Abstract
Variations in the gene that encodes the oxytocin receptor (OXTR) have been associated with many aspects of social cognition as well as several prosocial behaviors. However, potential associations of OXTR variants with reactions to betrayals of trust while cooperating for mutual benefit have not yet been explored. We examined how variations in 10 single-nucleotide polymorphisms on OXTR were associated with behavior and emotional reactions after a betrayal of trust in an iterated Prisoner’s Dilemma Game. After correction for multiple testing, one haplotype (C-rs9840864, T-rs2268494) was significantly associated with faster retaliation post-betrayal—an association that appeared to be due to this haplotype’s intermediate effect of exacerbating people’s anger after they had been betrayed. Furthermore, a second haplotype (A-rs237887, C-rs2268490) was associated with higher levels of post-betrayal satisfaction, and a third haplotype (G-rs237887, C-rs2268490) was associated with lower levels of post-betrayal satisfaction.
Keywords: oxytocin, cooperation, betrayal, prisoner’s dilemma, emotional reactions
The neuropeptide oxytocin appears to influence a wide range of social cognitive processes as well as prosocial behaviors (for review see Striepens et al., 2011). Intranasal administration of oxytocin has been found to increase trust (Kosfeld et al., 2005), perceived trustworthiness of strangers (Theodoridou et al., 2009), empathy (Hurlemann et al., 2010) and inference of others’ mental states from social cues (Domes et al., 2007). Administration of oxytocin may dampen neural systems subserving social fear (Kirsch et al., 2005; Petrovic et al., 2008), which might reduce aversion to betrayal (Baumgartner et al., 2008; De Dreu, 2012). Interestingly, the oxytocin system also influences maternal aggression in animals (for review see Campbell, 2008). Moreover, low levels of oxytocin in human cerebrospinal fluid have been associated with a history of aggression (Lee et al., 2009), and intranasal oxytocin increases defensive aggression toward outgroup members (De Dreu et al., 2010). Thus, a more nuanced picture of oxytocin’s influence on social processes has begun to emerge.
In addition to studying the role of endogenous and exogenous oxytocin in social processes (Bartz et al., 2011), recent research has examined how genetic differences in the oxytocin receptor gene (OXTR) may be associated with social cognition and behavioral functioning (Ebstein et al., 2012). OXTR is 17 kb in length, includes three introns and four exons and is located on chromosome 3p25–3p26.2 (for review see Gimpl and Fahrenholz, 2001). Israel et al. (2009) found both single- and multi-marker associations of OXTR with generosity and selflessness (as measured by the Dictator Game and the Social Value Orientations task). However, after multiple test correction, Apicella et al. (2010) found no associations among nine single-nucleotide polymorphisms (SNPs) on OXTR with a prosociality index created based on participants’ responses to a modified Dictator Game and a Trust Game, behavioral measures of generosity and trust. Findings to date are therefore mixed, but at least some of the results suggest that genetic variation on OXTR may be associated with prosocial behavior.
Studies have also begun to explore how variation on OXTR may be associated with affect and emotionality. For example, after multiple test correction, Kawamura et al. (2010) found a significant relationship between a seven-locus haplotype on OXTR and depressive affect in a non-clinical Japanese sample. In addition, Lucht et al. (2009) found nominal associations among single- and multi-marker loci on OXTR and both positive and negative affect. Most recently, variations in single- and multi-marker loci on OXTR were significantly associated with a history of aggression (Malik et al., 2012) in a sample of children (and these associations survived multiple test correction).
Although various kinds of prosocial behaviors as well as non-context-dependent state and trait emotionality have been studied as correlates of variations on OXTR, it is not yet known whether variation on OXTR is associated with cooperation in an iterated cooperative context (in which participants can modify their behavior on successive rounds of play based on their and their partners’ behavior on previous rounds) and emotional reactions to betrayals of trust in that context. The present study examined these questions using the most widely used behavioral game for studying cooperation: the iterated Prisoner’s Dilemma Game (PDG). We tested the role of OXTR by examining 10 SNPs (and haplotypes of these 10 SNPs) that we selected based on their previous associations with various aspects of social behavior (for review see Ebstein et al., 2012). In addition, based on previously discussed evidence suggesting that variation on OXTR is associated with individual differences in affect (e.g. Kawamura et al., 2010), we examined emotional responses to betrayals in trust. To do so, we measured both satisfaction and anger because these seem to be standard responses to receipt of benefits and harms, respectively, and hypothesized that certain emotions may mediate the relationship between the experience of being betrayed and the delay of retaliation.
