Abstract
Anxiety is the cognitive state related to the inability to control emotional responses to perceived threats. Anxiety is inversely related to brain activity associated with the cognitive regulation of emotions. Mindfulness meditation has been found to regulate anxiety. However, the brain mechanisms involved in meditation-related anxiety relief are largely unknown. We employed pulsed arterial spin labeling MRI to compare the effects of distraction in the form of attending to the breath (ATB; before meditation training) to mindfulness meditation (after meditation training) on state anxiety across the same subjects. Fifteen healthy subjects, with no prior meditation experience, participated in 4 d of mindfulness meditation training. ATB did not reduce state anxiety, but state anxiety was significantly reduced in every session that subjects meditated. Meditation-related anxiety relief was associated with activation of the anterior cingulate cortex, ventromedial prefrontal cortex and anterior insula. Meditation-related activation in these regions exhibited a strong relationship to anxiety relief when compared to ATB. During meditation, those who exhibited greater default-related activity (i.e. posterior cingulate cortex) reported greater anxiety, possibly reflecting an inability to control self-referential thoughts. These findings provide evidence that mindfulness meditation attenuates anxiety through mechanisms involved in the regulation of self-referential thought processes.
Keywords: anxiety, arterial spin labeling, fMRI, mindfulness meditation
INTRODUCTION
Anxiety is postulated to reflect the inability to govern ruminative cognitive processes and is associated with decreased executive-level brain activity in the bilateral prefrontal cortices (PFC) and anterior cingulate cortex (ACC; Bishop et al., 2004; Bishop, 2007, 2009; Harrison et al., 2008). The cognitive control of ruminative thought processes is mediated by activation in the PFC and ACC (Bishop et al., 2004; Raz and Buhle, 2006; Bishop, 2009). Successful attentional control of anxiety has been associated with modulating the intrinsic meaning of emotionally valenced sensory events (Bishop et al., 2004; Kalisch et al., 2006; Bishop, 2007; Hofmann et al., 2009). One area that has been implicated as a substrate for altering the contextual evaluation of affective processes is the ventromedial PFC (vmPFC; Urry et al., 2006; Hermann et al., 2009; Kompus et al., 2009; Kim et al., 2010; Roy et al., 2012). Activation in the vmPFC is associated with modulating higher-order affective appraisals, including cognitive regulation of negative emotions (Ochsner et al., 2004; Goldin et al., 2008; Goldin et al., 2009; Urry et al., 2009), self-directed cognition (Northoff and Bermpohl, 2004) and processing the value of affective events (Rangel and Hare, 2010; Schoenbaum et al., 2011; Roy et al., 2012).
Another cognitive mechanism related to anxiety is the default mode of functioning (Zhao et al., 2007). The default mode of brain activation, associated with activity in the posterior cingulate cortex (PPC) and the medial PFC, reflects a resting self-referential and emotional-processing brain state (Gusnard et al., 2001; Raichle et al., 2001). Clinical evidence supports this notion, as anxiety patients exhibit altered default mode brain activity, evidenced by decreased deactivation in the PCC and mPFC when compared to healthy controls (Zhao et al., 2007; Broyd et al., 2009; Gentili et al., 2009).
The process model of emotion regulation (Gross, 1998) highlights two commonly employed emotion regulation strategies—distraction and reappraisal. Both techniques are reported to reduce anxiety by distinct cognitive control mechanisms (de Fockert et al., 2001; Ochsner and Gross, 2005). Distraction allocates attentional resources away from emotionally provoking stimuli to attenuate negative mental states such as dysphoric mood (Van Dillen and Koole, 2007), pain (Legrain et al., 2009) and anxiety (Kalisch et al., 2006). Distraction-induced anxiety relief may be associated with brain mechanisms involved in working memory processes including the basal ganglia and ACC (Postle and D'Esposito, 1999; Lewis et al., 2004; Chang et al., 2007; Baier et al., 2010). Reappraisal reduces anxiety by cognitively altering the meaning of sensory events to down regulate the affective reaction to such events (Gross, 2002; McRae et al., 2009). Reappraisal of emotion activates executive control systems including vmPFC, ACC, as well as evaluative processing areas such as the anterior insula (Ochsner and Gross, 2005; Kalisch, 2009; Kanske et al., 2010).
Mindfulness meditation is premised on stabilizing attention, acknowledging discursive sensory events as ‘momentary’ and ‘releasing’ them without affective reaction (Gunaratana, 2002; Lutz et al., 2008). Training in mindfulness meditation has been found to significantly reduce anxiety in clinical (Kabat-Zinn et al., 1992; Miller et al., 1995; Goldin and Gross, 2010) and experimental settings (Zeidan et al., 2010b, c). Mindfulness meditation is hypothesized to regulate emotions by modifying cognitive and affective evaluations to sensory events by cognitive reappraisal processes (Shapiro et al., 2006; Garland et al., 2009; Garland et al., 2010a; Goldin and Gross, 2010). This form of reappraisal, also labeled ‘re-perceiving’ (Shapiro et al., 2006) may be associated with the meta-cognitive ability to monitor thoughts as they arise while maintaining a present-centered, non-evaluative awareness of self (Dunne, 2011; Vago and Silbersweig, 2012).
The goal of this study was to identify the brain mechanisms supporting mindfulness meditation-related anxiety relief. We hypothesized that mindfulness meditation would be more effective at reducing anxiety than simply attending to the breath (ATB) because mindfulness meditation would recruit mechanisms associated with cognitive control and emotion regulation. Therefore, we also postulated that mindfulness meditation-related anxiety relief would be associated with brain mechanisms associated with the cognitive regulation of emotions (e.g. ACC, vmPFC) when compared to ATB.
