A at concentrations of 0 and 0.2 μg/ml (C) for 7 d. Photographs of representative fields of the cultures are shown. (D) Percentage of cells bearing neurites treated with different concentrations of herbimycin or anti-CD29 antibody was counted using the same method as described in Table 2. Each data point represented the mean ± SEM (n = 3). Treatment with anti-CD29 antibody or herbimycin inhibited V1-induced differentiation.
Src is downstream of integrin signal and is activated during PC12 cell differentiation (Vaudry et al., 2002). To examine the effect of Src on V1-induced differentiation, the selective Src kinase inhibitor herbimycin A was used to block the signals. Because Src family kinases associate with both integrin-based focal adhesion complexes and receptor tyrosine kinases (Schlaepfer and Hunter, 1998), it is likely that signals originating from either locus would be sensitive to the Src inhibitor. V1-mediated differentiation was inhibited by low concentrations of herbimycin (Figure 8C and D). However, unlike β1-integrin blocking antibody, high concentrations of herbimycin caused cell death.
DISCUSSION
Versican V1 Induces Neuronal Differentiation
The PC12 cell line was derived from rat pheochromocytoma, a tumor arising from chromaffin cells of the adrenal medulla. The cells can proliferate for self-renewal, differentiate into neuronal-like cells (Vaudry et al., 2002), and/or undergo apoptosis (Foehr et al., 2000). Due to these special properties, the cell line has been a useful model for studying neuronal differentiation and signal transduction (Vaudry et al., 2002). NGF was the first, and to date, the strongest, factor identified in the induction of PC12 cell differentiation. Our results for the first time demonstrated that versican was able to initiate neuronal differentiation in PC12 cells. However, only the V1 isoform could induce mature neuronal differentiation, including fully developed neurites: V1-induced differentiation was as extensive as NGF-induced differentiation in terms of neurite length, number, and longevity. Previous studies have shown that different isoforms of versican exhibit striking and distinct features of expression (Bandtlow and Zimmermann, 2000). Versican V2 is present at relatively low levels during the late embryonic and early postnatal period, but increases dramatically during maturation. In contrast, the versican V1 levels double between E14 and birth, after which they decrease by >90% to reach a low “mature” level that remains stable throughout adulthood (Milev et al., 1998). Thus, versican V2, along with brevican, becomes a major chondroitin sulfate proteoglycan in the mature brain (Schmalfeldt et al., 1998). These evident and clear-cut changes suggest that versican V1 and V2 isoforms play important roles during neurogenesis and mature brain homeostasis.
The role of versican V1 in neuronal differentiation was further confirmed in neural stem cells. Previous work has shown that, under certain circumstances, neural stem cells can differentiate into neurons. When cocultured with versican V1-transfected astroglial cells, significantly increased number of neural stem cells differentiated into neurons. This indicated that versican V1 served as an inducing agent for neuronal differentiation or promoted the survival of differentiated neurons, further confirming the role of versican in neuronal development.
V1 Induces Differentiation through an EGFR- and Integrin-mediated Erk Pathway
PC12 cell differentiation is known to require the activation of growth factor receptors, such as NGF-induced TrkA activation (Vaudry et al., 2002) and FGF-induced FGF receptor phosphorylation (Hadari et al., 1998). Overexpression of EGFR or insulin receptor can also mediate PC12 cell differentiation (Traverse et al., 1994). However, activation of endogenous EGFR by EGF stimulates proliferation, rather than differentiation, of PC12 cells. The responses to NGF and EGF both require Erk, an MAPK. A strong mitogen-like signal is required for PC12 differentiation, and sustained mitogen signaling is required to maintain a continuous neurite outgrowth. In this sense, it is no surprise that V1 can induce a full cell differentiation and neurite outgrowth in PC12 cells, because V1 expression produced a strong and sustained mitogen-like signal activating EGFR and Erk.
To verify that sustained Erk phosphorylation is induced by V1 transfection in PC12 cells, blocking assays were performed, and we verified that signals upstream from integrins and EGFR were responsible for the V1-induced cell differentiation. This is consistent with our findings that in V1-expressing cells, integrin β1, α3, and α5, and EGFR were up-regulated at both protein levels and phosphorylation levels. The specific blockade of Src activity, which is associated with both integrin-based focal adhesion complexes (Schlaepfer and Hunter, 1998) and receptor tyrosine kinases (Medema and Bos, 1993), abolished V1-induced PC12 cell differentiation. Signals from integrins can mediate the integrin–EGFR physical interaction and enhanced ligand-induced EGFR activation (Yamada and Even-Ram, 2002). In the presence of EGF, overexpression of EGFR leads to partial differentiation of PC12 cells compared with the NGF-induced signal (Traverse et al., 1994). In our study, we found that V1 was as strong as NGF in inducing PC12 differentiation, and the integrin signals alone could not mediate PC12 differentiation. These results suggest that versican V1-mediated differentiation might be through an enhanced collaborative signal from both integrins and EGFR, because the versican G3 domain interacts with β1-integrin and is involved in EGFR functioning (Zhang et al., 1998b; Wu et al., 2002). However, the G3 domain alone was insufficient for a complete neuronal differentiation, and the CSβ domain seemed to be important in this action. The mechanism underlying this action awaits further investigation.
