Abstract
Failure of the amygdala to habituate, or decrease response intensity, to repeatedly presented faces may be one mechanism by which individuals with autism spectrum disorders (ASD) develop and maintain social symptoms. However, genetic influences on habituation in ASD have not been examined. We hypothesized that serotonin transporter-linked promoter region (5-HTTLPR) genotype affects change in amygdala response to repeated sad faces differently in individuals with ASD vs healthy controls. Forty-four youth with ASD and 65 controls aged 8–19 years were genotyped and underwent an event-related functional magnetic resonance imaging scan where they identified the gender of emotional faces presented for 250 ms. The first half of the run was compared with the second half to assess habituation. 5-HTTLPR genotype influences amygdala habituation to sad faces differently for individuals with ASD vs controls. The genotype-by-diagnosis-by-run half interaction was driven by individuals with ASD and low expressing genotypes (S/S, S/LG and LG/LG), who trended toward sensitization (increase in amygdala activation) and whose habituation scores significantly differed from individuals with ASD and higher expressing genotypes (LA/LA, S/LA and LA/LG) as well as controls with low expressing genotypes. Our results show that amygdala response to social stimuli in ASD, which may contribute to social symptoms, is genetically influenced.
Keywords: functional MRI, serotonin transporter gene, autism, amygdala, habituation, face
INTRODUCTION
The social impairment symptoms characteristic of autism spectrum disorders (ASD) may have their roots in altered processing of social stimuli, such as emotional faces. Altered activation of the amygdala, a brain structure that responds to faces and likely indexes emotional salience of stimuli (Adolphs, 2010), may contribute to social deficits in ASD.
There are two main views regarding the development of social symptoms in ASD and amygdala activation. The first view is that individuals with ASD may be disinterested in social stimuli, such as faces. If youth with ASD are disinterested in social stimuli, they may not seek out social stimuli and miss opportunities to develop social skills (Sasson, 2006). Supporting this view, a number of studies have found reduced amygdala activation in ASD in response to emotional faces (e.g. Kleinhans et al., 2011; for reviews see Volkmar, 2011 and Pelphrey et al., 2011). However, in these studies, the emotional face stimuli were presented for relatively long periods of time (several seconds) and attention to the faces was not monitored or controlled. However, when group differences in attention to faces are limited (Dalton et al., 2005; Monk et al., 2010; Weng et al., 2011) and when individuals with ASD initially fixate on the eyes (Kliemann et al., 2012), individuals with ASD have increased amygdala activation to faces. These studies support an alternative view that individuals with ASD are not disinterested in faces but rather find social stimuli to be aversive. Thus, individuals with ASD may actively avoid faces, and thus miss developmental opportunities to hone social skills. Indeed, children with ASD exhibit greater skin conductance responses to faces than healthy controls (Joseph et al., 2008) and actively avoid looking at the eyes of a face (Kliemann et al., 2010, 2012). Additionally, the more time spent looking at the eyes, the greater the amygdala activation in individuals with ASD (Dalton et al., 2005).
Increased amygdala activation in ASD (e.g. Weng et al., 2011) may be due to a failure to habituate to faces. Habituation refers to the decrease in neural response to repeated presentation of a stimulus (Rankin et al., 2009). Amygdala habituation may represent a learning process by which adaptive levels of arousal are maintained to social stimuli (Herry et al., 2007). In healthy controls, the amygdala quickly habituates to faces, decreasing responses as faces are repeatedly presented (e.g. Fischer et al., 2003). However, adults with ASD fail to habituate to faces (Kleinhans et al., 2009) and youth with ASD exhibit sensitization, or increase in response to faces (Swartz et al., 2013). Sustained activation to faces for the duration of the scan may be one reason previous studies found amygdala overactivation in ASD.
Genetic factors, particularly the serotonin transporter-linked polymorphic region variant (5-HTTLPR), may play a role in amygdala habituation. The S and LG alleles of 5-HTTLPR are associated with decreased serotonin transporter expression relative to the LA allele (A to G SNP in L allele, rs25531; Hu et al., 2006). A meta-analysis showed that individuals with the low expressing genotypes of 5-HTTLPR show greater amygdala activation (Murphy et al., 2013). This may be caused by a failure to habituate to socio-emotional stimuli, as healthy controls with low expressing genotypes, relative to high expressing genotypes, fail to habituate to faces (Lonsdorf et al., 2011). Since individuals with ASD, as a group, show reduced habituation to faces (Kleinhans et al., 2009; Swartz et al., 2013), this genetic effect of 5-HTTLPR on habituation may be heightened in the ASD group. There is evidence that the low expressing genotype may represent a subtype in ASD in terms of symptoms. Individuals with ASD and low expressing 5-HTTLPR genotypes exhibit worse social symptoms (e.g. Brune et al., 2006). However, the role of 5-HTTLPR in amygdala habituation in individuals with ASD has not yet been examined.
