Abstract
The interactionist approach to the study of exogenous oxytocin (OT) effects on prosocial behavior has emphasized the need to consider both contextual cues and individual differences. Therefore, an experiment was set up to examine the joint effect of intranasal OT, a salient social cue and the personality trait social value orientation on cooperative behavior in one-shot prisoner’s dilemma games. The outcome of these mixed-motive games is known to be highly dependent on values and on social information that might reveal the partner’s intent. Consistent with an a priori hypothesis, OT and social information interact significantly to affect the behavior of individuals with a proself value orientation: after prior contact with the game partner, OT enhances cooperative behavior, whereas in anonymous conditions, it exacerbates their intrinsic self-interested behavior. These effects of OT do not hold for individuals with a prosocial value orientation, whose cooperation levels appear to be more influenced by prior contact with the game partner. Follow-up hypotheses for why prosocial and proself individuals respond differently to exogenous OT were developed.
Keywords: oxytocin, cooperation, prisoner’s dilemma, social value orientation, prosocial behavior
INTRODUCTION
The neuropeptide oxytocin (OT), implicated in regulating reproduction and social attachment in mammals, is receiving increasing attention with respect to understanding the roots of human prosocial behavior. A recently published literature review by Bartz et al. (2011a) on the effects of exogenous OT calls for an interactionist approach. Their review reveals that both the environmental context and/or individual differences significantly influence how OT affects behavior, so that, more often than not, OT has a moderating (rather than a direct) effect on prosociality. These authors additionally suggest that OT increases the perceptual sensitivity to social cues, which affects downstream cognition and behavior that is dependent on interpreting these cues. Thus, by increasing people’s attention to salient social information in the environment, OT activates affiliative motivation in congruence with environmental demands. Contextual factors that are congruent with this motivation increase prosocial behavior, whereas contextual factors that work against the goal should decrease prosocial behavior (see also Declerck et al., 2010; De Dreu et al., 2010; Mikolajczac et al., 2010; De Dreu et al., 2011).
This interactionist approach has so far generated interesting results that highlight a more all-encompassing role of OT than was previously believed. Since Kosfeld’s seminal work showing that exogenous OT increases trusting behavior in an economic game (Kosfeld et al., 2005), other reports have indicated that this effect is not unconditional. For example, perceptions of the trustworthiness of the partner matter greatly in the relation between OT and trust-related or cooperative behaviors (Mikolajczac et al., 2010). OT is furthermore sensitive to group effects. It facilitates cooperation with in-group members but at the same time hinders cooperation with out-group members (De Dreu et al., 2010, 2011). Finally, OT appears to enhance coordination on the cooperative outcome in an assurance game, but this effect is dependent on participants having had prior contact with their game partners. If the interactions proceed completely anonymously, OT caused players to be more risk-averse (Declerck et al., 2010).
Evidence that OT interacts with individual differences is also accumulating. On the one hand, a number of research findings indicate that certain stable individual differences in social behaviors correspond to different endogenous levels of OT. For example, plasma levels of OT appear to vary with autism spectrum disorder (Modahl et al., 1998), parenting styles (Feldman et al., 2007; Gordon et al., 2010) and major depression (Parker et al., 2010). On the other hand, people also differ in how they respond to exogenous OT. It appears that the enhanced prosocial effects of intranasally administered OT are especially pronounced for individuals suffering from socio-emotional deficits, as is the case for individuals with Asperger syndrome (Andari et al., 2010) or high alexithymia (Luminet et al., 2011). In contrast, Bartz et al. (2011b) showed that intranasal OT significantly reduced cooperative expectations (trust) among a population of patients with borderline personality disorder. These authors further investigated if individual differences in attachment anxiety (sensitivity to rejection and abandonment) and avoidance (desire to avoid close relationships) moderated the effect of intranasal OT on cooperative behavior. Across borderline personality disorder and control participants, the effect of OT was most pronounced for anxiously attached individuals, but diverged depending on how avoidant they were: those with high avoidance responded to OT with decreased cooperative behavior, whereas those with low avoidance became more cooperative.
Few studies, if any, have examined the combined effect of contextual and individual differences on the relationship between OT and prosocial behavior. This endeavor might be especially fruitful in understanding cooperative behavior in social dilemmas, or in situations where people are torn between a collectively beneficial but costly outcome vs a self-serving outcome. Choice behavior in a social dilemma is not only highly influenced by individual values and motivation (Kollock, 1998; Camerer and Fehr, 2006; Fehr and Gintis, 2007) but also by social cues that decrease anonymity, reduce social distance or prime people to trust their partner (e.g., Hoffman et al., 1996; Bohnet and Frey, 1999; Burnham et al., 2000; Burnham, 2003). Exogenous OT effects on cooperative behavior in a social dilemma should then depend on both individuals’ inclinations (how they value cooperation) and on how they interpret the social cues. The purpose of this study is to attempt to unravel the moderating effects of individual differences and social cues on the relation between OT and cooperative behavior in a one-shot mixed-motive social dilemma. We propose that individuals who have a proself value orientation will be more affected by exogenous OT than people with a prosocial value orientation who are already naturally cooperative. In addition, if OT increases perceptual sensitivity, the positive effect of OT on proselfs’ behavior should be dependent on the presence of salient social cues that enhance social approach motivation. Before presenting the experiment, we elaborate on this hypothesis.
Cooperation in a prisoner’s dilemma: the influence of social value orientation
The prisoner’s dilemma (PD) game is a well-studied paradigm in social dilemma research. When both players make their decision simultaneously, motives of fear and greed pull people towards non-cooperation. A pay-off matrix for the PD game is shown in Figure 1.
Fig. 1.
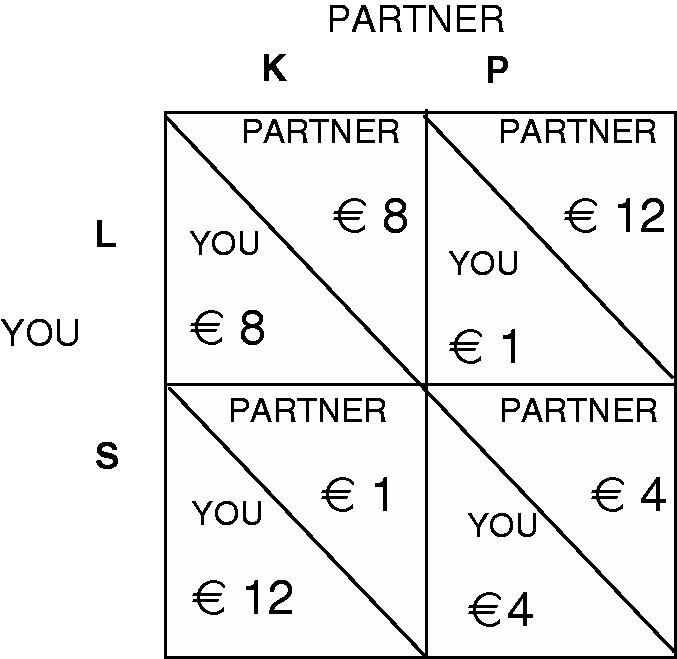
Pay-off matrix for a prisoner’s dilemma.