V1 Promotes Neurite Outgrowth
Previous studies have demonstrated that versican is distributed perineuronally in the brain (Oohira et al., 2000). In our study, we found that versican bound on the cell surface and surrounded the neurites. This distribution suggests that versican may play a role in promoting and supporting neurite outgrowth, and we confirmed that versican V1 promoted neurite outgrowth in both PC12 cells and primary hippocampal neurons, although the effects of V1 and V2 on hippocampal neurons was weaker. Enhanced EGFR staining was found on the neurites in V1-transfected PC12 cells. This is consistent with the findings that up-regulated EGFR levels and enhanced Erk phosphorylation were found in V1-transfected cells. Blockade of EGFR greatly inhibited neurite development. The results suggest that an EGFR-mediated signal is important for neurite outgrowth.
Our finding that V1 and V2 exhibited complementary effects on neurite outgrowth of primary hippocampal neurons may be important in understanding the roles of versican in neurite development and axonal regeneration after injuries. A previous study has shown that versican V2 is up-regulated after brain injury (Asher et al., 2002), and this isoform exerts an inhibitory effect on axon growth (Schmalfeldt et al., 2000). Versican V1 and V0 were not detected in the damaged tissue nor in the surrounding region after CNS injury (Fidler et al., 1999). In addition, coculture experiments show that versican isoforms V0 and V1 have no inhibitory effect on axon extension (Fidler et al., 1999). Our result suggests that the CSα domain of V2 is responsible for the inhibitory effect, whereas the CSβ domain of V1 is associated with the enhancement of neurite outgrowth.
Recent studies of spinal cord injury have demonstrated that delivery of chondroitinase to the lesioned dorsal columns promotes axonal regeneration (Bradbury et al., 2002). Chondroitinase can alter the biochemical composition of the perineuronal net (that surrounds neurons) by degrading its glycosaminoglycan components (Pizzorusso et al., 2002). These studies are consistent with our finding that versican V2, which is the predominant form of versican in the mature brain and is up-regulated after brain injury, plays an inhibitory role in neurite outgrowth. Treatment with chondroitinase might abolish the inhibitory effect of the CSα domain. Whereas versican V1 functions in regulating brain development, V2 may play a part in maintaining brain homeostasis. After CNS injury, genes favoring neuronal regeneration (e.g., V1) seem to not be expressed; instead, proteoglycans (e.g., V2) that normally expressed in the mature brain, and collagens, which have been believed to be important in blocking axon extension and path finding, are overexpressed. Treatment with chondroitinase may thus abolish V2 and other inhibitory proteoglycans' inhibitory effect on neurite outgrowth, and promote axonal regeneration.
Together, the successful cloning and expression of full-length versican V1 and V2 isoforms have allowed us, for the first time, to study their functions in defined conditions. Our results provided strong evidence that versican actively regulates neuronal differentiation, maturation, neurite outgrowth, and synaptic transmission. Further studies will shed light on the role of versican in CNS injury repair and the development of neuron-related diseases.
Acknowledgments
This work was supported by Grant NA-5127 from Heart and Stroke Foundation of Ontario and Grant MOP-62729 from Canadian Institutes of Health Research to B.B.Y.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0667. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–09–0667.
Abbreviations used: CNS, central nerve system; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; Erk, extracellular regulated kinase; FBS, fetal bovine serum; NGF, nerve growth factor.