The goal of the present study is to address this gap in the literature by examining whether 5-HTTLPR impacts amygdala habituation to sad faces differently in ASD. We specifically focused on sad faces for several reasons. First, compared with controls, individuals with ASD consistently show greater amygdala activation to sad faces (Monk et al., 2010; Weng et al., 2011). Moreover, individuals with ASD require more intense sad facial expressions to accurately identify the face as sad, and diminished sensitivity to sad faces is related to worse social impairment (Wallace et al., 2011). Next, evidence from controls indicates that 5-HTTLPR genotype affects amygdala activation to sad faces, but not happy or neutral faces (Dannlowski et al., 2010). Last, the amygdala in healthy controls may not reliably habituate to fearful faces, as one study found habituation with fearful faces (Fisher et al., 2009), another did not (Swartz et al., 2013), and a third found habituation in a single voxel in the amygdala (Fischer et al., 2003). Thus, to maximize potential group differences, sad faces presented early in the scan were compared with sad faces presented late in the scan. We hypothesized that 5-HTTLPR affects change in amygdala response to repeated sad faces differently in individuals with ASD vs healthy controls.
METHODS
Participants
Forty-four children and adolescents with ASD and 65 healthy controls, aged 8–19 years, were included (Table 1). Of 103 participants with ASD and 86 controls, all data from 56 participants with ASD and 21 controls were excluded because of head movement exceeding 2.25 mm translation or degrees rotation in any frame compared with the first, inability to complete the magnetic resonance imaging (MRI) scan, scoring <80% accuracy in identifying gender in the face task, failure to return a saliva sample for genotyping or technical problems with the MRI. Three participants with ASD were excluded as outliers, with amygdala responses more than 2.5 s.d. from the mean. Individuals were excluded if they had braces, medical conditions contraindicated for MRI or history of seizures or neurological disorders.
Table 1.
Participant characteristics
| ASD group |
Control group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low expressing genotypes |
Higher expressing genotypes |
Low expressing genotypes |
Higher expressing genotypes |
|||||||||
| S/S | S/LG | LG/LG | LA/LA | S/LA | LA/LG | S/S | S/LG | LG/LG | LA/LA | S/LA | LA/LG | |
| No. of participants | 10 | 4 | 1 | 8 | 20 | 1 | 19 | 2 | 1 | 20 | 22 | 1 |
| Total (N) | 15 | 29 | 22 | 43 | ||||||||
| Gender (F:M) | 1:14 | 4:25 | 5:17 | 11:32 | ||||||||
| Handednessa (L:R) | 3:11 | 4:20 | 4:18 | 4:36 | ||||||||
| fMRI task accuracy | 95.2% (4.17%) | 94.9% (5.00%) | 95.9% (4.53%) | 96.6% (3.56%) | ||||||||
| fMRI task RT (ms) | 799 (159) | 771 (125) | 687 (131) | 797 (148) | ||||||||
| DSM-IV-TR diagnosis | 10 AD; 5 AS | 23 AD; 4 AS | N/A | N/A | ||||||||
| Age | 12.9 (2.37) | 14.1 (2.24) | 15.3 (1.77) | 14.1 (3.28) | ||||||||
| Verbal CF | 115 (25.3) | 111 (18.5) | 114 (13.2) | 114 (14.1) | ||||||||
| Nonverbal CF | 109 (18.7) | 104 (20.9) | 105 (10.6) | 100 (14.0) | ||||||||
| SRS | 73.9 (11.9) | 77.1 (11.3) | 44.5 (7.51) | 42.5 (6.95) | ||||||||
| SCQ | 18.8 (7.20) | 20.8 (7.02) | 3.0 (2.55) | 3.2 (4.15) | ||||||||
| CDI | 7.67 (5.18) | 8.62 (6.07) | 5.41 (3.67) | 4.44 (5.37) | ||||||||
| CBCL-Internal | 63.4 (8.71) | 63.4 (9.01) | 46.3 (9.17) | 46.4 (8.82) | ||||||||
| OCI-R | 20.0 (16.4) | 17.0 (11.1) | 10.6 (8.02) | 10.1 (8.88) | ||||||||
| MASC | 42.5 (21.6) | 45.6 (16.2) | 32.1 (13.3) | 31.2 (15.3) | ||||||||
| Caucasian | 93% | 93% | 64% | 77% | ||||||||
Standard deviations are reported in parentheses next to means. fMRI task accuracy = accuracy in identifying gender of all emotional or neutral faces, fMRI task RT = reaction time in milliseconds to identify gender of all emotional or neutral faces, AD = autistic disorder, AS = Asperger’s syndrome, CF = cognitive functioning, SRS = Social Responsiveness Scale, SCQ = Social Communication Questionnaire – Lifetime. Supplementary Table S1 contains more details on subject characteristics.