METHODS AND MATERIALS
Subjects
Previously, we examined the effects of meditation on behavioral pain responses and pain-related brain activation (Zeidan et al., 2011). The present investigation employs the same subjects and imaging data in a new set of analyses designed to examine the brain mechanisms by which meditation reduces anxiety. There are no differences in the experimental procedures in the present manuscript when compared to the previous study (Figure 1; Zeidan et al., 2011). In brief, 15 healthy subjects without meditation experience, mood or affective disorders (six males, nine females; age range 22–35; mean age = 26 years) completed meditation training and MRI scanning. All subjects were right handed, 13 were White and there was one Hispanic and Asian. The Institutional Review Board at Wake Forest School of Medicine approved all experimental procedures.
Fig. 1.
The SAI was administered before and after each MRI session. In first half of MRI session one, 1 heat (49°C) and 1 neutral (35°) series was randomly presented in each fMRI block. Furthermore, subjects were instructed to close their eyes and reduce movement (rest), which served as the no-distraction condition. In the second half of MRI session one, 1 heat (49°C) and neutral (35°) series was randomly presented in each fMRI block. Before anatomical acquisition, subjects were instructed to ‘meditate by focusing on the changing sensations of the breath (ATB).’ ATB served as the self-distraction condition when compared to rest and mindfulness meditation after training. Subjects then participated in 4 days of mindfulness meditation training (see Mindfulness-Based Mental Training for more detail). After successful completion of meditation training, subjects returned for MRI session 2. In first half of MRI session 2, two heat (49°C) and two neutral (35°) series was randomly presented in each fMRI block. Similar to MRI session 1, in the first half of MRI session 2, subjects were instructed to close their eyes (rest). Afterwards, subjects were instructed to ‘begin meditating by focusing on the changing sensations of the breath (‘mindfulness-based attention to breath’—meditation). In the second half of MRI session 2, 2 heat (49°C) and 2 neutral (35°) series was randomly presented in each fMRI block. The presentation of noxious vs neutral scans was counterbalanced across subjects to minimize potential order effects.
The State Anxiety Inventory
The State Anxiety Inventory (SAI; Spielberger, 1983) is a 20-item subscale of the State Trait Anxiety Inventory. The SAI was administered to assess the effects of mindfulness meditation on state anxiety. The SAI was administered before and after each MRI and meditation training session. Higher SAI scores indicate greater levels of state anxiety.
The Freiburg Mindfulness Inventory
Subjects completed the Freiburg Mindfulness Inventory short-form (FMI), a 14-item assessment that measured levels of mindfulness before MRI session 1 and after MRI session 2. The FMI is a psychometrically validated instrument with high internal consistency (Cronbach’s alpha = 0.86)(Walach et al., 2006). Higher scores indicate higher levels of mindfulness.
Overview of experimental procedures
Experimental procedures are outlined in Figure 1 and reported in greater detail in our previous study (Zeidan et al., 2011). In both MRI sessions, a pulse oximeter was attached to each subject’s left index finger to assess heart rate, a transducer was placed around the chest to assess respiration rate, and a thermal probe (35°C, 16 × 16 mm2) was positioned on the posterior aspect of the right lower leg as part of the pain-related portion of the study (Zeidan et al., 2011).
MRI session 1
Subjects completed the SAI at the beginning of MRI session 1. In the first half of the experiment, subjects were instructed to keep their eyes closed and restrict movement (rest). Participants then received one MRI scan of neutral stimulation and one MRI scan of noxious stimulation in random order. In the second half, subjects were instructed to ‘meditate by focusing on the changing sensations of the breath’ until the end of the experiment. It is important to note that subjects had not yet undergone mindfulness meditation training at this point (i.e. participants were not taught to attend to discursive [distracting] sensory events and to regulate their emotional responses to those events). Thus, this condition reflects simple attention to breath [distraction] and serves as an active control condition for mindfulness meditation. During this condition, participants received one MRI scan of neutral stimulation and one MRI scan of noxious stimulation in random order. We then administered the SAI at the conclusion of MRI session 1.
Mindfulness-based mental training
Mindfulness meditation training was carried out in four separate, 20 min sessions conducted by a facilitator with over 10 years of experience leading similar meditation regimens. Subjects were informed that mindfulness meditation training was secular and had no spiritual/religious emphasis. Each training session was held with one to three participants.
Subjects completed the SAI before and after each meditation training session. On mindfulness meditation training day 1, subjects were encouraged to sit with a straight posture, eyes closed and to focus on the changing sensations of the breath. Instructions emphasized acknowledging distracting thoughts and feelings and to return their attention back to the breath sensation without emotional reaction whenever such discursive events occurred. Subjects were taught to evaluate discursive thoughts as ‘momentary and fleeting.’ On training day 2, participants were instructed to ‘follow the breath’ by mentally noting the rise and fall of the chest and abdomen. The last 10 min were held in silence so subjects could develop their meditative practice. On training day 3, the same basic principles of the previous sessions were reiterated. An audio recording of MRI scanner sounds was introduced during the last 10 min of meditation to familiarize subjects with the MRI environment. On the final training session (day 4), subjects received minimal meditation instruction but were required to lie in the supine position and meditate with the audio recording of the MRI sounds to simulate the scanner environment. Contrary to traditional mindfulness-based training programs, subjects were not required to practice outside of training.