References
- Alema, S., Casalbore, P., Agostini, E., and Tato, F. (1985). Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature 316, 557–559. [DOI] [PubMed] [Google Scholar]
- Ang, L.C., Zhang, Y., Cao, L., Yang, B.L., Young, B., Kiani, C., Lee, V., Allan, K., and Yang, B.B. (1999). Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J. Neuropathol. Exp. Neurol. 58, 597–605. [DOI] [PubMed] [Google Scholar]
- Asher, R.A., Morgenstern, D.A., Shearer, M.C., Adcock, K.H., Pesheva, P., and Fawcett, J.W. (2002). Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 22, 2225–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspberg, A., Binkert, C., and Ruoslahti, E. (1995). The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc. Natl. Acad. Sci. USA 92, 10590–10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow, C.E., and Zimmermann, D.R. (2000). Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 80, 1267–1290. [DOI] [PubMed] [Google Scholar]
- Barres, B.A., Silverstein, B.E., Corey, D.P., and Chun, L.L. (1988). Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1, 791–803. [DOI] [PubMed] [Google Scholar]
- Bradbury, E.J., Moon, L.D., Popat, R.J., King, V.R., Bennett, G.S., Patel, P.N., Fawcett, J.W., and McMahon, S.B. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640. [DOI] [PubMed] [Google Scholar]
- Chen, L., Wu, Y., Lee, V., Kiani, C., Adams, M.E., Yao, Y., and Yang, B.B. (2002). The folded modules of aggrecan G3 domain exert two separable functions in glycosaminoglycan modification and product secretion. J. Biol. Chem. 277, 2657–2665. [DOI] [PubMed] [Google Scholar]
- Chen, L., Yang, B.L., Wu, Y., Yee, A., and Yang, B.B. (2003). G3 domains of aggrecan and PG-M/versican form intermolecular disulfide bonds that stabilize cell-matrix interaction. Biochemistry 42, 8332–8341. [DOI] [PubMed] [Google Scholar]
- Dikic, I., Schlessinger, J., and Lax, I. (1994). PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr. Biol. 4, 702–708. [DOI] [PubMed] [Google Scholar]
- Fidler, P.S., et al. (1999). Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J. Neurosci. 19, 8778–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehr, E.D., Bohuslav, J., Chen, L.F., DeNoronha, C., Geleziunas, R., Lin, X., O'Mahony, A., and Greene, W.C. (2000). The NF-kappa B-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J. Biol. Chem. 275, 34021–34024. [DOI] [PubMed] [Google Scholar]
- Hadari, Y.R., Kouhara, H., Lax, I., and Schlessinger, J. (1998). Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18, 3966–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R.O. (1987). Integrins: a family of cell surface receptors. Cell 48, 549–554. [DOI] [PubMed] [Google Scholar]
- Isogai, Z., Aspberg, A., Keene, D.R., Ono, R.N., Reinhardt, D.P., and Sakai, L.Y. (2002). Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 277, 4565–4572. [DOI] [PubMed] [Google Scholar]
- Ito, K., Shinomura, T., Zako, M., Ujita, M., and Kimata, K. (1995). Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J. Biol. Chem. 270, 958–965. [DOI] [PubMed] [Google Scholar]
- Juliano, R.L., and Haskill, S. (1993). Signal transduction from the extracellular matrix. J. Cell Biol. 120, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima, H., Hirose, M., Hirose, J., Nagakubo, D., Plaas, A.H., and Miyasaka, M. (2000). Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 275, 35448–35456. [DOI] [PubMed] [Google Scholar]
- Kiani, C., Chen, L., Lee, V., Zheng, P.S., Wu, Y., Wen, J., Cao, L., Adams, M.E., Sheng, W., and Yang, B.B. (2003). Identification of the motifs and amino acids in aggrecan G1 and G2 domains involved in product secretion. Biochemistry 42, 7226–7237. [DOI] [PubMed] [Google Scholar]
- Kukekov, V.G., Laywell, E.D., Suslov, O., Davies, K., Scheffler, B., Thomas, L.B., O'Brien, T.F., Kusakabe, M., and Steindler, D.A. (1999). Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp. Neurol. 156, 333–344. [DOI] [PubMed] [Google Scholar]
- Landolt, R.M., Vaughan, L., Winterhalter, K.H., and Zimmermann, D.R. (1995). Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development 121, 2303–2312. [DOI] [PubMed] [Google Scholar]
- LeBaron, R.G., Zimmermann, D.R., and Ruoslahti, E. (1992). Hyaluronate binding properties of versican. J. Biol. Chem. 267, 10003–10010. [PubMed] [Google Scholar]
- Lee, V., Chen, L., Paiwand, F., Cao, L., Wu, Y., Inman, R., Adams, M.E., and Yang, B.B. (2002). Cleavage of the carboxyl tail from the G3 domain of aggrecan but not versican and identification of the amino acids involved in the degradation. J. Biol. Chem. 277, 22279–22288. [DOI] [PubMed] [Google Scholar]
- Lemire, J.M., Braun, K.R., Maurel, P., Kaplan, E.D., Schwartz, S.M., and Wight, T.N. (1999). Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1630–1639. [DOI] [PubMed] [Google Scholar]