aNine individuals missing handedness data, 4 missing non-verbal cognitive functioning, 1 missing SRS, 8 missing SCQ, 2 missing RT and 2 missing clinical consensus diagnostic category for DSM-IV-TR due to data failure; however, all participants received an ASD diagnosis via clinical consensus and met cutoffs for autism spectrum on both the ADI-R and ADOS.
Controls were recruited through flyers posted at community organizations. Clinicians at the University of Michigan Autism and Communication Disorders Center diagnosed participants with an ASD (autistic disorder, Asperger’s syndrome or pervasive developmental disorder—not otherwise specified) using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) and clinical consensus (Lord et al., 2006). The University of Michigan Institutional Review Board approved procedures. Participants older than 18 years and parents of minors gave written informed consent; participants younger than 18 years gave written assent.
Participants were given a battery of self-report and parent-report symptom and behavioral measures (Table S1). All control participants scored below clinical cutoffs for affected status. Individuals with the low and higher expressing genotypes did not differ in any of the symptom measures or cognitive functioning in either the ASD or control group. There was a significant diagnosis-by-genotype interaction predicting age and puberty; specifically, participants with ASD and the low expressing genotypes were younger and less advanced in pubertal development. Therefore, we conducted additional analyses controlling for age and pubertal status. Prior studies utilized portions of this dataset (Weng et al., 2010, 2011; Wiggins et al., 2011, 2012; Swartz et al., 2013).
Genetic analyses
5-HTTLPR genotype was assessed using established procedures (Wiggins et al., 2012). The Oragene DNA kit (DNA Genotek, Kanata, Canada) was used to collect saliva samples from each participant. PCR and agarose gel genotyping were utilized to discriminate between the S and L alleles. Subsequently, Sanger sequencing was used to determine the A to G SNP in the L allele (rs25531; Hu et al., 2006) and to confirm PCR genotyping.
Participants were grouped by expression level of genotype: low expressing genotypes (S/S, S/LG and LG/LG) vs medium plus high expressing genotypes (LA/LA, S/LA and LA/LG, referred to as ‘higher expressing’ genotypes). As the LG allele results in serotonin transporter expression equivalent to the S allele (Hu et al., 2006), the S and LG alleles were grouped together for the purpose of analysis. This genotype grouping is consistent with a number of non-ASD studies that found recessive effects of the low expressing 5-HTTLPR alleles, often in adolescent populations (e.g. Cicchetti et al., 2007; Surguladze et al., 2008; Benjet et al., 2010). Within the ASD group, there were 15 individuals with low and 29 with higher expressing genotypes. There were 22 controls with low and 43 with higher expressing genotypes. Hardy–Weinberg equilibrium was tested for low vs medium vs high expressing genotypes. Genotype frequencies were in Hardy–Weinberg equilibrium for the ASD group (χ2 = 1.49, df = 1, P = 0.222) and the control group (χ2 = 2.60, df = 1, P = 0.107).
Emotional faces task (in scanner)
We utilized a faces task known to reliably activate the amygdala (Weng et al., 2011). During image acquisition, participants were instructed to identify the gender of emotional and neutral faces from NimStim (model numbers: 1, 7, 10, 12, 15, 16, 17, 20, 23, 25, 30, 34, 38, 40 and 42; Tottenham et al., 2009). Each model was pictured four times, showing sad, happy, fearful and neutral expressions. Half of the models were male and half were female. Eight of the models were European American, four were African American and three were Asian American. Prior to the MRI scan, participants practiced the task with different faces in a mock scanner.
