Abstract
Introduction
Idiopathic intracranial hypertension (IIH) is increasingly recognized as a cause of spontaneous cerebrospinal (CSF) leak in the ENT and neurosurgical literature. The diagnosis of IIH in patients with spontaneous CSF leaks is classically made a few weeks after surgical repair of the CSF leak when symptoms and signs of elevated intracranial pressure (ICP) appear.
Methods
Case reports and literature review. Two young obese women developed spontaneous CSF rhinorrhea related to an empty sella in one, and a cribriform plate encephalocele in the other. Both patients underwent surgical repair of the CSF leak. A few weeks later, they developed chronic headaches and bilateral papilledema. Lumbar punctures showed elevated CSF-opening pressures with normal CSF contents, with temporary improvement of headaches. A man with a three-year history of untreated IIH developed spontaneous CSF rhinorrhea. He experienced improvement of his headaches and papilledema after a CSF shunting procedure, and the rhinorrhea resolved after endoscopic repair of the leak.
Results
These cases and the literature review confirm a definite association between IIH and spontaneous CSF leak based on: 1) similar demographics; 2) increased ICP in some patients with spontaneous CSF leak after leak repair; 3) higher rate of leak recurrence in patients with raised ICP; 4) patients with intracranial hypertension secondary to tumors may develop CSF leak, confirming that raised ICP from other causes than IIH can cause CSF leak.
Conclusions
CSF leak may occasionally keep IIH patients symptom-free; however, classic symptoms and signs of intracranial hypertension may develop after the CSF leak is repaired, exposing these patients to a high risk of recurrence of the leak unless an ICP-lowering intervention is performed.
Keywords: papilledema, rhinorrhea, cerebrospinal fluid leak, idiopathic intracranial hypertension
Introduction
CSF leaks have been traditionally classified as traumatic or non-traumatic (1,2). Non-traumatic CSF leak may be spontaneous in the absence of obvious cause, such as skull base abnormalities or bone erosion related to tumors or hydrocephalus (1,2,3). Spontaneous CSF leaks are sometimes referred to as high-pressure leaks when increased ICP contributes to the development of the CSF leak (2,3). Idiopathic intracranial hypertension (IIH) is increasingly recognized as a cause of spontaneous CSF leak in the ENT and neurosurgical literature (2-8). Over the past 20 years, a few authors have suggested that so-called “primary spontaneous CSF leaks” might represent a form of IIH (2-8). Some of these patients are asymptomatic or only have symptoms attributable to the CSF leak (such as rhinorrhea, CSF hypotension-related headaches, or bacterial meningitis) while the leak is active. The diagnosis of IIH is typically made a few weeks or months after surgical repair of the CSF leak, because of increased ICP resulting in classic IIH symptoms and signs (9). More rarely, patients with a known diagnosis of IIH may develop a spontaneous CSF leak, presumably directly secondary to the chronically raised ICP with skull base erosion and meningoceles (2).
Material and methods
Medical records and neuroimaging of the illustrative cases were reviewed. PubMed was searched for English-language articles published before January 2013 using the search terms “idiopathic intracranial hypertension”, “encephalocele”, “skull-base defect”, “spontaneous cerebrospinal fluid leak”, “CSF rhinorrhea”, and “CSF otorrhea”. The reference lists of identified articles were also searched for further relevant articles.