MRI session 2
After successful completion of meditation training, subjects participated in MRI session 2. Subjects completed the SAI before MRI session 2. Participants then received two MRI scans of neutral stimulation and two MRI scans of noxious stimulation in random order before meditation. After completing the first half of functional acquisition, subjects were again instructed to ‘meditate by focusing on the changing sensations of the breath’ until the end of the experiment. Participants received two MRI scans of neutral stimulation and two MRI scans of noxious stimulation in random order during meditation. Subjects then completed the SAI after MRI session 2.
MRI acquisition
Cerebral blood flow (CBF) images were acquired on a 1.5T General Electric Twin-Speed LX Scanner using the pulsed arterial spin labeled MRI technique (PASL, Q2TIPS-FAIR; Luh et al., 1999). Scan parameters for the PASL Q2TIPS-FAIR acquisition are as follows: TR = 2500 ms, TE = 17.9 ms, TI = 1700 ms, TI1 = 700 ms, TI2 = 1200 ms, total number of volumes = 140 and total scan time = 5 min 55 s. One CBF image was generated from each PASL series. Brain structure was assessed via an accelerated (2×) T1-weighted Inversion Recovery 3D Spoiled Gradient Echo (IR-3DSPGR) scan.
Statistical analyses of regional signal changes within the brain
The functional image analysis package FSL [Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Center for FMRIB, University of Oxford, Oxford, UK) was used for image processing and analyses. Functional data were movement corrected and spatially smoothed with a 8 mm full-width at half-maximum 3D isotropic Gaussian kernel. Each functional image was scaled by its mean global intensity (intensity normalization) to minimize confounds arising from global CBF fluctuations. All subjects’ structural images were spatially transformed nonlinearly into standard stereotaxic space. Functional images were registered to structural images using a six-parameter (rigid-body) transformation. Functional images were then transformed to standard space based on the nonlinear transformation of the structural data.
In order to identify brain activity significantly related to changes in anxiety within each MRI session, we first performed a first-level within subject fixed effects general linear model-based analysis (Woolrich et al., 2001) to identify differences between ATB and rest (MRI session 1) and meditation and rest (MRI session 2). Only series corresponding to neutral stimulation were employed for this analysis (MRI session 1: rest + neutral, ATB + neutral; MRI session 2: rest + neutral, meditation + neutral). For MRI session 1, each condition was presented once. For MRI session 2, each condition was acquired twice. Next, SAI scores obtained after the respective MRI scanning session were subtracted from those obtained before MRI scanning and divided by the pre-MRI scanning values to calculate SAI percent changes for each individual. The SAI percent changes were then demeaned and entered as a covariate of interest in a second-level random effects analysis in order to identify brain regions related to individual differences in SAI percent changes.
In order to compare brain mechanisms between meditation and ATB-related anxiety modulation, we performed a random effects analysis to determine if the relationship between SAI and regional brain signals, after meditation, is different from that of the change in SAI after ATB. To control for potential order effects to presentation of noxious and innocuous stimuli and to ensure equal statistical power across MRI sessions, we included all four scans from MRI session 1 (rest + neutral stimuli, rest + noxious stimuli, ATB + neutral stimuli, ATB + noxious stimuli) and four scans from MRI session 2 (rest + neutral stimuli, rest + noxious stimuli, meditation + neutral stimuli, meditation + noxious stimuli).
These multiple regression analyses were conducted on a voxel by voxel basis and yielded T/F statistic images. These T/F statistic images were then Gaussianized in order to ensure that data followed a normal distribution. This transformation allows the application of the Gaussian random field theory-based corrections for multiple comparisons. This cluster-based thresholding procedure corrects for familywise error and is considered more sensitive in detecting the ‘true signal’ when compared to voxelwise thresholding (Friston et al., 1996). All voxels in Gaussianized (T/F) statistic images were thresholded with a z-score of >2.3 to identify clusters of voxels. A cluster-forming threshold of >2.3 is considered a conservative level for accurate results (Petersson et al., 1999). According to Gaussian random field theory, the probability of a false positive is determined, in part, by the number of voxels in each cluster and the smoothness of the statistic image (Worsley, 2001). Clusters with a P-value <0.05 are considered statistically significant.
Analysis of behavioral and physiological data
A 2 (before/after) × 6 (session) RM ANOVA tested hypothesized SAI changes before and after each experimental session (SPSS Inc). For each MRI session, a simple regression analysis was conducted between demeaned percent changes in global CBF, heart rate, respiration rate and demeaned percent changes in SAI. RM ANOVA assessed hypothesized changes in FMI scores before MRI session 1 and after MRI session 2. Due to equipment malfunction, we employed listwise deletion of six subject’s heart rate data and five subject’s respiration data in MRI session 1 (Allison, 2002).
Psychophysical correlation analyses between anxiety and pain
In order to assess if anxiety was associated with pain as described previously (Zeidan et al., 2011), we conducted a bivariate regression analysis comparing raw pain ratings to raw SAI scores. Furthermore, since anxiety ratings were obtained only before and after each MRI session and not after each individual scan, it is important to determine if meditation-related changes in anxiety are distinct from meditation-related changes in pain. Therefore, separate bivariate regression analyses were conducted to compare ATB and meditation-related changes in SAI with ATB and meditation-related changes in pain ratings for each MRI session. The degree of change in each of these variables was calculated as a percent change [MRI session 1 (post ATB—pre ATB /pre-ATB) and MRI session 2 (post-meditation—pre-meditation/pre-meditation)].