While mutual cooperation is collectively the most efficient choice (outcome L, K in Figure 1),1 a greedy person can always obtain a better outcome by defecting (choosing S). Anticipating this, a cooperative but fearful person will defect too (choosing P). As a result, defect is the dominant strategy in the PD game, and mutual defection (outcome S, P) is the only Nash equilibrium. Public radio stations, blood banks or carpooling efforts are real-life examples of multiple-players PD games. They only continue to exist when enough people choose to support them through personal contributions rather than free-ride on the goodwill of others. Greed appears to be a stronger motive for free-riding in these types of dilemmas compared with fear (van de Kragt et al., 1983; Dawes et al., 1986). When players in a PD game decide sequentially, the ‘second mover’ already knows the decision of the ‘first mover’. This removes the fear of betrayal for the second player all together, so that greed remains as only obstacle for cooperation.
When played only once without the possibility of reputation benefits, the PD offers no cooperative incentives. Hence the outcome of this game is highly dependent on intrinsic values. The personality trait social value orientation (SVO) captures how a person intrinsically values outcomes for self vs other and tends to have much predictive value with respect to behavior in social dilemmas (reviewed in Bogaert et al., 2008). Although many different SVOs have been identified (Messick and McClintock, 1968), most experimental research distinguishes between two opposing types, proselfs and prosocials (Van Lange, 2000). A proself value orientation indicates that this individual has a stable preference for maximizing own benefits and prefers a self-serving option over a collectively efficient one. In contrast, an individual with a prosocial value orientation prefers equal outcomes the most (Van Lange, 2000; Simpson, 2004; Van Lange et al., 2007). Accordingly, in a one-shot anonymous PD game, proself individuals cooperate significantly less than prosocials, and this is true regardless of whether the game is played simultaneously or sequentially (Boone et al., 2010).
Developing a hypothesis regarding the moderating role of exogenous OT
The hypothesis that exogenous OT increases a player’s cooperative behavior in a PD game depending on both his/her SVO and the social context extends previous research indicating that OT enhances approach motivation in congruence with environmental demands. Our first postulate is that OT will especially affect proself individuals, given their low intrinsic motivation to cooperate. This proposition is in line with other findings that intranasally administered OT increases generosity (Zak et al., 2007) and approach behavior in socially deficient individuals (Andari et al., 2010; Luminet et al., 2011). However, we do not imply that proselfs are socially deficient (on the contrary), but rather that they are not naturally motivated to take others’ needs into account in social interactions. Proselfs do not enjoy a mutual cooperative outcome as much as prosocials do (Simpson, 2004), making them ‘cold’ in their rapport with others. In a PD game, they tend to make economic decisions that are lucrative, irrespective of what is good or bad for other people, thereby abiding by the collectively deficient Nash equilibrium (Boone et al., 2010). Because OT stimulates dopamine release in the mesolimbic dopaminergic reward system (Depue and Morrone-Strupinsky, 2005), it has been suggested that OT can positively influence prosocial behavior by linking approach behavior to the capacity to experience reward from social interaction (Insel, 2003; Campbell, 2008). Such a mechanism would motivate proselfs to cooperate by making it socially (rather than economically) rewarding.
From previous research, we also know that context matters in the relation between OT and prosocial behavior (Declerck et al., 2010; Bartz et al., 2011a; Radke and de Bruijn, 2012). Therefore, our second postulate is that if OT is to positively influence the affiliative motivation of proselfs, this will depend on the presence of salient social information that draws attention to others and to the social nature of the interaction. Increasing social approach behavior is not always warranted, and the finding that, at the cellular level, OT is a neuromodulator with both excitatory and inhibitory effects in neurons of the central amygdala (Huber et al., 2005) suggests that the behavioral effect of OT can go both ways. Indeed, OT appears to suppress activity in one region of the amygdala in response to fearful faces (lowering anxiety), but OT can also enhance activity for happy faces in another part of the amygdala (Gamer et al., 2010). OT regulates bonding as well as selective aggression in pair-bonded prairie voles (Young et al., 2008) and in lactating rats (Leng et al., 2008). Similarly, in humans, it would make sense that OT should only stimulate approach behavior when cues indicate that it is safe or when the interaction is likely to be pleasant. Already experimental results indicate that OT enhances the perceptual sensitivity to social cues. It increases the accuracy of people’s perceptual judgments of others’ emotions and intentions (Domes et al., 2007), improves recognition memory (Savaskan et al., 2008) and stimulates eye contact (Guastella et al., 2008). In children diagnosed with autism spectrum disorder, plasma OT administration tends to improve social information processing and increases the number of self-initiated social interactions (Hollander et al., 2007). If OT is to enhance the cooperative motivation of proselfs in a PD, it is expected to be conditional on the presence of a social cue that facilitates trusting or liking the other person. In the presence of such a positive social cue, we expect that proselfs, who are normally greedy, will become more prosocially motivated (by feeling more affiliation with the partner) when they receive OT. Instead of exploiting the social information generated by the social cue and succumbing to the temptation to defect in a PD game, OT will cause them to be more generous and cooperative. In an anonymous interaction, however, OT cannot enhance social motivation (because there is no identifiable partner to affiliate), but instead might reinforce their intrinsic self-interested nature.
In conclusion: we expect a three-way interaction between exogenous OT, social cues and SVO. Specifically, we hypothesize that OT enhances the cooperative decisions of proselfs more than prosocials in the presence of salient social cues. However, in anonymous interactions, OT is more likely to not affect or even to decrease the cooperative decisions of proselfs compared with prosocials.
METHODS
Participants were 259 university students (119 male, average age = 20.2, s.d. = 2.4) recruited from different departments through Web mail. Exclusion criteria for participating included any medical or psychiatric illness, pregnancy, substance abuse, smoking >15 cigarettes per day and the consumption of medication or alcohol during the past 12 h preceding the experiment. Participants were informed that this was a study investigating the effect of a hormone on decision making. Full anonymity was guaranteed at all times, and monetary incentives were emphasized. The study was approved by the University of Antwerp Ethics Advisory Commission.