Case reports
Case 1
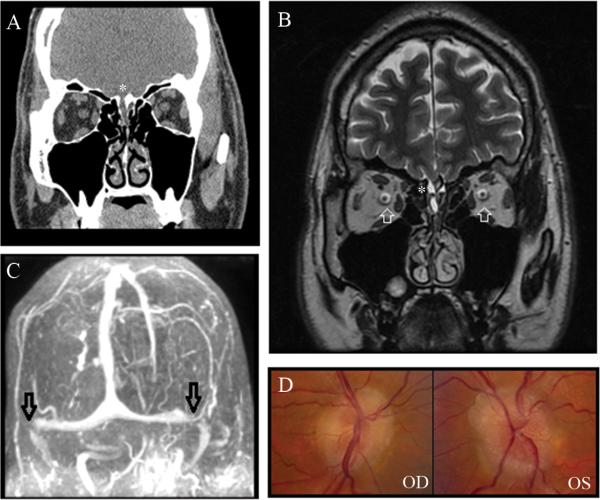
A 49-year old obese African-American woman developed isolated CSF rhinorrhea, which was initially mistaken for sinusitis. One year later, she developed a bacterial meningitis, and brain imaging demonstrated a skull-base defect in the right cribiform area causing meningocele with CSF leak (Fig. 1A). She underwent endoscopic sinus surgery with repair of the leak. Three months later, she complained of headaches and transient visual obscurations and was found to have bilateral florid papilledema (Fig. 1B). Visual fields showed bilateral enlarged blind spots and nasal steps (Fig. 1C). A lumbar puncture showed increased CSF opening pressure (OP) at 37 cmH2O, and resulted in temporary resolution of the headaches. She underwent a ventriculoperitoneal shunt with immediate resolution of her symptoms and signs of raised ICP. The CSF leak did not recur.
Figure 1.
A. Coronal view of CT of the head without contrast showing dehiscence of the right cribiform plate with soft tissue in the right olfactory recess, consistent with meningocele in this region (white arrow). B. Fundus photographs obtained after repair of the CSF leak, showing florid bilateral papilledema with obliteration of the central cup and congestion of the veins OU. C. Humphrey visual fields, performed on the SITA-fast 24-2 program, showing bilateral enlarged blind spots and bilateral nasal steps, OS greater than OD.
Case 2
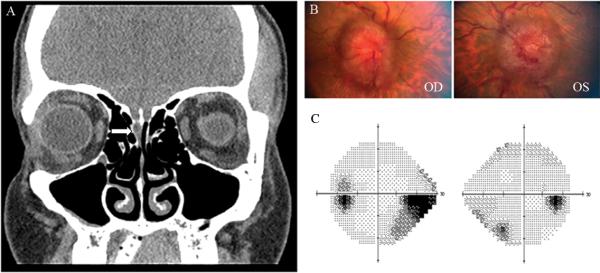
A 32 year-old obese white woman was found to have isolated CSF rhinorrhea after presenting with mild postural headaches. Brain imaging showed an empty sella and intrasellar bone erosion (Fig. 2A and 2B). She underwent endoscopic surgical repair of the CSF leak and her headaches resolved after the procedure. However, a few weeks later, persistent headaches developed, and she experienced transient visual obscurations. She was found to have bilateral optic disc edema (Fig. 2C) with enlarged blind spots on visual fields. A lumbar puncture showed elevated CSF OP at 42 cmH2O and resulted in temporary resolution of the headaches. She subsequently underwent a CSF shunting procedure with complete resolution of all symptoms and signs of intracranial hypertension. The CSF leak did not recur.
Figure 2.
A. T1 sagittal view of brain MRI with gadolinium showing an empty sella (white arrow). B. Coronal CT of the head showing intrasellar bone erosion (asterisk). C. Fundus photographs obtained after repair of the CSF leak, showing moderate papilledema OU.