RESULTS
Mindfulness meditation reduces state anxiety
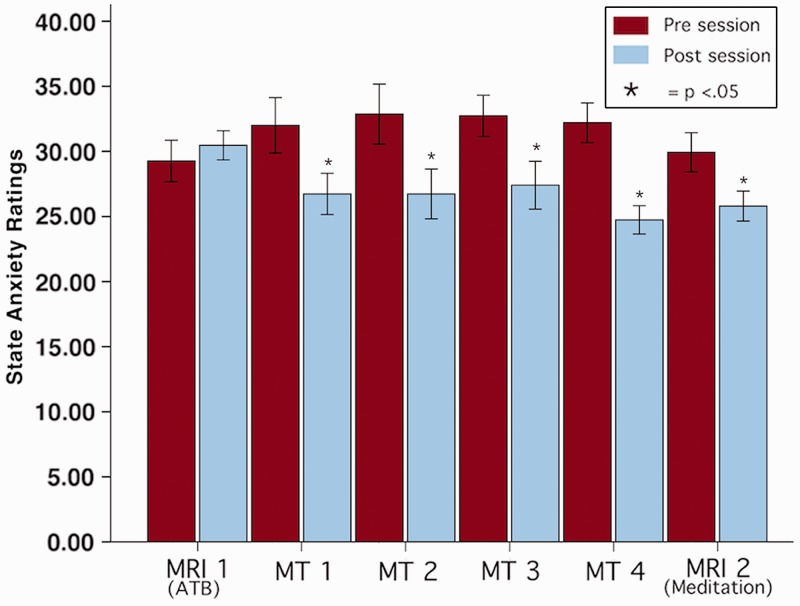
Twenty minutes of mindfulness meditation significantly reduced state anxiety in each session that meditation was practiced (Figures 2 and 3). RM ANOVA revealed that there was no significant effect across sessions, F(5, 70) = 0.96, P = 0.45, η2 = 0.06, although there was a significant main effect within sessions (i.e. pre vs post session), F(1,14) = 31.72, P < 0.001, η2 = 0.69. The interaction effect, F(5,70) = 5.96, P < 0.001, η2 = 0.30 was driven by a significant decrease in state anxiety after each session in which subjects meditated (Figure 2) as compared to MRI 1 where subjects simply attended to their breath. Meditation significantly reduced (P < 0.05) state anxiety in each meditation training session with decreases ranging from 15% to 22% (Figures 2 and 3). Meditating in MRI session 2 reduced state anxiety scores by 12%. In contrast, ATB in MRI session 1 did not alter state anxiety when compared to rest (P > 0.05; Figure 2).
Fig. 2.
State anxiety was significantly reduced in every session in which subjects meditated. ATB did not significantly reduce state anxiety. * = P < 0.05. MRI = MRI session, MT = Meditation Training.
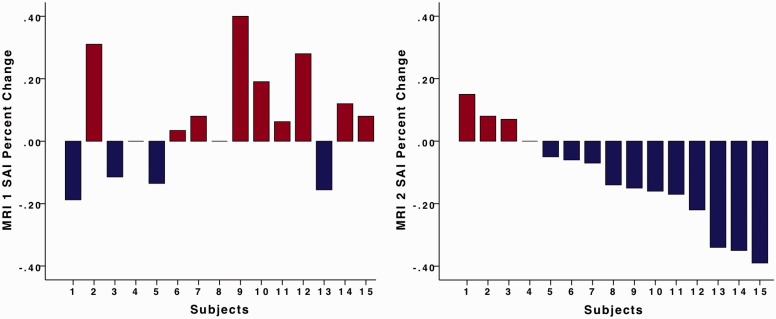
Fig. 3.
Figure 3 illustrates the individual percent state anxiety changes in MRI sessions 1 and 2. Subject numbers in MRI session 1 correspond with subject numbers in MRI session 2. Four subjects reported decreases in state anxiety in MRI session 1. There were nine subjects that reported decreases in state anxiety in MRI session 2.
Four days of meditation training significantly increased mindfulness levels
A RM ANOVA found that 4 days of mindfulness-based mental training significantly increased mindfulness levels after training, F(1,14) = 11.62, P = 0.004, η2 = 0.46. Average mindfulness ratings increased by 14% after training (M = 51.80, S.E.M. = 2.00) when compared to before training (M = 45.33, S.E.M. = 1.95).
Brain imaging analyses
MRI session 1
Correlations with ATB and SAI
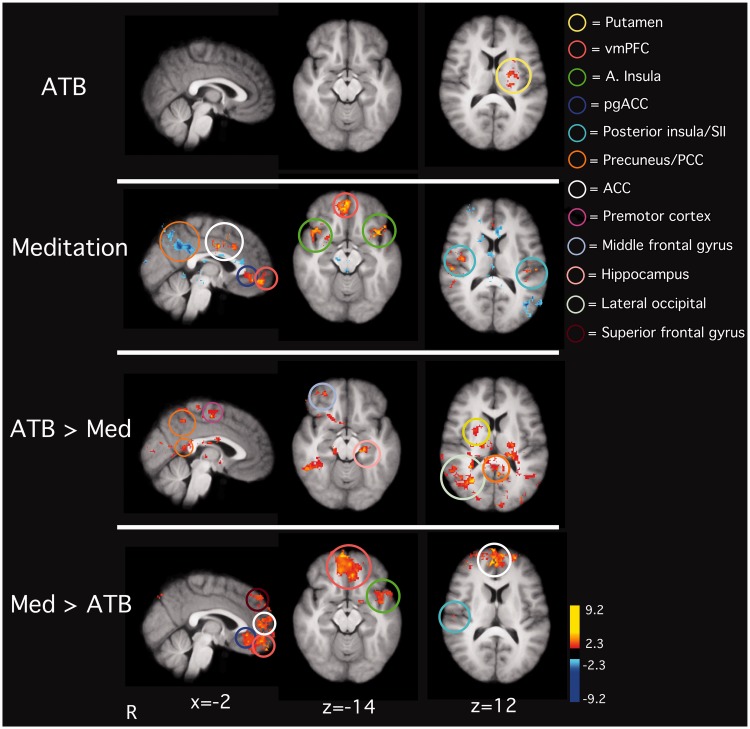
To assess brain activity associated with inter-individual variability in ATB-related anxiety relief, a regression analysis was conducted on the mean regional brain signals between ATB and rest and demeaned percent changes in SAI ratings. Individuals with the greatest reductions in state anxiety during ATB exhibited the greatest activity in the left putamen (Figure 4).