MATERIALS AND METHODS
Participants and procedure
Participants were 165 (82 female, 83 male; mean age = 19.21 years, SD = 1.79, range = 17–37) undergraduates at the University of Miami who played an iterated PDG with another ‘partner’ (some of these data have also been used in McCullough et al., 2013; Study 2, Tabak et al., 2012). The ‘partner’ was actually a pre-programmed computer strategy. The research, therefore, involved deception, without which it would have been unfeasible (see Supplementary material). The ‘partner’s’ gender, race and age were not disclosed to participants. The sample was restricted to those who self-identified as non-Hispanic White to prevent spurious results due to variations in allele frequencies among subgroups of different ancestry (Risch et al., 2002; Cardon and Palmer, 2003). The original sample included 21 additional participants who were removed for the following reasons: using the default criterion in Haploview version 4.2 (Barrett et al., 2005; http://www.broadinstitute.org.mpg/haploview), seven participants were removed because they had <75% complete genotype data. During post-task debriefing, 10 participants stated that they did not believe that they had been playing against another person, and thus were omitted. Four participants cooperated no more than once during the first 12 rounds of the PDG and were also omitted, as participants who cooperated at such a low rate were viewed as not having initially trusted their partners, making it impossible for us to look at reactions to violations of trust. After these four participants were removed, the lowest rate of initial cooperation was 33%. Participants received credit toward a course requirement and between $7 and $10, depending on their performance in the PDG.
Participants came to the laboratory in groups of 1–20 (see Supplementary material; analyses were re-run controlling for group size and results remained unchanged). At the beginning of the session, they learned that they would be playing a computerized decision-making game consisting of 20–40 rounds with an anonymous networked partner (randomly assigned). In actuality, all participants played against the same pre-programmed computer strategy described later in the text. Participants played for points and were told that those points would be later converted to money (10 points = $1), which they would be paid at the end of the session. While participants followed along, a research assistant read aloud a 10-min tutorial that provided instructions on how to play the PDG (modified from Rilling et al., 2002; Rilling et al., 2007). The research assistant did not begin the PDG until receiving confirmation from all group members that they understood all instructions. After the PDG, participants underwent either blood draw or saliva collection and completed a series of self-reports (described later). Finally, they were debriefed and paid for their participation.
Iterated PDG
The iterated PDG is commonly used to model mutual benefit cooperation in interpersonal interactions (Axelrod and Hamilton, 1981; West et al., 2011). This study used a modified version of the PDG of Rilling et al. (2002, 2007) using E-prime software (Psychology Software Tools, Pittsburgh, Pennsylvania). Participants believed that they were playing the PDG in dyads. On each round, they chose to either cooperate or defect independently of their partner by choosing the letter ‘C’ or ‘D’. Participants’ choices earned them a certain number of points per round, conditional on the choices made simultaneously by their partner (see Figure 1).
Fig. 1.
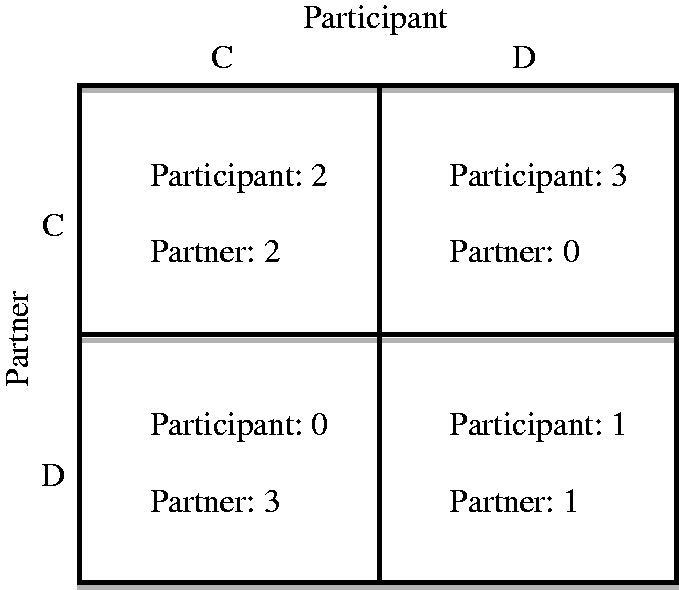
Iterated Prisoner’s Dilemma Game. Each round had four possible outcomes: mutual cooperation (CC), the participant cooperated and their partner defected, and vice versa (CD or DC) or mutual defection (DD). Each outcome yielded a different number of points: CC represented the highest collective payoff (2, 2), DD represented the lowest collective payoff (1, 1) and DC or CD represented the maximum payoff for the defector and zero payoff for the cooperator (3, 0 or 0, 3).