Case 3
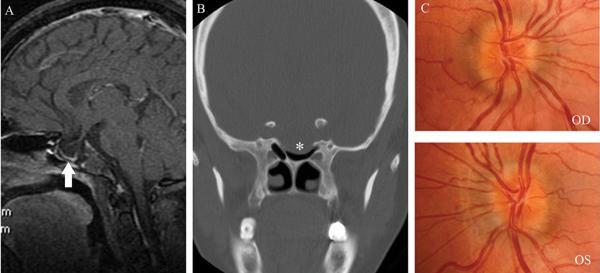
A 43 year-old mildly overweight white man was followed for 3 years for mildly-symptomatic IIH treated only with episodic acetazolamide. He had moderate bilateral papilledema with enlarged blind spots, but no or very mild headaches, and was most often doing well without treatment. He consulted again for worsening of headaches, which were occurring daily and were associated with tinnitus, and transient visual obscurations. Repeat neuro-ophthalmic examination showed persistent, stable mild papilledema OU (Fig. 3D) and enlarged blind spots OU. Repeat lumbar puncture showed elevated CSF OP at 33.5 cmH2O. His headaches improved, but around the same time he noticed CSF rhinorrhea. Repeat brain imaging showed a right cribriform plate encephalocele responsible for the CSF leak, in addition to previously known empty sella and bilateral transverse sinus stenoses (Fig. 3A, 3B, 3C). He was placed on prophylactic antibiotics and underwent a ventriculoperitoneal shunt procedure aimed at decreasing his ICP. The CSF rhinorrhea markedly decreased after the procedure, and endoscopic repair of the leak was subsequently performed. One month later, he remained asymptomatic, with improvement of his papilledema.
Figure 3.
A. Coronal view of CT of the head without contrast showing dehiscence of the right cribiform plate (asterisk) with soft tissue in the right olfactory recess, consistent with meningocele in this region; B. T2 coronal view of brain MRI showing hyperintense fluid and soft tissue in the right olfactory recess (asterisk) and distension of the peri-optic nerve subarachnoid CSF space (white arrows); C.Head MRV showing bilateral distal transverse stenoses (black arrows). D. Bilateral chronic papilledema.
Review of the literature
Table 1 presents a summary of reports in the English literature of spontaneous CSF leaks associated with presumed or diagnosed IIH (2-23).
Table 1.
Reports in the English literature of CSF leaks associated with symptoms and signs of presumed idiopathic intracranial hypertension
| Case series (reference) | Number of cases | % Women | Mean age (years) | BMI (kg/m2) | CSF OP (cmH2O) | Clinical presentation | Site of leakage (N° of patients) | Treatment | Diagnosis of IIH |
|---|---|---|---|---|---|---|---|---|---|
| Brisman et al 1970* (10) | 1 | 100 | 44 | ? | “Elevated” | Rhinorrhea | Cribiform plate | Surgical (craniotomy + sealing of the cribiform plate with muscle) | Yes |
| Applebaum and Desai 1980 (11) | 1 | 100 | 52 | “Moderately obese” | ? | Rhinorrhea, previous meningitis | Sella turcica | Surgical repair | Maybe |
| Eljamel and Foy 1991 (12) | 2 | ? | ? | ? | “High” | Rhinorrhea | ? | LP shunt in one of the patients, unknown for the other patient | Yes |
| Clark et al 1994 (2) | 4 | 100 | 38 | ? | 32 | Rhinorrhea | Cribiform plate | Surgical repair/Lumbar punctures/Diuretics | Yes |
| Camras et al 1998 (13) | 1 | 100 | 46 | “Obese” | 25 | Rhinorrhea, headaches | Anterior cranial fossa | Bifrontal craniotomy + fascia lata graft | Yes |
| Mokri 2002 (9) | 4 | 100 | 27 | ? | 30.8 | Orthostatic headaches-after repair: elevated ICP related-symptoms | Spinal | Surgical repair | Maybe |
| Owler et al 2003 (14) | 1 | 100 | 38 | ? | ? | Rhinorrhea, visual disturbances, headaches | Anterior cranial fossa | Craniotomy + LP shunt; transverse sinus stenting | Yes |