Procedure
The experiment was conducted in eight sessions (with a minimum of 30 and a maximum of 36 participants per session). Participants in each session first gathered in one large classroom to fill out a consent form and a short questionnaire that included the SVO assessment. Next, they were then split into four groups (approximately eight per group) and guided to another classroom for the OT administration and actual experiment. Using a double-blind procedure, each participant received a nasal spray containing either OT (four IU per puff) or a placebo (containing only the carrier and no active ingredients). One puff was administered in each nostril, and this was repeated three times in 5-min intervals (24 IU OT total). To allow sufficient time for OT to diffuse through the brain–blood barrier, participants waited for an additional 30 min after the last puff (Born et al., 2002). During this entire time, participants did not talk to each other. An experimenter who was blind to the purpose of the experiment supervised the procedure and stayed in the room the entire time.
Manipulation of the social cue
Following the procedures of Boone et al., (2008), the presence or absence of a social cue was manipulated by allowing one group to have a brief moment of prior contact with their game partner, whereas the other group did not. During the 30-min waiting period, participants in half of the experimental sessions (n = 128) were called one-by-one out of the experimental room to meet in another group of eight inside a conference room. Thus each participant met seven other people who were participating in the experiment but were sitting in different rooms. They were asked to introduce themselves by name, state their favorite hobby and shake hands with everybody. The interaction lasted around 5 min. This is the ‘prior contact’ group. The ‘anonymous’ group remained in the experimental room at all times and spoke to nobody other than the room supervisor.
Previous research has shown that this prior contact manipulation significantly increases game players’ expectation of reciprocity, as well as overall cooperation levels in repeated public good games. This research also suggests that prior contact may serve as a trust signal, as players also showed more trusting behavior by investing more in a trust game (Boone et al., 2008).
The PD games
All participants played three one-shot interactive games: a simultaneously played PD (hereafter referred to as simPD), a sequentially played PD (or seqPD) and an assurance game. Only the results of the two PD games are reported here. An earlier study (Declerck et al., 2010) comparing only the effects of OT and prior contact on cooperation reported a significant interaction for the assurance game, but not for the PD. The current study follows up on the latter finding and tests if the previously reported null effect in the PD might be due to individual differences in SVO. To increase statistical power and external validity, we pooled the data of the simPD and the seqPD. Both games have the exact same pay-off structure but differ in their procedure (explained later). By including both games into the analysis, we accomplish two goals. First, adding variability to a predicting factor increases the generalizability of statistically significant results. Second, by analyzing two decisions per individual in panel data form, we also account for within-subject variability. The data of the assurance game are not included in this analysis because the pay-off structure of this game differs substantially from that of a PD. The temptation to defect is replaced with a cooperative incentive, hence the assurance game is not suited to test hypotheses with respect to greed. In contrast, greed is a major reason to defect in the simPD, and the only reason to defect for a second player in the seqPD (van de Kragt et al., 1983; Dawes et al., 1986), further justifying pooling the data from the two games to test if OT increases the social motivation of self-interested individuals who typically defect. Furthermore, in experiments that make use of a repeated PD game format, the data from all trials are also typically combined, despite the fact that the trials are not conceptually equivalent. The first trial is devoid of any information regarding the partner, and hence resembles our one-shot anonymous PD. But in subsequent trials, the partner’s general behavior is revealed by the feedback of his/her decision in the previous round, somewhat similar to a sequential PD but played with the same partner in each round (e.g., Boone et al., 1999).
Before playing the games, written instructions were given to each participant and these were additionally read out loud by the room supervisor. They were told that for each game, they were pre-matched with a different partner who was sitting in another room. Pre-matching occurred on the basis of the OT bottle numbers. In each game, they were to make one decision for which real money could be earned. How much would depend on their decision as well as on the decision of their partner. The money they earned based on their combined decisions would be computed at the end of the experiment and paid to them in full. In the prior contact condition, the instructions also explained that participants had been matched with one of the people they had just met but was now sitting in a different room. Note that this prior contact manipulation does not allow exact identification of the partner, which prevents possible retaliation or reputation effects. To ensure that all participants adequately understood the monetary pay-offs, the game instructions also included eight questions that had to be answered correctly before continuing with the actual experiment. The answers were checked by the room supervisor.
For each game, participants received a booklet depicting the pay-off matrix shown in Figure 1. They recorded their decision (choosing the cooperative or the defect option) directly in the booklet, which was subsequently collected by the experimenter to compute the pay-offs. Separate booklets were provided for each game, emphasizing the fact that the games comprised single interactions with different partners.
The order in which the simPD and the assurance games were played was reversed in half the sessions. In neither of these games was the decision of the other player revealed, so the outcome of one game could not have affected decisions in the other games. The seqPD was always played last and thus could also not have affected decisions in the former. Instructions in the seqPD booklet explained that the participant was now the ‘second player’ and that the decision of the ‘first player’ would be revealed. Because we were only interested in how the participant reciprocated a cooperative decision, every participant responded to a pre-determined decision indicating that the first player had cooperated.
Participants were paid in truth their actual earnings. Except for the seqPD, pay-offs were computed based on actual partner matching. Debriefing occurred by sending each participant an e-mail referring them to a Web site with a full description of the intent, procedures and results of the experiment.
Variables and analysis
The dependent variable is a bivariate indicating whether- the participant cooperated in the one-shot simPD and seqPD. Cooperation is coded 1, defection coded 0. The independent variables are OT (coded 1, vs placebo, coded 0), prior contact (coded 1, vs anonymous, coded 0) and SVO (prosocial, coded 1, vs proself, coded 0).
SVO was assessed using the 9-item, triple dominance decomposed measure, which distinguishes between a cooperative, individualistic and competitive orientation. Respondents are classified into one of these three orientations if they make six of nine choices consistent with one orientation (e.g., Van Lange et al., 1997; Van Lange, 2000). As is usually done, the individualistic and competitive orientations are combined to form the proself orientation. Thirty-nine participants (15%) did not make consistent choices and could therefore not be classified.2 Following standard practice, we dropped these subjects from subsequent analyses (leaving n = 220). The reliability and validity of the 9-item decomposed measure has proven to be adequate in previous research and is reviewed elsewhere (Au and Kwong, 2004; Bogaert et al., 2008).
Several control variables are included in the logistic regressions that test the main hypothesis; these are the order in which the games were played (0 if the PD was played first, 1 if it was played second), sex (0 = female; 1 = male) and age. The latter two variables are included because previous studies have reported age (Van Lange et al., 1997) and sex effects (Van Vugt et al., 2007) on cooperative behavior in a PD game. The type of game is added as a dummy (simPD coded 1, seqPD coded 0).