Fig. 4.
Anxiety relief during ATB was associated with greater activity in areas such as the left putamen (1st row). Meditation-related anxiety relief was associated with greater activity in areas such as the anterior insula, ACC and the ventro-medial medial prefrontal cortex (vmPFC; 2nd row). Increases in state anxiety were associated with increased PCC activation (2nd row). ATB-related anxiety changes were associated with activation in PCC, middle frontal gyrus, hippocampus, lateral occipital lobe and the right putamen when compared to meditation-related anxiety changes (3rd row). When contrasted to brain regions involved in ATB-related anxiety changes, brain regions involved in meditation-related anxiety alterations were found in the ACC, anterior insula, putamen and SII (4th row). Note: fMRI series with noxious stimulation were not included in analyses corresponding to the first two rows. One noxious stimulation series, per condition (3rd row: rest + neutral, rest + heat, ATB + neutral, ATB + heat; 4th row: rest + neutral, rest + heat, meditation + neutral, meditation + heat), was included for the analyses corresponding with the last two rows of the figure.
MRI session 2
Meditation reduces anxiety by activating higher-order brain mechanisms
A regression analysis examining inter-individual differences was conducted between regional brain signals during meditation as compared to rest and demeaned percent decreases in state anxiety scores. Meditation-related anxiety relief was associated with greater activation in the vmPFC, ACC, perigenual ACC (pgACC), bilateral anterior insula and SII (Figure 4). Morever, individuals who reported increases in state anxiety after meditating exhibited increased brain activity in the PCC.
Mindfulness meditation compared to ATB
Brain activity related to mindfulness meditation-related anxiety alterations were found in the ACC (BA 24, 32), vmPFC, left anterior insula, superior frontal gyrus, pgACC and right SII when compared to brain regions involved in ATB-related anxiety changes (Figure 4). When compared to mindfulness meditation, ATB-related anxiety changes were associated with greater brain activity in the right occipital cortex, middle frontal gyrus, hippocampus, right putamen and PCC/precuneus (Figure 4).
No relationships between global CBF, respiration rate, heart rate and state anxiety
A simple bivariate regression analysis was employed to determine if percent changes in global CBF, respiration rate and heart changes are associated with changes in state anxiety. There was no relationship between global CBF and SAI in MRI session 1 (r = 0.11, P = 0.71) or MRI session 2 (r = 0.34, P = 0.21; Table 1). There was also no relationship found between respiration rate and state anxiety in MRI 1 (r = −0.34, P = 0.25) or MRI 2 (r = 0.27, P = 0.35), and no correlation between changes in heart rate and SAI scores in MRI session 1 (r = −0.12, P = 0.72) or MRI session 2(r = −0.39, P = 0.19; Table 1).
Table 1.
CBF, respiration rate and heart rate means (S.E.M.) across conditions and sessions
| CBF | Respiration rate | H.R. | |
|---|---|---|---|
| Session 1 | |||
| Rest | 74.12(3.01) | 19.97(1.29) | 72.53(2.33) |
| ATB | 70.69(3.56) | 17.05(1.00)a | 70.46(1.79) |
| Session 2 | |||
| Rest | 68.57(3.17) | 16.72(.82)b | 74.82(3.08) |
| Meditation | 65.09(3.59) | 11.55(.74)c | 73.62(2.77) |
There were no differences in global CBF between rest and ATB in Session 1 or between rest and meditation in session 2.
aSubjects exhibited lower respiration rate during ATB when compared to rest in session 1.
bOverall respiration rate was lower in session 2 when compared to session 1.
cRespiration rate was lower during meditation when compared to rest in session 2 but was not associated with anxiety changes.
State anxiety and pain
A simple bivariate regression analysis comparing raw pain ratings to raw SAI scores revealed that higher state anxiety ratings in pre-scan MRI session 1 were related to lower pre-ATB pain intensity (r = −0.60, P = 0.02) and pain unpleasantness (r = −0.71, P = 0.003) ratings (Table 2). However, there was no significant relationship between post-scan SAI ratings and post-ATB (MRI session 1), pre-meditation, or post-meditation (MRI session 2) pain ratings (Table 2).
Table 2.
The relationship between raw SAI scores and pain ratings for each MRI session
| MRI Session 1 | Pain intensity pre-ATB | Pain unpleasantness pre-ATB |
| SAI pre-scan | r = −0.60, P = 0.02* | r = −0.71, P = 0.003* |
| MRI session 1 | Pain intensity post-ATB | Pain unpleasantness post-ATB |
| SAI post-scan | r = −0.16, P = 0.56 | r = −0.06, P = 0.83 |
| MRI session 2 | Pain intensity pre-meditation | Pain unpleasantness pre-meditation |
| SAI pre-scan | r = −0.44, P = 0.10 | r = −0.45, P = 0.10 |
| MRI session 2 | Pain intensity post-meditation | Pain unpleasantness post-meditation |
| SAI post-scan | r = −0.04, P = 0.89 | r = 0.13, P = 0.64 |
Note: * = Statistically significant (P < 0.05).