| Schlosser et al 2003 (4) | 16 | 81 | 49.6 | 35.9 | 26.5 | Rhinorrhea, symptoms of elevated ICP | Lateral sphenoid recess (8), central sphenoid (4), ethmoid roof (4), cribiform (2), supraorbital/posterior frontal recess (2), frontal sinus (2) | Surgical repair | Yes |
| Schlosser and Bolger 2003 (15) | 16 | 81 | 49.6 | 35.9 | 28.3 | Rhinorrhea, symptoms of elevated ICP | Lateral sphenoid recess (8), central sphenoid (4), ethmoid roof (4), cribiform (2), supraorbital/posterior frontal recess (2), frontal sinus (2) | Surgical repair | Yes |
| Mirza et al 2005 (16) | 11 | ? | ? | ? | ? | Rhinorrhea, meningitis | Sinonasal | Surgical repair (+ CSF shunting in 3 patients) | Yes |
| Rudnick 2005 (8) | 1 | 100 | 33 | 48.8 | 26 | Rhinorrhea | Cribiform plate | Gastric bypass (weight loss) | Yes |
| Dunn et al 2005 (17) | 15 | 93 | 50 | “Obese” | ? | ? | Roof of the ethmoid (6), sphenoid (5), cribiform plate (4) | Endoscopic repair | Maybe |
| Schlosser et al 2006 (5) | 16 | 81 | 49.6 | 35.9 | 31.1 | Rhinorrhea, symptoms of elevated ICP | Lateral sphenoid recess (8), central sphenoid (4), ethmoid roof (4), cribiform (2), supraorbital/posterior frontal recess (2), frontal sinus (2) | Surgical repair | Yes |
| Prichard et al 2006 (18) | 8 | 50 | 58 | 34.9 | ? | Hearing loss, meningitis, otorrhea, rhinorrhea | Posterior fossa | Surgical repair; LP shunt (in 1 patient) | Maybe |
| Ransom et al 2006 (19) | 1 | 100 | 53 | ? | “Elevated” | Postural headaches, rhinorrhea | Roof of the ethmoid sinus | Revision of VP shunt | Maybe |
| Suryadevara et al 2007 (6) | 2 | 100 | 47 | “Obese” | 26 | Rhinorrhea, headaches, rhinorrhea, meningitis | Cribiform plate | Surgical repair | Yes |
| Woodworth et al 2008 (20) | 55 | 78 | 61 | 43 patients: >30, only one was <25 | 27 | ? | Lateral sphenoid sinus (23), ethmoid roof (17), cribiform plate (12), central sphenoid (7), frontal sinus (7) | Surgical | Maybe |
| Stangherlin et al 2008 (21) | 1 | 100 | 45 | 48 | ? | Rhinorrhea | Left posterior ehtmoidal cell | Gastric banding (weight loss) | Maybe |
| Seth et al 2010 (22) | 39 | 85 | 57.7 | 38.5 | 24 | ? | Cribiform plate, sphenoid lateral pterygoid recess, ethmoid roof | Surgical repair (+ acetazolamide in 9 patients) (+ CSF shunting in 6 patients) | Maybe |
| Reh et al 2010 (23) | 12 | 92 | ? | 40 | Monitoring: > 25 (at least 4% of the time) | Rhinorrhea, headaches, tinnitus | Sphenoid, ethmoid, cribiform | Endoscopic repair + lumbar drain with continuous CSF pressure monitoring | Maybe |
| Yang et al 2011 (3) | 21 | 86 | 53 | 31.2 | 25.5 | Rhinorrhea | Ethmoid sinus (13), lateral sphenoid sinus (7), frontal sinus (1) | Surgical repair + oral diuretics (in some cases) | Yes |
| Brainard et al 2012 (7) | 9 | 89 | 57 | 41 | 24.5 | Otorrhea | ? | ? | Yes |
This study also reports 4 additional women with spontaneous CSF leaks and empty sella, but no diagnosis of IIH.
BMI: body mass index; CSF OP: cerebrospinal opening pressure; LP: lumboperitoneal; IIH: idiopathic intracranial hypertension; VP: ventriculoperitoneal
Discussion
Idiopathic intracranial hypertension is increasingly recognized as a cause of primary spontaneous CSF leaks. Over the past two decades, several articles on this topic have been published mostly in the ENT and neurosurgical literature (2-24), and have highlighted the similarities between the demographics of patients with IIH and those with spontaneous CSF leaks supporting a relationship between IIH and so-called spontaneous CSF leak (Table 1).