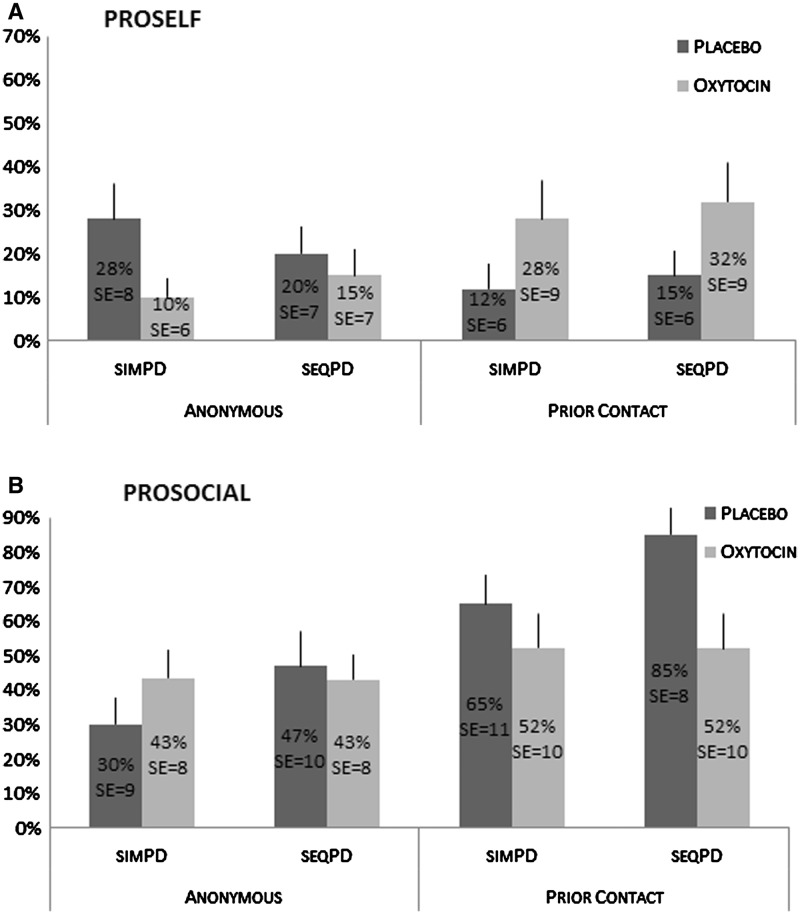
To test the main and interaction effects of the independent and control variables on cooperative decisions, the data from the two games are pooled, yielding 440 observations (2 decisions × 220 individuals). Pooling data is recommended to increase statistical power and the efficiency of the statistical estimates (Wooldridge, 2010). However, as both decisions cannot be assumed to be independent, we test the effect of our variables by means of logistic regressions for panel data (STATA 9, xtlogit command). To control for unobserved heterogeneity between variables, we use a random effects model that accounts for clustering of observations per individual. Pooling the data of both games is the optimal strategy to test the proposed hypothesis if we can show (i) that the type of game does not interact with OT to affect cooperation, and (ii) that the pattern of cooperative decisions made by prosocials and proselfs in the various experimental conditions remains stable across games. Fulfillment of the latter requirement is illustrated by the decomposed figure in Appendix A. The main effect of game and the interactive effect of OT × game are tested with logistic regressions (Table 1).
Table 1.
Unstandardized coefficients for the logistic regressions with random effects performed on panel data and clustered on each individual
| Predictor | Dependent variable: cooperation |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Order | 0.32 (0.39) | 0.32 (0.39) | 0.32 (0.39) | 0.43 (0.39) |
| Sex | −0.52 (0.39) | −0.52 (0.39) | −0.54 (0.39) | −0.44 (0.39) |
| Age | 0.26** (0.08) | 0.26** (0.08) | 0.25** (0.08) | 0.25** (0.08) |
| Game | −0.38 (0.28) | −0.61 (0.39) | −0.38 (0.28) | −0.39 (0.28) |
| Prior contact | 0.86 (0.39) | 0.86 (0.39) | −0.20 (0.69) | −1.09 (0.80) |
| OT | −0.24 (0.39) | −0.46 (0.47) | −0.25 (0.68) | −1.48 (0.79) |
| SVO | 2.42** (0.42) | 2.43** (0.42) | 2.17** (0.68) | 1.02 (0.74) |
| OT × Game | 0.46 (0.55) | |||
| SVO × prior contact | 0.94 (0.79) | 3.42** (1.15) | ||
| SVO × OT | −0.43 (0.78) | 1.83 (1.06) | ||
| OT × prior contact | 0.40 (0.78) | 2.87* (1.13) | ||
| SVO × OT × prior contact | −4.88** (1.60) | |||
| Constant | −7.65** (1.73) | −0.757** (1.73) | −7.16** (1.79) | −6.62** (1.76) |
| Wald χ2 | 44.18 | 44.53 | 45.38 | 48.61 |
| N | 440 obs. (220 Ind.) | 440 obs. (220 ind.) | 440 obs. (220 ind.) | 440 obs. (220 ind.) |
OT, oxytocin (coded 1; placebo coded 0); SVO, social value orientation (prosocial coded 1, proself 0); prior contact (coded 1; anonymous condition coded 0); game (simultaneous PD coded 1; sequential PD coded 0). Cooperation is the dependent variable (1 = cooperate, 0 = defect). Age, sex (1 = male; 0 = female), the order in which the games were played (0 if PD played first; 1 otherwise) and a dummy controlling for the type of game are added to the model as control variables. Standard errors of the regression coefficients are added in parentheses. *P < 0.05, **P < 0.01, two-tailed.