A simple bivariate regression analysis comparing the percent change in SAI and the percent change in pain ratings revealed no differences in the relationship between the percent change in SAI scores and pain ratings in MRI session 1 or MRI session 2 (Table 3).
Table 3.
The relationship between the percent change SAI scores and pain ratings for each MRI session
| Pain intensity percent change | Pain unpleasantness percent change | |
|---|---|---|
| MRI 1 | ||
| SAI percent change | r = 0.10, P = 0.74 | r = 0.20, P = 0.48 |
| MRI 2 | ||
| SAI percent change | r = −0.32, P = 0.24 | r = −0.21, P = 0.45 |
DISCUSSION
Mindfulness meditation is postulated to regulate emotions by stabilizing attentional processes, enhancing awareness of discursive sensory events, and disengaging from corresponding affective appraisals (Kabat-Zinn et al., 1992; Gunaratana, 2002; Vago and Silbersweig, 2012). The present findings provide evidence for these principles by identifying the brain mechanisms involved in mindfulness meditation-related anxiety relief. Meditation significantly reduced state anxiety in every session that subjects meditated (Figure 2). Anxiety is inversely related to activity in a brain network involved in cognitive and affective control (Bishop et al., 2004; Bishop, 2009). Meditation-related activation of this network was clearly associated with anxiety reduction (Figure 4). When compared to ATB, meditation reduced anxiety by engaging brain mechanisms involved in sensory evaluation (SII) and the cognitive control of emotions (ACC, vmPFC) (Figure 4; Ochsner et al., 2002; Ochsner and Gross, 2005; Kalisch, 2009; Kanske et al., 2010; Zeidan et al., 2011). Although it is possible that our findings during meditation could be related to practice effects associated with training in ATB, the decrease in anxiety across each meditation session suggests that mindfulness meditation training is exerting a different effect. After distraction with ATB, anxiety ratings went up (Figure 2). However, after the first meditation training session where mindfulness meditation was introduced, anxiety went down (Figure 2).
Brain mechanisms associated with mindfulness meditation-related anxiety relief
Consistent with the instructions of mindfulness-based mental training, meditation activated brain areas associated with the mindfulness practice of Shamatha and Vipassana (Lutz et al., 2008; Manna et al., 2010). Our findings confirm that mindfulness meditation modulates state anxiety by engaging a network of brain regions including the ACC, anterior insula and vmPFC. We postulate that the engagement of these regions by mindfulness meditation regulates anxiety through multiple potential mechanisms.
Meditation-related anxiety relief was associated with greater activity in a distinct network of brain regions involved in cognitive reappraisal processes (Ochsner and Gross, 2005; Eippert et al., 2007; Wager et al., 2008; Figure 4). Greater reductions in anxiety during meditation were associated with increased vmPFC activity. Furthermore, the vmPFC is crucially involved in successfully down regulating negative emotions and is associated with enhanced cognitive control, working memory processing and modifying appraisals of sensory events (Teasdale et al., 1999; Urry et al., 2006; Hermann et al., 2009; Kompus et al., 2009; McRae et al., 2009). The relationship between the vmPFC and meditation-related anxiety relief is consistent with the act of monitoring and reappraising cognitive and affective states. Creswell et al. (2007) found that greater activity in the vmPFC was directly associated with higher levels of dispositional mindfulness as well as down regulation of amygdala activity during an affect-labeling paradigm (Creswell et al., 2007). Moreover, when compared to experienced Zen meditators, subjects with brief meditation training successfully downregulated amygdala activation in response to negative emotional pictures (Taylor et al., 2011). The direct relationship between vmPFC and anxiety relief provides unique mechanistic insight into the regulation of self-referential processing by mindfulness meditation. Greater activation in the anterior insula and ACC was associated with larger decreases in state anxiety (Figure 4). These areas are not only important to the cognitive control of emotion and sensory evaluation (Ochsner et al., 2002; Ochsner et al., 2004; Ochsner and Gross, 2005), but also in integrating cognitive, affective and sensory representations to produce a continuous and fluctuating awareness of self (Critchley et al., 2004; Medford and Critchley, 2010).
Mindfulness meditation also reduced brain activity in areas associated with ruminative thought processes (i.e. default mode). Mind wandering has been associated with negative disposition (Smallwood et al., 2009; Smallwood and O'Connor, 2011). The 14% increase in mindfulness levels and the negative relationship between activity in the anterior insula and anxiety relief during meditation indicates that enhancement of self-awareness processes may also play a role in anxiety relief (Critchley et al., 2004; Farb et al., 2010). Notably, greater increases in state anxiety during meditation were related to increased activity in default mode-related brain activity (e.g. PCC; Figure 4)(Raichle et al., 2001), suggesting that individuals who were less successful at regulating self-referential thoughts were less able to reduce anxiety. Mindfulness meditation is postulated to increase awareness of emotion-evoking thought processes (Farb et al., 2010). We postulate that mindfulness meditation-related improvements in anxiety may be related to acknowledging discursive thought processes accompanied by the intention to sustain a present focused, non-reactive mental stance. These mechanisms are remarkably consistent with the premise that mindfulness meditation involves enhanced awareness in the present moment and the cognitive reappraisal of emotionally salient sensory events (Wallace, 2006; Lutz et al., 2008; Garland et al., 2009; Grant et al., 2010; Zeidan et al., 2011).