- Matsumoto, K., Shionyu, M., Go, M., Shimizu, K., Shinomura, T., Kimata, K., and Watanabe, H. (2003). Distinct interaction of versican/PG-M with hyaluronan and link protein. J. Biol. Chem. 278, 41205–41212. [DOI] [PubMed] [Google Scholar]
- Medema, R.H., and Bos, J.L. (1993). The role of p21ras in receptor tyrosine kinase signaling. Crit. Rev. Oncog. 4, 615–661. [PubMed] [Google Scholar]
- Milev, P., Maurel, P., Chiba, A., Mevissen, M., Popp, S., Yamaguchi, Y., Margolis, R.K., and Margolis, R.U. (1998). Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem. Biophys. Res. Commun. 247, 207–212. [DOI] [PubMed] [Google Scholar]
- Mongiat, M., Fu, J., Oldershaw, R., Greenhalgh, R., Gown, A.M., and Iozzo, R.V. (2003). Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J. Biol. Chem. 278, 17491–17499. [DOI] [PubMed] [Google Scholar]
- Olin, A.I., Morgelin, M., Sasaki, T., Timpl, R., Heinegard, D., and Aspberg, A. (2001). The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J. Biol. Chem. 276, 1253–1261. [DOI] [PubMed] [Google Scholar]
- Oohira, A., Matsui, F., Tokita, Y., Yamauchi, S., and Aono, S. (2000). Molecular interactions of neural chondroitin sulfate proteoglycans in the brain development. Arch. Biochem. Biophys. 374, 24–34. [DOI] [PubMed] [Google Scholar]
- Paulus, W., Baur, I., Dours-Zimmermann, M.T., and Zimmermann, D.R. (1996). Differential expression of versican isoforms in brain tumors. J. Neuropathol. Exp. Neurol. 55, 528–533. [DOI] [PubMed] [Google Scholar]
- Pizzorusso, T., Medini, P., Berardi, N., Chierzi, S., Fawcett, J.W., and Maffei, L. (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Santiago-Garcia, J., Kodama, T., and Pitas, R.E. (2003). The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J. Biol. Chem. 278, 6942–6946. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., and Hunter, T. (1998). Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8, 151–157. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt, M., Bandtlow, C.E., Dours-Zimmermann, M.T., Winterhalter, K.H., and Zimmermann, D.R. (2000). Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 113, 807–816. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt, M., Dours-Zimmermann, M.T., Winterhalter, K.H., and Zimmermann, D.R. (1998). Versican V2 is a major extracellular matrix component of the mature bovine brain. J. Biol. Chem. 273, 15758–15764. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Nishida, Y., Ito, K., and Kimata, K. (1993). cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J. Biol. Chem. 268, 14461–14469. [PubMed] [Google Scholar]
- Song, H., Stevens, C.F., and Gage, F.H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. [DOI] [PubMed] [Google Scholar]
- Traverse, S., Seedorf, K., Paterson, H., Marshall, C.J., Cohen, P., and Ullrich, A. (1994). EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr. Biol. 4, 694–701. [DOI] [PubMed] [Google Scholar]
- Vaudry, D., Stork, P.J., Lazarovici, P., and Eiden, L.E. (2002). Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648–1649. [DOI] [PubMed] [Google Scholar]
- Walker, H.A., Whitelock, J.M., Garl, P.J., Nemenoff, R.A., Stenmark, K.R., and Weiser-Evans, M.C. (2003). Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol. Biol. Cell 14, 1941–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Chen, L., Zheng, P.S., and Yang, B.B. (2002). beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J. Biol. Chem. 277, 12294–12301. [DOI] [PubMed] [Google Scholar]
- Wu, Y., et al. (2001). Identification of the motif in versican G3 domain that plays a dominant-negative effect on astrocytoma cell proliferation through inhibiting versican secretion and binding. J. Biol. Chem. 276, 14178–14186. [DOI] [PubMed] [Google Scholar]
- Yamada, K.M., and Even-Ram, S. (2002). Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75–E76. [DOI] [PubMed] [Google Scholar]
- Yang, B.L., Cao, L., Kiani, C., Lee, V., Zhang, Y., Adams, M.E., and Yang, B.B. (2000). Tandem repeats are involved in G1 domain inhibition of versican expression and secretion and the G3 domain enhances glycosaminoglycan modification and product secretion via the complement-binding protein-like motif. J. Biol. Chem. 275, 21255–21261. [DOI] [PubMed] [Google Scholar]
- Yang, B.L., Zhang, Y., Cao, L., and Yang, B.B. (1999). Cell adhesion and proliferation mediated through the G1 domain of versican. J. Cell Biochem. 72, 210–220. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Cao, L., Kiani, C.G., Yang, B.L., and Yang, B.B. (1998a). The G3 domain of versican inhibits mesenchymal chondrogenesis via the epidermal growth factor-like motifs. J. Biol. Chem. 273, 33054–33063. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Cao, L., Yang, B.L., and Yang, B.B. (1998b). The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J. Biol. Chem. 273, 21342–21351. [DOI] [PubMed] [Google Scholar]
- Zimmermann, D.R., and Ruoslahti, E. (1989). Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 8, 2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]