RESULTS
The sample comprised 96 prosocials (48 men and 48 women, average age 20.35 ± 2.1) and 124 proselfs (59 men and 65 women, average age 20.15 ± 2.5). Consistent with previous studies (reviewed in Bogaert et al., 2008), cooperation levels are higher for prosocials. They cooperated 50% of the time, whereas proselfs cooperated 18% of the time (χ2 = 51.90, P < 0.001, n = 440 decisions).3
The results of the logistic regression analyses are reported in Table 1. Model 1 shows that neither the type of game, nor prior contact, nor OT has a significant main effect on cooperation. SVO is the main predictor of cooperation (B = 2.42, P < 0.001). The results of Model 2, showing that OT does not affect cooperation differently according to the type of game, justifies pooling the data to test the remaining interactive effects.4 Model 3 indicates that none of the two-way interactions between SVO, prior contact, and OT is significant. Thus OT does not significantly increase the cooperative behavior of proselfs when social cues are not taken into account. Model 4 shows that the estimate of the hypothesized SVO × OT × prior contact interaction is strongly significant (B = −4.88, P < 0.002).5
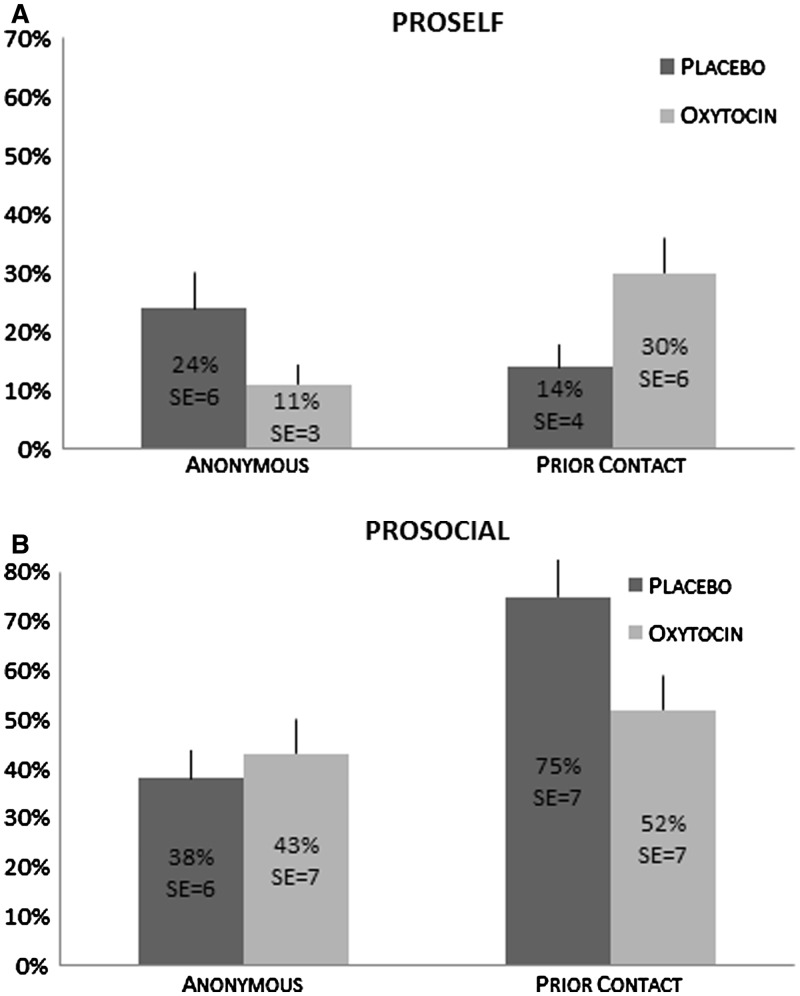
This interaction is illustrated in Figure 2A. For proselfs, OT tends to enhance cooperation when there is prior contact (30% cooperative decisions with OT, and only 14% cooperative decisions with a placebo, χ2 = 4.64, P < 0.05, n = 116 decisions), whereas it hinders cooperation in the anonymous condition (11% with OT, vs 24% with placebo, χ2 = 3.94, P < 0.05, n = 132 decisions), supporting our hypothesis that OT changes the social motivation of proselfs in congruence with the social cue (prior contact vs anonymous).
Fig. 2.
Percent cooperative decisions made by proselfs (A) and prosocials (B) in the four experimental conditions. Each individual made two decisions (one in the simultaneous and one in the sequential prisoner’s dilemma game). SEs represent standard errors of average percentages. Sample sizes for proselfs (A): anonymous condition with placebo, n = 50; with oxytocin, n = 82; prior contact condition with placebo, n = 66; with oxytocin, n = 50. Sample sizes for prosocials (B): anonymous condition with placebo, n = 60, with oxytocin, n = 46, prior contact condition with placebo, n = 40; with oxytocin, n = 46.
For prosocials in the anonymous condition, there is no difference in cooperation between the OT and the placebo group, 43% of the decisions with OT are cooperative, vs 38% with the placebo (χ2 = 2.86, P = 0.59, n = 106 decisions). Unlike hypothesized, their cooperation levels in the prior contact condition differ significantly. Prosocials cooperate less when they received OT (52%) in comparison with the placebo (75%, χ2 = 4.77, P < 0.05, n = 86 decisions). Inspection of Figure 2 and of the decomposed figure in Appendix 1 reveals that prosocials tend to increase their cooperation levels especially in response to prior contact, but that cooperation remains relatively stable across conditions in response to OT.
Surprisingly, and unlike in a previous study, which reports on a repeated social dilemma game (Boone et al., 2008), there was no main effect of prior contact. Instead, we find a complex interactive effect with SVO and OT. For proselfs, prior contact is essential for exogenous OT to boost cooperation, whereas in its absence, OT significantly decreases cooperation (see the significant three-way interaction in Table 1, Model 3). For prosocials, prior contact by itself suffices to increase the level of cooperation in the placebo condition. But, unexpectedly, prior contact in combination with OT reduces their cooperation level back to baseline.6
Sex has neither a main effect on cooperation (see Table 1, Model 1; B = −0.52, P = 0.18), nor does it interact with OT (B = 0.42, P = 0.58, n = 440 decisions), with SVO (B = 0.59, P = 0.45, n = 440 decisions) or with prior contact (B = −0.1.42, P = 0.075, n = 440 decisions).
DISCUSSION
This study adds to the growing body of evidence that the relation between OT and cooperation is not straightforward. By investigating the combined effect of an important social cue (prior contact) and a stable individual difference (SVO), we have shown that, at least in a social dilemma situation where greed is known to affect behavior, it is crucial to consider both these influences to reveal how OT affects cooperation. Specifically, the data show that, compared with a placebo, OT-exposed proselfs (who are by nature selfish) become more cooperative in a PD when they had prior contact with their potential game partners, whereas they become even less cooperative when their partner remains completely anonymous. These results corroborate the a priori hypothesis that exogenous OT increases both the perceptual sensitivity to social cues and the social motivation of otherwise greedy proselfs. When the partner is made salient through the combination of prior contact and OT, proselfs become more generous. In contrast, the combination of OT and an anonymous partner exacerbates their self-interested motive.