Pre-programmed computer strategy for the PDG
The program cooperated in the first round. During rounds 2–12, the computer played a ‘generous-tit-for-tat’ strategy (Nowak and Sigmund, 1992)—cooperating 100% of the time after player cooperation in the previous round, and cooperating 50% of the time after player defection in the previous round (modified from the 33% cooperation after defection used by Rilling et al., 2007). To further elicit cooperation, participants received a message ‘lets keep cooperating’ (sic) from their anonymous partner after eight rounds of the generous-tit-for-tat strategy. The program then defected 100% of the time during rounds 13–18 (breaches in trust that occur during later rounds more reliably increase negative emotional reactions and decrease cooperation than those that occur earlier; Bottom et al., 2002).
Measures during the PDG
Initial defection
As described earlier, the computer program (the anonymous partner) played a cooperative and forgiving ‘generous tit-for-tat’ strategy during the first 12 rounds of the iterated PDG. Because participants generally cooperate with—and rarely defect against—cooperators in the iterated PDG, the resulting distribution of the number of rounds participants cooperate tends to be severely non-normal. To the extent that this is the case, statistical models that assume normality will fit poorly. However, models that assume count distributions, which are generally more appropriate for modeling rare events—in the present case, defection—tend to fit better (Atkins and Gallop, 2007). Thus, a count of the number of rounds in which participants defected in response to this strategy (i.e. participants’ choices during rounds 2–13) was used to represent baseline rates of defection, with higher values indicating higher levels of initial defection.
Delay of retaliation
After 12 rounds of cooperative play, the simulated partner defected on seven consecutive rounds. Following the same logic as aforementioned, a count of the number of rounds in which participants cooperated before defecting for the first time in response to this series of defections (i.e. rounds 14–19) was used to represent delay of retaliation in response to betrayal. Thus, lower values during the series of defections indicate faster retaliation.
Emotions
Participants rated their emotional experiences on two occasions (after round 7 and round 18) in response to these instructions: ‘Next, we would like you to please rate how YOU are feeling about your partner’s behavior at this time. How are you currently feeling about your partner’s behavior so far in the game’? Ratings were made on three positive emotions (happy, content and relaxed), hereafter ‘satisfaction’, and five negative emotions (insulted, disrespected, bitter, resentful and angry), hereafter ‘anger’. Responses were made on a 7-point Likert-type scale (1 = not at all, 7 = extremely). A principal components analysis with oblimin rotation was conducted on all eight items to determine whether satisfaction and anger could be considered separate factors. Results indicated that initial levels of satisfaction and anger could be represented with one factor, whereas post-betrayal satisfaction and anger represented two separate factors. Table 2 shows that although initial and post-betrayal satisfaction and anger are highly negatively correlated, they are not perfect inverses. For this reason, we retained two separate scales, which demonstrated good internal consistency at both the first (satisfaction, α = 0.81; anger, α = 0.91) and second (satisfaction, α = 0.77; anger, α = 0.87) measurements.
Table 2.
Means, s.d. and correlations among major study variables
| Measure | Mean (SD) | Pb satisfaction | Initial anger | Pb anger |
|---|---|---|---|---|
| Initial defection | 1.7 (2.38) | |||
| Pb delay of retaliation | 1.15 (1.39) | |||
| Initial satisfaction | 5.52 (1.24) | −0.09 | −0.67** | 0.14 |
| Pb satisfaction | 3.2 (1.2) | 0.21** | −0.58** | |
| Initial anger | 1.72 (1.12) | −0.08 | ||
| Pb anger | 3.97 (1.48) |
Correlations are not presented for count variables.
Pb, post-betrayal.
*P < 0.05; **P < 0.01.
Biological assessment
After the PDG, participants provided three 6-ml vacutainer tubes of blood or 2 ml of saliva using the Oragene DNA saliva kit (DNAGenotek, Ottawa, Ontario, Canada). Blood samples were placed in a standard refrigerator for approximately 2 days before being transported to the John P. Hussman Institute for Human Genomics at the University of Miami Miller School of Medicine for DNA extraction and genotyping. The Oragene samples were kept at room temperature until transported.
SNP genotyping was conducted using Taqman allelic discrimination assays from Applied Biosystems (ABI). Three nanograms of genomic DNA, extracted from whole blood or saliva according to established protocols, was used in the amplification reaction. Cycling was performed on GeneAmp PCR Systems 9700 thermocyclers, with conditions recommended by ABI. End-point fluorescence was measured on the ABI 7900 HT system. Genotype discrimination of experimental results was then conducted using ABI’s 7900 HT Sequence Detection System version 2.3 analysis software. To ensure genotyping accuracy, 32 quality control samples per 384-well plate, which matched within and across plates, were included.