Each trial consisted of a fixation cross displayed for 500 ms, followed by a face for 250 ms and a blank screen for 1500 ms. Any time after the face appeared, participants pressed a button with their right hand to indicate whether the face was male or female. We minimized group differences in attention to the faces by presenting the face very briefly (250 ms) and having participants do a task (identify gender) immediately following the face presentation. Inter-trial intervals were jittered between 0 and 6000 ms at intervals of 2000 ms. The blank screen displayed between trials served as baseline. E-prime (Psychological Software Tools, Pittsburgh, PA) presented stimuli and recorded responses. Sixty trials (15 trials of each emotion) were presented in a different randomized order for each participant. No picture (model displaying a particular emotion) was presented more than once.
fMRI data acquisition
Details on MRI acquisition have been previously published (Weng et al., 2011). High-resolution spoiled gradient images and T2*-weighted blood oxygen level-dependent (BOLD) images, using a reverse spiral sequence (Glover and Law, 2001) to ensure maximum coverage of the amygdala, were acquired.
fMRI data analysis
Data preprocessing
The University of Michigan fMRI Center’s standard pre-processing procedure was applied to the functional images, which includes removing outliers from the raw k-space data, reconstructing the k-space data to image space, applying a field map correction to reduce artifacts from susceptibility regions and correcting for slice timing. To address head motion, functional images were realigned to the 10th image (for details see Monk et al., 2010).
Using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk), anatomical images were co-registered to the functional images. Functional images were smoothed using an isotropic 8 mm full width at half maximum Gaussian kernel and normalized to the Montreal Neurological Image space.
Contrast images for habituation
We examined habituation by comparing activation to early faces (in the first half of the run) with activation to late faces (the second half of the run). This approach allowed us to control for all other activity related to the viewing of faces or specific face models, yielding differences in activation between early faces and late faces due to the timing of the faces. Habituation occurs if activation to late faces is less than activation to early faces; the converse is sensitization.
Face conditions were modeled with SPM8’s canonical hemodynamic response function. The individual-level model included separate regressors for each of the face emotions. Additionally, trials in which gender was incorrectly identified were modeled as a separate regressor and excluded from further analyses. Two contrast images were generated for each participant, early sad faces vs baseline and late sad faces vs baseline, by estimating the contrast value at every voxel. These images, which convey how much activation differed between the two conditions (seeing either early or late sad faces vs a blank baseline screen) at every voxel in the brain for that individual, were then used in group-level analyses.
Group-level analyses
The images for early sad faces vs baseline and late sad faces vs baseline were then entered into second-level analyses in SPM8. To address our hypothesis, a voxel-wise model was created to examine the three-way interaction of genotype [low (S/S, S/LG, LG/LG) vs higher expressing (LA/LA, S/LA, LA/LG)] by diagnosis (ASD vs control) by run half (early sad faces vs late sad faces). Genotype and diagnosis were between-subjects factors, and run half was a within-subjects factor. All lower order terms, as well as the three-way interaction, were included in the model. A t contrast was used in the group level model to assess the β of the three-way interaction. A small-volume correction using the bilateral amygdala from the automated anatomical labeling (AAL) atlas in the Wake Forest PickAtlas (Maldjian et al., 2002) was applied to test the three-way interaction. This small-volume correction restricted the search for voxels with a significant interaction to the bilateral amygdala and applied a family-wise error correction based on the size of the bilateral amygdala (Worsley et al., 1996).
Post hoc analyses were conducted to characterize the interaction in SPSS. Values from a 4 mm sphere around the peak voxel (xyz = −30, −6, −14) were extracted and averaged for the image representing the first half of the run and the image representing the second half of the run, then exported to SPSS. The low and higher expressing genotypes were compared on habituation scores within the ASD and control groups, and individuals with ASD and controls were compared within the low and higher expressing groups. Significance testing was corrected with the Holm–Bonferroni method with an initial α of 0.05/4 = 0.0125 (Holm, 1979). Post hoc tests were also performed to compare the activation change from the early faces to late faces for the four groups.
Emotion recognition task (outside scanner)
After the scan, participants also performed a computer task to assess accuracy in identifying emotional facial expressions. The same face stimuli were used in the fMRI task, as well as an additional 15 faces from NimStim (Tottenham et al., 2009). Each trial consisted of a 500 ms fixation cross, then a face for 250 ms, followed by an instruction screen asking participants to indicate the emotion of the face by pressing a button corresponding to fearful, happy, sad or neutral. There were 120 trials, 30 of each emotion.