An unexpected but particularly interesting finding of this study is that prosocials respond to OT and social cues in the opposite way of proselfs, and that, compared with a placebo, OT appears to decrease cooperation in the prior contact condition. However, this does not necessarily mean that OT makes prosocials greedier, as the decomposed figure in the Appendix reveals that (i) the level of cooperation of prosocials remains the same (and higher than that of proselfs) in all four OT conditions (even in the seqPD where greed would lead to defect), and (ii) cooperation of prosocials increases in the prior contact condition. Thus the relevant question is why OT would partly counteract the positive effect of social cues among prosocials when there has been prior contact. The latter finding suggests that exogenous OT would make prosocials less sensitive to context, which seems to contradict OT’s presumed role of increasing the saliency of social cues reported earlier (e.g., Declerck et al., 2010). Further inspection of the Appendix shows that, in the prior context condition of this study, the different cooperation levels between prosocials and proselfs seem to diminish under the influence of OT. From an evolutionary point of view, it may make sense that OT would have such a ‘leveling-off’ effect on social behavior that varies depending on an individual’s intrinsic personal inclinations. To avoid extreme asocial behavior of selfishly inclined individuals, OT would make them more sensitive to cues that enhance their social motivation. To avoid extreme gullibility of naturally cooperative prosocials, OT might cause them to be more cautious, even to ignore social cues. This explanation is compatible with other recent studies that have reported reduced sensitivity to specific contextual cues after OT administration. Compared with a placebo, participants given OT were less sensitive to cues that stimulated perspective taking of an anonymous partner in an interactive economic game (Radke and de Bruijn, 2012). The authors explain this finding as a protective response to avoid being generous outside of one’s in-group. An earlier study by Baumgartner et al. (2008) reported that participants in a trust game tend to discount feedback information regarding their partner’s unreciprocal behavior more so when they are given intranasal OT compared with a placebo. This effect occurred after several positive interactions in a trust game, a condition that has previously been shown to boost endogenous OT (Zak et al., 2004). A possibility worth exploring in future research is to test if this ‘leveling-off’ effect of exogenous OT occurs when endogenous OT levels are high, and whether this is mainly the case for prosocial individuals.
The finding that cooperative behavior can be negatively influenced by OT in certain conditions is consistent with recent research pointing to other anti-social effects of OT, such as parochialism and out-group aggression (De Dreu et al., 2010, 2011), increased envy and schadenfreude (Shamay-Tsoory et al., 2009) and decreased generosity towards strangers (Radke and de Bruijn, 2010). Together these research results contradict the previously held notion that OT only or primarily boosted prosociality, and further highlights the importance of considering the combination of contextual influences (Radke and de Bruijn, 2010) and individual differences (Bartz et al. 2011b). The diverging pattern of behavior shown by proselfs and prosocials in this experiment suggests that it is essential to understand more basal processes mediated by OT, such as changes in social motivation and/or perceptual sensitivity, to explain downstream cognition that in turn influences cooperative decision making (Churchland and Winkielman, 2012).
An alternative explanation for the finding that OT stimulates cooperation in the prior contact condition among proselfs—brought to our attention by a reviewer—is that OT reduced social anxiety of proself individuals, which then facilitated cooperation. Although we cannot rule this out, reduced anxiety cannot be a sufficient reason accounting for the increased level of cooperation in proselfs. First, in the seqPD, there is no possibility of betrayal and hence no reason to be anxious when cooperating. Second, proselfs differ from prosocials in their willingness to cooperate. In a PD, they therefore choose to defect even when they trust their partner to cooperate (Boone et al., 2010). Hence anxiety due to betrayal aversion is not likely to be an important motive for proselfs.7
A major contribution of this study is that the results generate new research questions. First, these results point at the importance of considering extrinsically motivating factors in the environment when investigating the prosocial influences of OT. One such factor is the type of incentives that are present in a decision context. The three-way interaction between OT, prior contact and SVO reported here is specific to the PD and is not replicated in an assurance game. In this game, the pay-off structure has been changed so that the temptation to defect is replaced by a strong incentive to cooperate. Because in this game prosocials and proselfs are equally motivated to cooperate, it is not surprising that SVO has no additional influence beyond the OT × prior contact effect reported earlier (Declerck et al., 2010). Only in the absence of cooperative incentives, when mixed motives encourage defection, do individual differences in SVO matter. This is an important conclusion that might also explain why De Dreu et al. (2010) found no moderating role of SVO on the relation between OT and cooperative behavior in an intergroup prisoner’s dilemma (IPD) game. These authors report that in the presence of an out-group, OT facilitated cooperation in the in-group, and this effect was similar for prosocials and proselfs. We believe this to be due to the structural changes generated by the IPD, which, compared with the PD, enhance the motivation to cooperate. By introducing competition between the in-group and the out-group, the optimal strategy in the IPD is for all in-group members to contribute, which results in the largest payoff to the in-group (Bornstein, 2003). Knowing that each player’s contribution becomes critical to win the competition incentivizes rational players to cooperate in the IPD (reviewed in Bornstein 2003), and these extrinsic incentives should hold for proselfs and prosocials alike. The idea that cooperative incentives in economic games override the moderating effect of SVO on the relation between OT and behavior could further be tested in an iterated PD game. The accruing benefits during repeated interactions make mutual cooperation much more attractive in the long run and are therefore a great incentive to cooperate, as has been documented in the literature many times before (e.g., Boone et al., 1999). We hypothesize that, similar to the assurance game and the IPD, cooperation levels in an iterated PD may be enhanced by OT and salient social cues, but that there will be no additional moderating effect of SVO.
Second, future neuroimaging studies should try to disentangle how the different neural pathways by which OT affects social behavior are related to individual differences. On the one hand, OT is known to stimulate dopamine release in the nucleus accumbens, by which it may directly improve approach motivation (Depue and Morrone-Strupinsky, 2005; Skuse and Gallagher, 2008). On the other hand, there are several reports that OT reduces social anxiety by attenuating the amygdala response to fearful social signals (Kirsch et al., 2005; Baumgartner et al., 2008). If the interpretations of the data set forth in this article are correct, proselfs would be more sensitive than prosocials when it comes to OT’s actions on brain regions involved in social approach motivation, such as the nucleus accumbens and the subgenual area. Prosocials on the other hand might be more sensitive to OT’s effect on the amygdala–hypothalamus stress axis, and find relief in OT’s stress-reducing actions. It would be interesting to test if indeed individual differences in neural sensitivities to social stress vs social motivation explain why exogenous OT evokes different responses in a heterogeneous population.