Similar to IIH patients, spontaneous CSF leak patients are often young or middle-age obese women (4,24). Review of published cases suggests that a large majority of patients with spontaneous CSF leak are women with a mean BMI higher than 30 kg/m2 (Table 1). Such overlap in the demographics is also shared by the primary empty sella syndrome, an endocrinologic entity in which chronically increased ICP may play a role (25,26). In a retrospective study of 11 patients with β-2 transferrin-proven spontaneous CSF leaks, 72% of patients met the criteria for the diagnosis of IIH (5). Interestingly, obesity has been suggested as an independent risk factor for the development of spontaneous CSF leaks and spontaneous encephaloceles, and the BMI of these patients is significantly higher than in those of patients developing CSF leaks for other reasons. In one study specifically performed to evaluate the role of obesity (BMI ≥ 30 kg/m2) in spontaneous encephaloceles and CSF leak, the mean BMI of the patients with spontaneous encephaloceles was 33.4 kg/m2 vs. 27.0 kg/m2 in the group of non-spontaneous encephaloceles (27).
The presenting symptoms of spontaneous CSF leaks vary greatly depending on multiple factors such as the location and activity of the leak, and the presence of concurrent signs of raised ICP. If the leak is active, symptoms and signs of intracranial hypotension (e.g. orthostatic headaches, neck stiffness) may occur (9). Depending on the location of the CSF leak, CSF otorrhea and conductive hypoacusia may be a presenting sign in patients with bone defects in the posterior fossa, whereas CSF rhinorrhea usually develops in patients with defects of the cribiform plate (6,8,28). Bacterial meningitis is sometimes the initial presentation leading to the discovery of the CSF leak. Some patients may have symptoms of increased ICP even when the leak is active (including headache, tinnitus, visual disturbances, and papilledema) (2), but most often, patients develop symptoms and signs of intracranial hypertension only after the CSF leak has been repaired (9), likely because the leak acts as a route of spontaneous CSF diversion (29).
The factors predisposing some IIH patients to develop spontaneous CSF leaks remain unclear, although it is likely that chronically elevated ICP is necessary, as demonstrated by our third patient who had chronic increased ICP for 3 years before he developed a cribriform plate defect. Some patients with increased ICP secondary to intracranial tumors (distant from the skull base) or hydrocephalus may also develop CSF leak, confirming that raised ICP in itself can cause CSF leaks (1). It is speculated that persistent elevated ICP can cause remodeling of the skull base, with resultant meningo-encephaloceles and CSF leak if the increased ICP persists (2). Indeed, primary spontaneous CSF leaks have been associated with a higher rate of encephalocele formation compared to other etiologies of CSF leaks (30), presumably because of underlying increased ICP that favors both remodeling of the weakest and thinner bony areas of the skull base, and bulging of the meninges through normal orifices in the skull base (e.g. foramen rotundum). Furthermore, skull base encephaloceles and empty sella turcica are often found in IIH patients, as well as in CSF leak patients (30,31).
Computed tomography and MRI of the brain and skull base are always performed to identify skull base defects responsible for CSF leaks; interestingly, these imaging studies also often show radiologic signs associated with increased ICP (31). A retrospective study evaluating the prevalence of empty sella in patients with CSF leaks showed that 100% of patients with spontaneous CSF leak had a completely or partially empty sella turcica on imaging, compared to 11% of patients with non-spontaneous CSF leaks, and 5% to 6% of the general population without CSF leaks (15). All patients in the spontaneous CSF leak group had elevated CSF-OP. Tortuosity of the optic nerves, increased CSF around the optic nerves, arachnoid pits and dural ectasias are other radiological findings often observed in IIH and in patients with spontaneous CSF leaks (30). Skull base defects are the most frequent location for spontaneous CSF leaks in patients with clinical and/or radiologic findings of raised ICP, with the ethmoid and the lateral lateral wall of the sphenoid sinus being the most common locations (3). However, there are reports of increased ICP after the repair of spinal spontaneous CSF leaks, suggesting that even spinal CSF leak might be associated with increased ICP (9). There are no specific studies addressing the radiologic findings of the intracranial venous system in patients with spontaneous CSF leaks, but extrapolating the available data on imaging, similar venous changes as seen in patients with IIH (e.g. transverse venous sinus stenosis) might be expected. Given the overlapping clinical and radiological presentation of primary spontaneous CSF leaks and IIH, some authors have even gone so far as to theorize that patients with primary spontaneous CSF leaks have a variant of IIH (5).