RESULTS
The four groups (individuals with ASD and low expressing genotypes, individuals with ASD and higher expressing genotypes, controls with low expressing genotypes and controls with higher expressing genotypes) did not differ in their accuracy (F1,103 = 1.261, P = 0.264, controlling for age and gender) nor in their reaction time (F1,101 = 2.512, P = 0.116, controlling for age and gender) to identify face gender during the faces task in the scanner. In the emotion recognition task outside the scanner, the four groups did not differ in accuracy to identify sad faces (F1,99 = 0.009, P = 0.923, controlling for age and gender). Neither did they significantly differ in accuracy to identify other emotions (fearful: F1,99 = 0.001, P = 0.970; happy: F1,99 = 1.155, P = 0.285; neutral: F1,99 = 1.504, P = 0.223; each analysis controlling for age and gender). The number of faces shown in the first half vs second half of the run did not differ across the four groups for sad (F1,105 = 0.448, P = 0.505), fearful (F1,105 = 0.732, P = 0.394), happy (F1,105 = 0.395, P = 0.531) and neutral (F1,105 = 1.234, P = 0.269) faces. Cognitive functioning did not differ across the four groups, and individuals with the low and higher expressing genotypes did not differ on symptom measures within both the control group and the ASD group (supplementary Table S1).
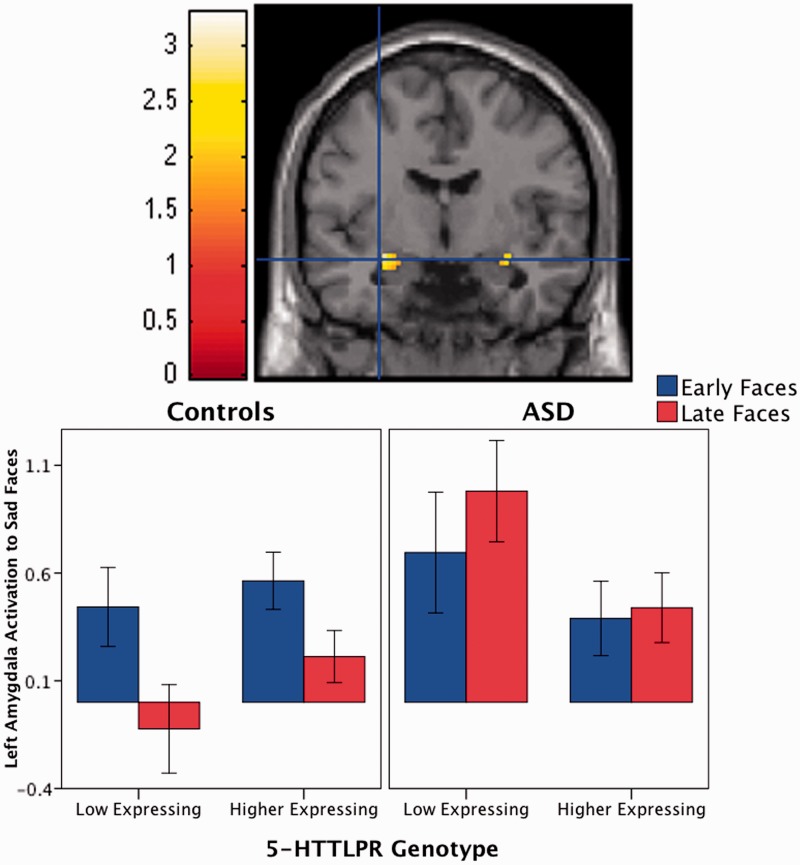
Our hypothesis that the relationship between 5-HTTLPR genotype and amygdala habituation to sad faces differs in the ASD group vs controls was confirmed. There was a significant genotype-by-diagnosis-by-run half interaction predicting left amygdala activation to sad faces (xyz = −30, −6, −14, cluster size = 27, t210 = 3.31, P = 0.023, corrected for multiple comparisons within bilateral amygdala; Figure 1). Specifically, the impact of 5-HTTLPR genotype on amygdala habituation was different for individuals with ASD vs controls. Post hoc analyses indicated that individuals with ASD and low expressing genotypes failed to habituate and displayed a trend toward sensitization (i.e. greater activation to late faces compared with early faces, P = 0.065). Additionally, individuals with ASD and low expressing genotypes had greater increases in amygdala activation from the early to late sad faces compared with individuals with ASD and higher expressing genotypes (P = 0.012) as well as controls with low expressing genotypes (P = 0.013).