Third, effort should be directed at understanding how individual differences in responses to exogenous OT are related to both endogenous levels of OT and genetic polymorphisms. Already there are many indications that stable individual differences in prosocial tendencies have a genetic root and that they correspond to polymorphisms in the oxytocin receptor (OXTR) gene. A number of single-nucleotide polymorphisms have been associated with differences in prosociality (Bakermans-Kranenburg & Van Ijzendoorn, 2008, Rodrigues et al., 2009; Riem et al.,2010; Tost et al., 2010). The SVO construct itself has been characterized by a specific haplotype of the OXTR gene (Israel et al., 2009). At least three single-nucleotide polymorphisms and one four-locus haplotype appear to be significant predictors of the prosocial–proself dichotomy. However, how exactly OT receptor genes relate to endogenous OT on the one hand and to neuropeptide functions in the central nervous system on the other hand is greatly in need of further research. Currently, endogenous OT assessment methods (Szeto et al., 2011) and interpretations of the relation between plasma OT levels and corresponding central nervous system functions are still contested (Meyer-Lindenberg et al., 2011; Churchland and Winkielman, 2012). Incorporating the role of individual differences might be promising in this domain. For example, there are some reports that in women, peripheral OT reactivity serves as a marker of relational distress (Tabak et al., 2011), and it has been suggested that these high OT levels in conjunction with positive affiliative contact may serve to motivate approach behavior to seek new friendships and reinstate normal social functioning after conflicts (Bartz and Hollander, 2006; Taylor, 2006). For future research, we suggest investigating if (i) prosocials show greater endogenous OT reactivity and (ii) if the effect of exogenous OT on prosocials also varies depending on the level of conflict in a situation. This would give further insights into the possible ‘leveling-off’ effects of exogenous OT (as suggested earlier) to avoid extreme asocial or social behaviors.
Finally, the integrationists approach to the study of OT will benefit by taking into account several modulating influences to approach the complexity of human interactions. Gaining understanding in how endogenous and exogenous OT interact, together with the underlying neural pathways and genetics, will also spur progress in translational medicine (Bora et al., 2009; Meyer-Lindenberg et al., 2011), whereby exogenous OT could serve as a therapeutic agent for individuals with social deficits. Realizing that exogenous OT may have negative as well as positive influences on prosocial behavior depending on context and personality is a major breakthrough for research in this domain.
Acknowledgments
This work was supported by an NOI grant (#1044) from the University of Antwerp. The authors thank Erik Fransen from STAT-UA statistics consulting services for his expert advice on analyzing pooled data.
APPENDIX
Fig. 3.
Percent cooperative decisions made by proselfs (A) and prosocials (B) in the four experimental conditions and decomposed for the simultaneous and the sequential prisoner’s dilemma game. Each individual made one decision per game. SEs represent standard errors of average percentages. Sample sizes for proselfs: anonymous condition with placebo, n = 25; with oxytocin, n = 41; prior contact condition with placebo, n = 33; with oxytocin, n = 25. Sample sizes for prosocials: anonymous condition with placebo, n = 30; with oxytocin, n = 23; prior contact condition with placebo, n = 20; with oxytocin, n = 23.
Footnotes
1 The letters L, K, S and P were chosen at random to denote the options that participants in an experiment can choose given the pay-off matrix shown in Figure 1. We purposefully avoided using the more typical ‘option A versus option B’ (or option 1 vs 2) to avoid possible experiential biases. For similar reasons, we avoided using ‘C’ for cooperate and ‘D’ for defect.
2 Typically, around 10% of the participants in experiments remain unclassified using the triple dominance decomposed measure (Au & Kwong, 2004). Although information loss is a drawback of this method, most research in this domain uses the bivariate SVO variable rather than a continuous measure (see Bogaert et al., 2008 for a review).
3 Cooperation in one-shot simultaneously played PD games is highly influenced by the pay-off to defect and individual differences. The cooperation levels in this study fall well within the range of previously published results. For example, Frank et al. (1993) reported between 28% and 47% cooperation depending on whether participants were economic majors or not. Simpson (2004) reported 26% cooperation for proselfs and 63% for prosocials. Kiyonari et al. (2000) reported an overall cooperation level of 37.5% for a population of Japanese students, whereas Boone et al. (1999) report 17% for Dutch economic students.
4 We also repeated this analysis without the control variables and separately for the four experimental conditions (prosocials vs proselfs in anonymous vs prior contact conditions). In none of these models did the variable ‘game’ or the interaction ‘game × OT’ exert a significant effect on cooperation.
5 As a robustness check, we repeated the logistic regression analyses using the ‘number of prosocial decisions (values 1 through 9)’ as a continuous variable. The results are nearly identical. The main effect of ‘number of prosocial decisions’ on cooperation (computed exactly as in Model 1, Table 1) gives an unstandardized B = .30 (P < 0.001). The three-way interaction effect of ‘number of prosocial decisions’, OT and prior contact on cooperation (computed as in Model 3, Table 1, n = 518) gives an unstandardized B = −0.51 (P < 0.005). Note that in these analyses, n = 518 (259 individuals × 2 decisions) because no data are lost on unclassified individuals. These results are consistent with previous results that have compared analyses using either a bivariate or a continuous SVO measure, and found no statistically significant differences (Declerck & Bogaert, 2008; Sheldon, 1999).
6 In the placebo condition, prosocials decided 75% on the cooperative decision with prior contact, and only 38% in the anonymous condition, and this difference is significant (χ2 = 12.95, P < 0.001, n = 100 decisions). In the oxytocin condition, this difference was not significant (52% cooperative decisions in the prior contact condition and 43% in the anonymous condition, χ2 = .70, P = 0.40, n = 92 decisions).
7 The relation between anxiety, betrayal aversion and OT has proven to be complex and may further depend on attachment styles (Baltz, 2012). If OT modulates how particular individuals deal with social anxiety, then the more betrayal-averse prosocials might be more sensitive to OT in situations that elicit anxiousness (such as the simPD, see Appendix). Future measurements of anxiety together with SVO might reveal additional reasons for why prosocials and proselfs respond differently to OT.