It has also been postulated that mindfulness meditation produces a sense of emotional detachment from experienced sensory events (Kabat-Zinn et al., 1992; Gunaratana, 2002; Farb et al., 2007; Grant et al., 2011; Taylor et al., 2011). Mindfulness meditation practitioners can decouple transitory appraisals of ‘self’ with corresponding sensory events (Kabat-Zinn, 1982; Astin, 1997; Farb et al., 2007; Zeidan et al., 2011). To this extent, the present findings provide support for this hypothesis, evidenced by meditation-related anxiety relief and activation in brain areas associated with sensory processing (i.e. posterior insula, SII), cognitive control (ACC) and higher-order evaluation (vmPFC, anterior insula; Figure 4).
ATB does not modulate anxiety
Similar to previous studies (Kalisch et al., 2006; Zeidan et al., 2010a), we did not find that self-distraction (ATB) attenuated state anxiety. Some may argue that focusing on the sensations of the breath is a form of concentrative meditation, associated with other meditative techniques such as Transcendental, yogic, Zen and Shamatha (Cahn and Polich, 2006). As such, subjects in MRI session 1 may have been practicing a form of concentrative meditation. ATB’s failure to reduce anxiety may be due to the lack of the actual mindfulness component and/or the lack of training with this attentional task. However, consistent with previous findings, cognitive techniques using reappraisal are more effective at attenuating anxiety when compared to those employing distraction (Kalisch et al., 2006; McRae et al., 2009). Regression analyses revealed that the limited number of individuals who reported anxiety relief by self-distraction (ATB; Figure 3) did so by activating brain mechanisms (putamen) related to working memory processes specific to filtering irrelevant thought processes (Figure 4; Packard and Knowlton, 2002; McNab and Klingberg, 2008; Baier et al., 2010). In contrast, mindfulness meditation has been found to recruit brain mechanisms that are distinct from those activated during ATB. Such areas include the ACC and vmPFC, which are both involved in processing emotionally weighted sensory events (Holzel et al., 2007). The present study found similar patterns of activation during the cognitive state of meditation. We provide direct evidence that the ability to be aware of and regulate task-irrelevant self-referential processes can attenuate state anxiety.
Meditation-related anxiety relief is distinguishable from meditation-related pain relief
The present investigation employed the same subjects and imaging data as our previous study examining the brain mechanisms associated with meditation-related pain relief (Zeidan et al., 2011). However, the present study focuses on MRI series obtained during innocuous (neutral) thermal stimulation (35°C). Therefore, one potential confound is that the present findings could be influenced by the presentation of noxious thermal stimulation (49°C) that was interleaved with neural stimulation series. Accordingly, it is important to determine if the brain mechanisms and behavioral ratings associated with meditation-related anxiety relief are distinguishable from those associated with meditation-induced pain reductions. To address these important issues, we conducted a number of analyses. For one, we assessed if changes in self-reported state anxiety corresponded with changes in pain ratings. In addition, we conducted two separate regression analyses examining the neural correlates of meditation-related anxiety relief while controlling for the variance associated with meditation-induced pain reductions in the presence of innocuous or noxious thermal stimulation (see Supplementary Material). Additionally, we conducted conjunction analyses (Nichols et al., 2005) to determine whether there is significant overlap between brain regions supporting meditation-related anxiety and pain relief in the presence of innocuous or noxious thermal stimulation (see Supplementary Material).
In MRI session 1, higher pre-scanning anxiety ratings were associated with lower pain intensity and unpleasantness ratings during scanning (Table 2). This inverse relationship has been previously reported with healthy participants (Starr et al., 2010). However, it is important to note that there was no significant relationship between meditation-related anxiety and pain reductions as measured by the SAI and behavioral pain ratings, respectively (Tables 2 and 3). These behavioral findings are consistent with imaging findings that indicate that brain activation related to meditation-related anxiety reduction is distinguishable from brain activation involved with meditation-related pain intensity and unpleasantness reductions (see Supplementary Material). Further support for these findings is provided by conjunction analyses that revealed no significant overlap between brain regions associated with meditation-related anxiety relief and meditation-related pain relief.
Considerations for mindfulness meditation-related anxiety relief
Inclusion of a sham meditation group in the present study could have provided additional insight into possible demand characteristics and social support effects related to meditation training. However, we have found that comparable meditation training regimens produced similar improvements in anxiety and mood even when compared to relaxation and sham meditation regimens (Zeidan et al., 2010a, b, c). Moreover, in order to control for potential confounds from different experimental directives (McRae et al., 2009), we employed the same experimental directive before and after meditation training.
Additionally, our findings could simply be related to practice effects associated with training in ATB. Therefore, subjects may have been able to reduce anxiety simply by becoming more proficient at ATB. However, this effect is likely not the case due to a lack of mPFC activation during ATB as compared to rest (Kalisch et al., 2006; Denkova et al., 2010). Along a similar line, order effects related to the end of the experiment could be associated with reductions in anxiety observed in MRI session 2. However, reductions in anxiety were seen after each meditation training session (in the middle of the experiment). In addition, order effects related to the end of the MRI scanning session are also unlikely to account for anxiety reductions, since anxiety did not decrease at the end of MRI session 1, when subjects did not practice mindfulness meditation.