Fig. 1.
Impact of 5-HTTLPR genotypes on amygdala habituation is different in youth with ASD vs controls. A significant genotype-by-diagnosis-by-run half interaction in the amygdala is depicted in the coronal section of the brain (top). Color on brain image indicates places where change in response to face presentation early in the task vs later in the task is differentially influenced by 5-HTTLPR in the ASD group compared with controls. For illustration purposes, the threshold was set at P < 0.05 and the image masked for the bilateral amygdala. Crosshairs are at the peak voxel (xyz = −30, −6, −14). Contrast values from the whole left amygdala were extracted and plotted (bottom). Higher expressing 5-HTTLPR genotypes consisted of LA/LA, S/LA and LA/LG; low expressing 5-HTTLPR genotypes consisted of S/S, S/LG and LG/LG. Error bars indicate standard error of the mean.
Other emotion contrasts
To determine whether the hypothesized effect was specific to sad faces, additional analyses to examine potential genotype-by-diagnosis-by-run half interactions were conducted with the other emotional face types. We reran the model using early and late faces for fearful vs baseline, happy vs baseline and neutral vs baseline images. None of these models yielded significant voxels within the bilateral amygdala for the genotype-by-diagnosis-by-run half interaction (fearful: xyz = −28, 4, −18, t210 = 2.29, P = 0.215; happy: xyz = −26, 4, −22, t210 = 2.29, P = 0.249; neutral: xyz = −26, −8, −12, t210 = 1.40, P = 0.701, all corrected for multiple comparisons within the bilateral amygdala).
Additional analyses
In imaging and genetic studies with disordered populations, head motion, developmental differences, population stratification, psychotropic medication status, allele grouping, and MRI preprocessing steps (i.e., slice timing correction) are potential factors influencing associations. Thus, additional analyses were performed to determine whether these factors account for our main result, a significant genotype-by-diagnosis-by-run half interaction for sad faces. To summarize, when taking into account each of these factors, the results still stood. Details on the analyses are in Supplementary Material.
DISCUSSION
This is the first study, to our knowledge, to examine genetic influences on amygdala function in ASD. We found that 5-HTTLPR impacted changes in amygdala response to repeated sad face presentation differently in individuals with ASD compared with controls. Specifically, while our previous work found that, overall, individuals with ASD fail to habituate to sad faces (Swartz et al., 2013), the present study found that the degree to which individuals with ASD fail to habituate to sad faces depends on genotype. Individuals with ASD and low expressing genotypes failed to habituate to the sad faces and in fact displayed a statistical trend toward sensitization, or increase in activation over time; these individuals sensitized more than individuals who also have ASD but with higher expressing genotypes.
Our finding of lack of habituation and a trend toward increased sensitization to sad faces in the individuals with ASD and low expressing genotypes provides support for the theory that individuals with ASD experience faces as aversive (Weng et al., 2011). In avoiding faces, individuals with ASD may miss opportunities to develop social skills and maintain deficits in social communication. Our results suggest that this mechanism by which social impairment develops and is maintained is genetically influenced. Specifically, previous findings of sensitization (Swartz et al., 2013) and lack of habituation (Kleinhans et al., 2009) in the ASD group may be driven by individuals with ASD and the low expressing genotype.