Spontaneous CSF leak patients may develop raised ICP once the leak is repaired, as did two of our cases (9). Some studies have shown the mean CSF OP to be elevated (between 25 cmH2O and 32.5 cmH2O) after surgical repair of the leak (3,24). A small prospective study measured ICP through lumbar catheters after surgical repair of spontaneous CSF leaks (29). Despite the difficulty of measuring reliable CSF-OP using a lumbar drain, the authors concluded that elevated ICP was observed in 7 out of 8 patients, whereas none of the 3 patients with traumatic CSF leaks (used as controls) had abnormal ICP values (29). Although spontaneous resolution of spontaneous CSF leak may occur after treatment of increased ICP, the high risk of bacterial meningitis in cases of chronic CSF leaks usually requires additional endoscopic surgical repair of the bony dehiscence responsible for the leak (2,20). Furthermore, the prevalence of spontaneous resolution in primary spontaneous CSF leaks is low and endonasal endoscopic repair is the standard of care (16,24,32). Interestingly, the rate of leak recurrence after surgical repair is much higher with spontaneous CSF leaks than with other causes of leaks, with recurrences ranging from 25% to 87% for spontaneous CSF leaks (2,3,20). This high risk of leak recurrence likely reflects the excessive elevation of ICP that occurs after leak repair in those patients with presumed IIH (29). Interestingly, other factors associated with failure of the CSF leak repair include obesity (common in IIH) and difficult visualization of the site of leak (e.g. lateral sphenoid leaks) (21). Since increased ICP is the main cause of failure, appropriate ICP-lowering management is suggested to improve the success rates of a definite repair of the CSF leak. Once there is appropriate control of the ICP, the rate of success of the spontaneous CSF leak repair closely resembles the rate of success of the surgical repair of CSF leaks of other etiologies (95%) (20). This has led some authors to suggest that interventions to lower the ICP (either medical therapy with weight loss and acetazolamide, or CSF diversion procedures) be performed before, or at the time of, surgical repair of spontaneous CSF leaks (20,24). In patients with known increased ICP at the time of the diagnosis of CSF leak (or in patients with an intracranial mass), a CSF shunting procedure is usually done prior to repairing the leak itself in order to decrease the risk of leak recurrence, as illustrated by our third patient. Monitoring of the CSF pressure through a lumbar drain at the time of the leak repair has been suggested prior to recommending either medical therapy or a shunting procedure base on real-time response to a therapeutic trial with acetazolamide (23).
Because elevated BMI is a risk factor for both IIH and primary spontaneous CSF leaks, weight loss should likely be recommended in this patient population. There are a few case reports describing resolution of spontaneous CSF leaks after bariatric surgery, but the data are currently not robust enough to support this approach as the only treatment in patients with spontaneous CSF leaks (22,24).
The strong association between IIH and spontaneous CSF leaks in the literature, reinforced by our illustrative cases, supports systematic screening for symptoms and signs of increased ICP within weeks after surgical repair of a presumed spontaneous CSF leak. Early identification of these patients is warranted to prevent failure of the CSF leak repair, and to prevent visual loss from papilledema. Additionally, IIH patients with chronically raised ICP likely warrant close followup for the development of a CSF leak.