REFERENCES
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4389–94. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au WT, Kwong JY. Measurement and effects of social value orientation in social dilemmas: a review. In: Suleiman R, Budescu D, Fischer I, Messick D, editors. Contemporary Psychological Research on Social Dilemmas. Cambridge: Cambridge University Press; 2004. pp. 71–98. [Google Scholar]
- Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–34. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA. Oxytocin, attachment, betrayal, and self-interest: a commentary on “Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion” by Carsten K. W. DeDreu. Psychoneuroendocrinology. 2012;37:1106–10. doi: 10.1016/j.psyneuen.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50:518–28. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN. Social effcts of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011a;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience. 2011b;6:556–63. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bogaert S, Boone C, Declerck CH. Social value orientation and cooperation in social dilemmas: a review and conceptual model. British Journal of Social Psychology. 2008;47:453–80. doi: 10.1348/014466607X244970. [DOI] [PubMed] [Google Scholar]
- Bohnet I, Frey BS. The sound of silence in prisoner’s dilemma and dictator games. Journal of Economic Behavior & Organization. 1999;38:43–57. [Google Scholar]
- Boone C, De Brabander B, van Witteloostuijn A. The impact of personality on behavior in five Prisoner's Dilemma games. Journal of Economic Psychology. 1999;20:343–77. [Google Scholar]
- Boone C, Declerck CH, Kiyonari T. Inducing cooperative behavior among proselfs versus prosocials: the moderating role of incentives and trust. Journal of Conflict Resolution. 2010;54:799–824. [Google Scholar]
- Boone C, Declerck CH, Suetens S. Subtle social cues, explicit incentives, and cooperation in social dilemmas. Evolution and Human Behavior. 2008;29:179–88. [Google Scholar]
- Bora E, Yucel M, Allen NB. Neurobiology of human affiliative behaviour: Implications for psychiatric disorders. Current Opinion in Psychiatry. 2009;22:320–5. doi: 10.1097/YCO.0b013e328329e970. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bornstein G. Intergroup conflict: individual, group, and collective interests. Personality and Social Psychology Review. 2003;7:129–45. doi: 10.1207/S15327957PSPR0702_129-145. [DOI] [PubMed] [Google Scholar]
- Burnham CT. Engineering altruism: a theoretical and experimental investigation of anonymity and gift giving. Journal of Economic Behavior & Organization. 2003;50:133–44. [Google Scholar]
- Burnham T, McCabe K, Smith VL. Friend-or-foe intentionality priming in an extensive form trust game. Journal of Economic Behavior & Organization. 2000;43:57–73. [Google Scholar]
- Camerer CF, Fehr EF. When does “economic man” dominate social behavior? Science. 2006;311:47–52. doi: 10.1126/science.1110600. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Hormones and Behavior. 2012;61:392–9. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes RM, Orbell JM, Simmons RT, van de Kragt AJC. Organizing groups for collective action. The American Political Science Review. 1986;80:1171–85. [Google Scholar]
- Declerck CH, Bogaert S. Social Value Orientation is related to empathy and the ability to “read the mind in the eye.”. The journal of Social Psychology. 2008;148:711–26. doi: 10.3200/SOCP.148.6.711-726. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under uncertainty: the moderating influence of incentives and social information. Hormones and Behavior. 2010;57:378–4. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences. 2011;108:1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;11 doi: 10.1126/science.1189047. 328, 1408–11. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–95. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mindreading” in humans. Biological Psychiatry. 2007;61:731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Sciences. 2007;18:965–70. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fehr E, Gintis H. Human motivation and social cooperation: experimental and analytical foundations. Annual Review of Sociology. 2007;33:43–64. [Google Scholar]
- Frank RH, Gilovich T, Regan DT. Does studying economics inhibit cooperation? Journal of Economic Perspectives. 1993;7:159–71. [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences. 2010;108:3092. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68:377–82. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biological Psychiatry. 2008;64:256–8. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Hoffman E, McCabe K, Smith VL. Social distance and other-regarding behavior in dictator games. The American Economic Review. 1996;86:653–60. [Google Scholar]
- Hollander E, Bartz JA, Chaplin W, et al. Oxytocin increases retention of social cognition in autism. Biological Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology and Behavior. 2003;79:351–7. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. Plos One. 2009;4:1–9. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonari T, Tanida S, Yamagishi T. Social exchange and reciprocity: confusion or a heuristic? Evolution and Human Behavior. 2000;21:411–27. doi: 10.1016/s1090-5138(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Kollock P. Social Dilemmas: the anatomy of cooperation. Annual Review of Sociology. 1998;24:183–214. [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Current Opinion in Pharmacology. 2008;8:731–4. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Luminet O, Grynberg D, Ruzette N, Mikolajczak M. Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. Biological Psychology. 2011;87:401–6. doi: 10.1016/j.biopsycho.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Messick D, McClintock CG. Motivational bases of choice in experimental games. Journal of Experimental Social Psychology. 1968;4:1–25. [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mikolajczac M, Gross JJ, Lane A, Corneille O, de Timary P, Luminet O. Oxytocin makes people trusting, not gullible. Biological Psychology. 2010;85:182–4. doi: 10.1177/0956797610377343. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, et al. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Henna HA, Zeitzer JM, et al. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research. 2010;178:359–62. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, de Bruijn ER. The other side of the coin: oxytocin decreases the adherence to fairness norms. Frontiers in Human Neuroscience. 2012;6:193. doi: 10.3389/fnhum.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem ME, Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social, Cognitive, and Affective Neuroscience. 2011;6:294–300. doi: 10.1093/scan/nsq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, Oliver PJ, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–74. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Sheldon KM. Learning the lessons of tit-for-tat: even competitors can get the message. Journal of Personality and Social Psychology. 1999;77:1245–53. [Google Scholar]
- Simpson B. Social values, subjective transformations, and cooperation in social dilemmas. Social Psychology Quarterly. 2004;67:385–95. [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Hariri H, Perarch-Bloom N, Levkowitz Y. Intranasal administration of oxytocin increases envy and shadenfreude. Biological Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences. 2008;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Bation DA, et al. Evaluation of enzyme immunoassay and radioimminuoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational stress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–22. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Tend and Befriend. Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15:273–7. [Google Scholar]
- Tost H, Kolachana B, Hakimi S, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107:13936–41. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kragt AJC, Orbell JM, Dawes RM. The minimal contributing set as a solution to public good problems. The American Political Science Review. 1983;77:112–22. [Google Scholar]
- Van Lange PAM. Beyond self-interest: a set of propositions relevant to interpersonal orientations. In: Stroebe W, Hewstone M, editors. European Review of Social Psychology. Vol. 11. New York: Wiley; 2000. pp. 297–331. [Google Scholar]
- Van Lange PAM, De Cremer D, Van Dijk E, Van Vugt M. Self-interest and beyond: Basic principles of social interaction. In: Kruglanski AW, Higgings ET, editors. Social Psychology: Handbook of Basic Principles. New York: Guilford; 2007. pp. 540–61. [Google Scholar]
- Van Lange PAM, Otten W, De Bruin EMN, Joireman JA. Development of prosocial, individualistic, and competitive orientations: theory and preliminary evidence. Journal of Personality and Social Psychology. 1997;73:733–46. doi: 10.1037//0022-3514.73.4.733. [DOI] [PubMed] [Google Scholar]
- Van Vugt M, De Cremer D, Janssen DP. Gender differences in cooperation and competition. The male warrior hypothesis. Psychological Science. 2007;18:19–23. doi: 10.1111/j.1467-9280.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- Wooldridge JM. Econometric Analysis of Cross Sections and Panel Data. 2nd edn. Cambridge, Massachusetts: MIT Press; 2010. [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comparative Biochemistry and Physiology, Part C. 2008;148:401–10. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. The neurobiology of trust. Annual Review of the New York Academy of Sciences. 2004;1032:224–227. doi: 10.1196/annals.1314.025. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton A, Ahmadi S. Oxytocin increases generosity in humans. Plos One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]