The failure to habituate in individuals with ASD and the low expressing genotype was specific to sad faces; this effect was not found in fearful, happy or neutral faces. It is possible that amygdala response increases to sad faces because they are more ambiguous for those individuals to interpret. The amygdala is known to activate in ambiguous situations (Hsu et al., 2005). However, in our study, groups did not differ on accuracy to identify the emotion in sad faces, providing evidence against the idea that individuals with ASD and low expressing genotypes experienced sad faces as more ambiguous. Of note, the face task inside the scanner involved implicit processing of the emotion (instructions were to identify the gender of the face), whereas the face task outside the scanner required explicit processing (instructions were to identify the emotion on the face). It is possible that individuals with ASD can correctly identify sad emotion faces when explicitly instructed to do so, but have difficulties implicitly processing the same stimuli. Another explanation for the failure to habituate is that individuals with ASD and low expressing genotypes find sad faces either anxiety-provoking or distressing. However, self-report [Multidimensional Anxiety Scale for Children (MASC), Obsessive-Compulsive Inventory-Revised (OCI-R) and Children’s Depression Inventory (CDI)] and parent-report [Internalizing subscale of the Child Behavior Checklist (CBCL-Internalizing)] anxiety and depression symptom measures were not significantly correlated with amygdala habituation within the ASD group (MASC: r = −0.195, P = 0.234; OCI-R: r = −0.159, P = 0.327; CDI: r = −0.174, P = 0.259; CBCL-Internalizing: r = −0.206, P = 0.201) or the control group (MASC: r = 0.062, P = 0.663; OCI-R: r = 0.116, P = 0.358; CDI: r = −0.068, P = 0.589; CBCL-Internalizing: r = −0.078, P = 0.543). These findings are consistent with Swartz et al. (2013; overlapping sample). Although our study was not designed to investigate why sad faces in particular might be an effective probe for group differences, we offer the following possibility regarding the internal experiences of the emotional faces which could be evaluated in future research. When confronted with happy or fearful faces, the social protocol is clearer: happy faces are an invitation to socially interact with the other person, and fearful faces are a sign to scan the environment for threat. However, the social protocol for sad faces is less clear. When confronted with a sad person, should one comfort them or give them space? Not knowing exactly what the social protocol is may be distressing or anxiety-provoking, particularly for individuals with ASD. Perhaps because dealing with sad faces can be difficult even for typically developing individuals, this is the probe that revealed group differences. Future research should explore these possibilities to better understand the role of sad faces in genetically influenced lack of habituation and sensitization in ASD.
Of note, individuals with ASD and the low vs higher expressing genotypes did not differ on any of the symptom measures nor on accuracy or reaction times in the fMRI task, although it is important to note that as a forced-choice task (e.g. identify male or female gender), performance may be inflated. Moreover, the genotype groups within ASD did not differ on DSM-IV-TR diagnoses (Fisher’s exact test, P = 0.242). Participants did differ on brain activation patterns however, such that individuals with ASD and higher expressing genotypes failed to habituate but did not sensitize as much as individuals with ASD and the low expressing genotypes. When groups are equivalent on the behavioral measures, it suggests that genotype is not simply acting as a proxy for symptoms. The brain differences we found in the absence of statistically significant differences on the symptom measures speak to the possibility that the brain measures may have been more sensitive to genetic effects than current parent- or self-report measures. Our study, which examined individuals homogenous in terms of parent or self-report symptom measures but heterogeneous in terms of brain and genetic profiles, represents a step toward identifying subtypes based on brain and genetic profiles within ASD. Moreover, the development of more finely tuned behavioral measures and tasks, used in combination with brain and genetic information, may aid identification of subtypes. Identifying subtypes is important in heterogeneous disorders such as ASD to tease apart multiple pathways to developing the disorder, as subtypes may represent different etiologies for the same disorder. Additionally, different subtypes may be associated with different prognoses and treatment responses. Longitudinal analyses will be necessary to determine what the outcomes are for individuals with ASD and low compared with high expressing genotypes. If individuals who display sensitization to sad faces are more suited toward some medical and behavioral treatments, early identification based on genotype could increase the efficacy of treatment plans.
This study has several limitations. First, our sample size is modest. Within the ASD group, we had 15 individuals with low expressing and 29 with higher expressing genotypes, and 22 controls with low and 43 with higher expressing genotypes. This sample size is comparable with other 5-HTTLPR and neuroimaging studies with controls (e.g. 15 low and 15 high expressing adults, 31 lower and 20 high expressing children, 13 lower and 6 high expressing children in Battaglia et al., 2011, respectively; Roiser et al., 2009; Thomason et al., 2010) and with an ASD sample (two cohorts from different sites: 6 low and 23 higher expressing children, 3 low and 12 higher expressing children in Wassink et al., 2007). However, replication with a larger sample is necessary to make our results more generalizable.
Second, our groups differed in age and pubertal status. Because of this, we covaried age as well as pubertal status to determine whether development accounted for the genotype-by-diagnosis-by-run half interaction predicting amygdala response. We found that even after controlling for these developmental measures, our results still stood, which makes it unlikely that age and puberty are driving our results (see Supplementary Material).
Third, our groups differed in mean head motion as calculated according to Van Dijk et al. (2012). However, when removing variance associated with head motion, our hypothesis was still confirmed (Supplementary Material).