Acknowledgments
Funding: This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). Dr. Bruce receives research support from the NIH/NEI (K23-EY019341). Dr. Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award. Dr. Pérez receives support from a scholarship-loan program by COLFUTURO (Bogotá DC, Colombia)
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Ommaya AK. Cerebrospinal Fluid Rhinorrhea. Neurology. 1964;14:106–113. doi: 10.1212/wnl.14.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Clark D, Bullock P, Hui T, et al. Benign intracranial hypertension: a cause of CSF rhinorrhoea. J Neurol Neurosurg Psych. 1994;57:847–849. doi: 10.1136/jnnp.57.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Wang B, Wang C, et al. Primary spontaneous cerebrospinal fluid rhinorrhea: a symptom of idiopathic intracranial hypertension? J Neurosurg. 2011;115:165–170. doi: 10.3171/2011.3.JNS101447. [DOI] [PubMed] [Google Scholar]
- 4.Schlosser RJ, Wilensky EM, Grady MS, et al. Elevated intracranial pressures in spontaneous cerebrospinal fluid leaks. Am J Rhinol. 2003;17:191–195. [PubMed] [Google Scholar]
- 5.Schlosser RJ, Woodworth BA, Wilensky EM, et al. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol. 2006;115:495–500. doi: 10.1177/000348940611500703. [DOI] [PubMed] [Google Scholar]
- 6.Suryadevara AC, Fattal M, Woods CI. Nontraumatic cerebrospinal fluid rhinorrhea as a result of pseudotumor cerebri. Am J Otol. 2007;28:242–246. doi: 10.1016/j.amjoto.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Brainard L, Chen DA, Aziz KM, et al. Association of benign intracranial hypertension and spontaneous encephalocele with cerebrospinal fluid leak. Otol & Neurotol. 2012;33:1621–1624. doi: 10.1097/MAO.0b013e318271c312. [DOI] [PubMed] [Google Scholar]
- 8.Rudnick E, Sismanis A. Pulsatile tinnitus and spontaneous cerebrospinal fluid rhinorrhea: indicators of benign intracranial hypertension syndrome. Otol & Neurotol. 2005;26:166–168. doi: 10.1097/00129492-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mokri B. Intracranial hypertension after treatment of spontaneous cerebrospinal fluid leaks. Mayo Clin Proc. 2002;77:1241–1246. doi: 10.4065/77.11.1241. [DOI] [PubMed] [Google Scholar]
- 10.Brisman R, Hughes JE, Mount LA. Cerebrospinal fluid rhinorrhea. Arch Neurol. 1970;22:245–252. doi: 10.1001/archneur.1970.00480210055007. [DOI] [PubMed] [Google Scholar]
- 11.Applebaum EL, Desai NM. Primary empty sella syndrome with CSF rhinorrhea. JAMA. 1980;244:1606–1608. [PubMed] [Google Scholar]
- 12.Eljamel MS, Foy PM. Non-traumatic CSF fistulae: clinical history and management. Br J Neurosurg. 1991;5:275–279. doi: 10.3109/02688699109005187. [DOI] [PubMed] [Google Scholar]
- 13.Camras LR, Ecanow JS, Abood CA. Spontaneous cerebrospinal fluid rhinorrhea in a patient with pseudotumor cerebri. J Neuroim. 1998;8:41–42. doi: 10.1111/jon19988141. [DOI] [PubMed] [Google Scholar]
- 14.Owler BK, Allan R, Parker G, et al. Pseudotumour cerebri, CSF rhinorrhoea and the role of venous sinus stenting in treatment. Br J Neurosurg. 2003;17:79–83. doi: 10.3109/02688690309177979. [DOI] [PubMed] [Google Scholar]
- 15.Schlosser RJ, Bolger WE. Significance of empty sella in cerebrospinal fluid leaks. Otolaryngology--head and neck surgery. 2003;128:32–38. doi: 10.1067/mhn.2003.43. [DOI] [PubMed] [Google Scholar]
- 16.Mirza S, Thaper A, McClelland L, et al. Sinonasal cerebrospinal fluid leaks: management of 97 patients over 10 years. Laryngoscope. 2005;115:1774–1777. doi: 10.1097/01.mlg.0000175679.68452.75. [DOI] [PubMed] [Google Scholar]