Long-term training in mindfulness meditation has been found to improve cognitive processes that subsequently improve a wide spectrum of health outcomes (Davidson et al., 2003; Grossman et al., 2004; Grant and Rainville, 2009; Garland et al., 2010a, b). The present findings verify that brief mindfulness meditation training can reliably attenuate anxiety, even in the absence of a mood-inducing manipulation or a generalized anxiety disorder. While anxiety medications have been found to reduce anxiety reports by 50% in affective disorder patients (Haider, 1972), they do not improve anxiety and other mood constructs in healthy subjects (Deijen et al., 1991; Murphy et al., 2008). In contrast, we found that 20 min of meditation reduced anxiety by as much as 22% in healthy subjects (Figure 2). We postulate that if the benefits of mindfulness meditation can be realized after a brief training format, then patients may feel more inclined to continue to practice and clinicians may not feel as reluctant to recommend mindfulness meditation to their patients.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Acknowledgments
The authors would like to thank Dr John Dunne for his helpful comments in the development of this manuscript. This work was supported by the Mind and Life Institute’s Francisco J. Varela Grant, the Biomolecular Imaging Center at Wake Forest School of Medicine, and the National Institute of Health grant NS3926.
REFERENCES
- Allison PD. Missing Data. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Astin JA. Stress reduction through mindfulness meditation. Effects on psychological symptomatology, sense of control, and spiritual experiences. Psychotherapy and Psychosomatics. 1997;66:97–106. doi: 10.1159/000289116. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Muller NG. Keeping memory clear and stable–the contribution of human basal ganglia and prefrontal cortex to working memory. Journal of Neuroscience. 2010;30:9788–92. doi: 10.1523/JNEUROSCI.1513-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and Biobehavioral Reviews. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–69. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–6. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Deijen JB, Heemstra ML, Orlebeke JF. Residual effects of lormetazepam on mood and performance in healthy elderly volunteers. European Journal of Clinical Pharmacology. 1991;40:267–71. doi: 10.1007/BF00315207. [DOI] [PubMed] [Google Scholar]
- Denkova E, Wong G, Dolcos S, et al. The impact of anxiety-inducing distraction on cognitive performance: a combined brain imaging and personality investigation. PLoS ONE. 2010;5:e14150. doi: 10.1371/journal.pone.0014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne J. Toward an understanding of non-dual mindfulness. Contemporary Buddhism. 2011;12:69–86. [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, et al. Upward spirals of positive emotions counter downward spirals of negativity: insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010a;30:849–64. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010b;42:177–92. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E, Gaylord S, Park J. The role of mindfulness in positive reappraisal. Explore (NY) 2009;5:37–44. doi: 10.1016/j.explore.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, et al. Beyond amygdala: default Mode Network activity differs between patients with social phobia and healthy controls. Brain Research Bulletin. 2009;79:409–13. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66:1091–9. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, et al. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–6. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Grant JA, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosomatic Medicine. 2009;71:106–14. doi: 10.1097/PSY.0b013e31818f52ee. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Gunaratana H. Mindfulness in Plain English. Boston: Wisdon Publications; 2002. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider I. Lorazepam in the treatment of anxiety. Current Medical Research and Opinion. 1972;1:70–3. doi: 10.1185/03007997209111147. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Schafer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Heering S, Sawyer AT, Asnaani A. How to handle anxiety: the effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behavior Research and Therapy. 2009;47:389–94. doi: 10.1016/j.brat.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry. 1992;149:936–43. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18:1266–76. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2010;21:1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex. 2010;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neuroscience Letters. 2009;467:76–80. doi: 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144:230–2. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. The European Journal of Neuroscience. 2004;19:755–60. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine. 1999;41:1246–54. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A, Raffone A, Perrucci MG, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Research Bulletin. 2010;82:46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2009;22:248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. General Hospital Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Downham C, Cowen PJ, Harmer CJ. Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology (Berl) 2008;199:503–13. doi: 10.1007/s00213-008-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Nichols TE, Poline JB, Holmes AP. Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. Philosophical transactions of the Royal Society of London. 1999;354:1261–81. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Cognitive Brain Research. 1999;8:107–15. doi: 10.1016/s0926-6410(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nature Reviews. Neuroscience. 2006;7:367–79. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16:147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;62:373–86. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion. 2009;9:271–6. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- Smallwood J, O'Connor RC. Imprisoned by the past: unhappy moods lead to a retrospective bias to mind wandering. Cognition and Emotion. 2011;25:1–10. doi: 10.1080/02699931.2010.545263. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI-Form Y) Palo Alto, CA: Consulting Psychology Press; 1983. [Google Scholar]
- Starr CJ, Houle TT, Coghill RC. Psychological and sensory predictors of experimental thermal pain: a multifactorial model. Journal of Pain. 2010;11:1394–402. doi: 10.1016/j.jpain.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, et al. Functional MRI study of the cognitive generation of affect. The American Journal of Psychiatry. 1999;156:209–15. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience. 2012;6:296. doi: 10.3389/fnhum.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: a working memory model of distraction from negative mood. Emotion. 2007;7:715–23. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmuller V, Kleinknecht N, Schmidt S. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI) Personality and Individual Differences. 2006;40:1543–55. [Google Scholar]
- Wallace BA. The Attention Revolution: Unlocking the Power of the Focused Mind. Somerville: Wisdom Publications; 2006. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York, NY: Oxford University Press Inc; 2001. [Google Scholar]
- Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. Journal of Pain. 2010a;11:199–209. doi: 10.1016/j.jpain.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition. 2010b;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. Journal of Alternative and Complementary Medicine. 2010c;16:867–73. doi: 10.1089/acm.2009.0321. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Wang PJ, Li CB, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. European Journal of Radiology. 2007;63:373–8. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.