The present study lays a foundation for future studies to better understand the brain and genetic mechanisms involved in the etiology and maintenance of ASD symptoms. We found that individuals with ASD and low expressing genotypes did not display habituation to repeated sad faces; conversely, they exhibited a trend toward sensitization, unlike individuals with ASD and higher expressing genotypes and controls of any 5-HTTLPR genotype. Future research could expand on these findings by designing studies to understand amygdala habituation and sensitization within the context of a network, using functional connectivity and diffusion tensor imaging tools. Additionally, future researchers may wish to include measures of stress and the individuals’ environments, as 5-HTTLPR may act in conjunction with environmental input (Belsky et al., 2009). Such studies could be used to examine potential gene-by-environment interactions in predicting amygdala habituation and sensitization in ASD. To conclude, the findings from our study open a path to better understand genetic influences on brain function in ASD.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank our research assistants and are grateful to the families for participating. This work was supported by an Autism Speaks Predoctoral Fellowship (4773 to J.L.W.) and grant (2573 to C.S.M.); Michigan Institute for Clinical Health Research Predoctoral Fellowship (UL1RR024986 to J.L.W.) and National Institutes of Health grants (R01NS54784 and R01DC009410 to D.M.M. and U19HD35482 and MH066496 to C.L.). C.L. receives royalties from the publisher of diagnostic instruments used on participants. All profits are given to charity. Other authors declare no conflict of interest.
REFERENCES
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Zanoni A, Taddei M, et al. Cerebral responses to emotional expressions and the development of social anxiety disorder: a preliminary longitudinal study. Depression and Anxiety. 2011;29(1):54–61. doi: 10.1002/da.20896. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–54. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(2):173–9. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr. 5-HTTLPR Genotype-specific phenotype in children and adolescents with autism. American Journal of Psychiatry. 2006;163(12):2148–56. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19(4):1161–80. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Kugel H, et al. Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. Neuroimage. 2010;53(3):893–8. doi: 10.1016/j.neuroimage.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: A functional MRI study. Brain Res Bull. 2003;59(5):387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fischer PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cerebral cortex. 2009;19(11):2499–507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46(3):515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, et al. Processing of temporal unpredictability in human and animal amygdala. The Journal of Neuroscience. 2007;27(22):5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–3. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14(6):947–55. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. The American Journal of Psychiatry. 2009;166(4):467–75. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Johnson LC, et al. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54(1):697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. The Journal of Neuroscience. 2012;32(28):9469–76. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Steimke R, Heekeren HR. Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. The Journal of Neuroscience. 2010;30(37):12281–7. doi: 10.1523/JNEUROSCI.0688-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstom KM, Fransson P, Schalling M, Ohman A, et al. 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biological Psychology. 2011;87(1):106–12. doi: 10.1016/j.biopsycho.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft R. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. Neuroimage. 2002;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, et al. Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry and Neuroscience. 2010;35(2):105–14. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, et al. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Molecular Psychiatry. 2013;18(4):512–20. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(6):631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92(2):135–8. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, et al. A genetically mediated bias in decision making driven by failure of amygdala control. The Journal of Neuroscience. 2009;29(18):5985–91. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ. The development of face processing in autism. Journal of Autism and Developmental Disorders. 2006;36(3):381–94. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Elkin A, Ecker C, et al. Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes, Brain, and Behavior. 2008;7(5):543–51. doi: 10.1111/j.1601-183X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Henry ML, et al. Neural and behavioral responses to threatening emotion faces in children as a function of the short allele of the serotonin transporter gene. Biological Psychology. 2010;85(1):38–44. doi: 10.1016/j.biopsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR. Understanding the social brain in autism. Developmental Psychobiology. 2011;53(5):428–34. doi: 10.1002/dev.20556. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Case LK, Harms MB, Silvers JA, Kenworthy L, Martin A. Diminished sensitivity to sad facial expressions in high functioning autism spectrum disorders is associated with symptomatology and adaptive functioning. Journal of Autism and Developmental Disorders. 2011;41(11):1475–86. doi: 10.1007/s10803-010-1170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Archives of General Psychiatry. 2007;64(6):709–17. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research. 2010;1313:202–14. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Bedoyan JK, Peltier SJ, et al. The impact of serotonin transporter (5-HTTLPR) genotype on the development of resting-state functional connectivity in children and adolescents: a preliminary report. Neuroimage. 2012;59(3):2760–70. doi: 10.1016/j.neuroimage.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Research. 2011;1380:187–97. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.