- 17.Dunn CJ, Alaani A, Johnson AP. Study on spontaneous cerebrospinal fluid rhinorrhoea: its aetiology and management. J Laryngol Otol. 2005;119:12–15. doi: 10.1258/0022215053222833. [DOI] [PubMed] [Google Scholar]
- 18.Prichard CN, Isaacson B, Oghalai JS, et al. Adult spontaneous CSF otorrhea: correlation with radiographic empty sella. Otolaryngology--head and neck surgery. 2006;134:767–771. doi: 10.1016/j.otohns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Ransom ER, Komotar RJ, Mocco J, et al. Shunt failure in idiopathic intracranial hypertension presenting with spontaneous cerebrospinal fluid leak. J Clin Neurosci. 2006;13:598–602. doi: 10.1016/j.jocn.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Woodworth BA, Prince A, Chiu AG, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. Otolaryngology--head and neck surgery. 2008;138:715–720. doi: 10.1016/j.otohns.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Stangherlin P, Ledeghen S, Scordidis V, et al. Benign intracranial hypertension with recurrent spontaneous cerebrospinal fluid rhinorrhoea treated by laparoscopic gastric banding. Acta chirurgica Belgica. 2008;108:616–618. doi: 10.1080/00015458.2008.11680302. [DOI] [PubMed] [Google Scholar]
- 22.Seth R, Rajasekaran K, 3rd, Luong A, et al. Spontaneous CSF leaks: factors predictive of additional interventions. Laryngoscope. 2010;120:2141–2146. doi: 10.1002/lary.21151. [DOI] [PubMed] [Google Scholar]
- 23.Reh DD, Gallia GL, Ramanathan M, et al. Perioperative continuous cerebrospinal fluid pressure monitoring in patients with spontaneous cerebrospinal fluid leaks: presentation of a novel technique. Am J Rhinol & Allergy. 2010;24:238–243. doi: 10.2500/ajra.2010.24.3465. [DOI] [PubMed] [Google Scholar]
- 24.Wang EW, Vandergrift WA., 3rd Schlosser RJ. Spontaneous CSF Leaks. Otolaryngol Clinics North Am. 2011;44:845–856. doi: 10.1016/j.otc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 25.De Marinis L, Bonadonna S, Bianchi A, et al. Primary empty sella. J Clin Endocrinol Metabol. 2005;90:5471–5477. doi: 10.1210/jc.2005-0288. [DOI] [PubMed] [Google Scholar]
- 26.Guitelman M, Garcia Basavilbaso N, Vitale M, et al. Primary empty sella (PES): a review of 175 cases. Pituitary. 2012 Aug 9; doi: 10.1007/s11102-012-0416-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Stucken EZ, Selesnick SH, Brown KD. The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otology & Neurotology. 2012;33:1412–1417. doi: 10.1097/MAO.0b013e318268d350. [DOI] [PubMed] [Google Scholar]
- 28.Jindal M, Hiam L, Raman A, et al. Idiopathic intracranial hypertension in otolaryngology. Eur Arch Oto-Rhino-Laryngol. 2009;266:803–806. doi: 10.1007/s00405-009-0973-0. [DOI] [PubMed] [Google Scholar]
- 29.Schlosser RJ, Wilensky EM, Grady MS, et al. Cerebrospinal fluid pressure monitoring after repair of cerebrospinal fluid leaks. Otolaryngology--head and neck surgery. 2004;130:443–448. doi: 10.1016/j.otohns.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Silver RI, Moonis G, Schlosser RJ, et al. Radiographic signs of elevated intracranial pressure in idiopathic cerebrospinal fluid leaks: a possible presentation of idiopathic intracranial hypertension. Am J Rhinol. 2007;21:257–261. doi: 10.2500/ajr.2007.21.3026. [DOI] [PubMed] [Google Scholar]
- 31.Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Op Otolaryngol & Head and Neck Surg. 2007;15:28–34. doi: 10.1097/MOO.0b013e328011bc76. [DOI] [PubMed] [Google Scholar]
- 32.Mattox DE, Kennedy DW. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857–862. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]