RESULTS
Statistical analysis
We tested 10 OXTR SNPs—nine of which were identified by Israel et al. (2009) as haplotype tagging single-nucleotide polymorphisms (htSNPs; maximally informative markers on a particular region of the gene of interest; Carlson et al., 2004) (for reviews see Ebstein et al., 2009; Meyer-Lindenberg et al., 2011). According to the International HapMap Consortium (2005), the use of htSNPs, or tagging SNPs, improves the ability to identify relevant markers in association studies by taking advantage of the redundancy associated with SNPs that are highly correlated and that tend to be close to each other. The use of htSNPs freed us from investigating the >30 SNPs that have been identified in OXTR to date (Insel, 2010). As Table 1 shows, adherence to Hardy–Weinberg equilibrium was confirmed for all genotype distributions by PLINK version 1.07 (Purcell et al., 2007; http://pngu.mgh.harvard.edu/purcell/plink).
Table 1.
OXTR descriptive statistics
| Measure | Position | Genotype | Genotype frequency | Minor allele | mAF | HapMap CEU mAF | HWE |
|---|---|---|---|---|---|---|---|
| rs53576 | 8779371 | AA/AG/GG | 17/61/84 | A | 0.29 | 0.26a | 0.26 |
| n = 162 | |||||||
| rs237887 | 8772042 | GG/AG/AA | 23/80/62 | G | 0.38 | 0.41 | 0.87 |
| n = 165 | |||||||
| rs237888 | 8772095 | CC/CT/TT | 2/17/146 | C | 0.09 | 0.08 | 0.13 |
| n = 165 | |||||||
| rs237889 | 8777483 | TT/CT/CC | 22/74/59 | T | 0.38 | 0.37 | 1 |
| n = 155 | |||||||
| rs237897 | 8783285 | AA/AG/GG | 25/72/66 | A | 0.37 | 0.39 | 0.5 |
| n = 163 | |||||||
| rs2254298 | 8777228 | AA/AG/GG | 1/35/127 | A | 0.11 | 0.07 | 0.7 |
| n = 163 | |||||||
| rs2268494 | 8777046 | AA/AT/TT | 4/26/135 | A | 0.10 | 0.05 | 0.07 |
| n = 165 | |||||||
| rs2268490 | 8772085 | TT/CT/CC | 2/40/123 | T | 0.13 | 0.12 | 0.74 |
| n = 165 | |||||||
| rs9840864 | 8773477 | CC/GC/GG | 11/68/85 | C | 0.27 | 0.23 | 0.7 |
| n = 164 | |||||||
| rs1042778 | 8769545 | TT/GT/GG | 28/78/57 | T | 0.41 | 0.37 | 0.87 |
| n = 163 |
mAF, minor allele frequency; HapMap CEU, Utah residents with Northern and Western European ancestry; HWE, Hardy–Weinberg equilibrium.
aValue obtained from HapMap pilot 1 CEU.
Crawford and Nickerson (2005) noted that when many SNPs are intercorrelated, haplotype analyses are potentially valuable. Haplotypes are ‘combinations of alleles at different loci on the same chromosome’ that can be used to ‘capture the correlation structure of SNPs in regions of little recombination’ (Balding, 2006, p. 781). Examining haplotypes, based on patterns of linkage disequilibrium (LD), or non-random statistical associations of alleles at two or more loci (Balding, 2006), may increase power in tests of genetic association (de Bakker et al., 2005). Determination of LD between SNPs was analyzed using Haploview version 4.2 (Barrett et al., 2005; http://www.broadinstitute.org/mpg/haploview), and haplotype block identification was conducted using the Gabriel method (Gabriel et al., 2002). PLINK version 1.07 (Purcell et al., 2007; http://pngu.mgh.harvard.edu/purcell/plink) was then used to estimate haplotype distributions and frequencies using the expectation-maximization algorithm (Dempster et al., 1977). Owing to the relatively small sample size, only haplotypes with frequencies >0.15 were analyzed (Kawamura et al., 2010). The correlation among individual SNPs and haplotypes led us to conduct separate Bonferroni corrections for multiple testing for single-marker analyses (based on 10 individual SNPs, P = 0.005) and for multi-marker analyses (based on four specific haplotypes, P = 0.0125).
Linear regression analyses were performed with PLINK version 1.07 (Purcell et al., 2007; http://pngu.mgh.harvard.edu/purcell/plink) and SPSS version 19 to evaluate single-marker and haplotype associations with satisfaction and anger after betrayal (see Supplementary material for additional exploratory analyses). The behavioral dependent variable (initial defection) was based on a count of the number of defections during rounds 2–13, and behavioral reaction to betrayal was based on a count of the number of cooperative choices before defection during rounds 14–19 (i.e. delay of retaliation). The distributions of these count variables were non-normal, zero-inflated and overdispersed (i.e. the variances were larger than the means). To deal with these distributions, zero-inflated negative binomial regression analyses were performed (Atkins and Gallop, 2007) using the statistical package R version 2.14.0 (R Development Core Team, 2011) to evaluate single-marker and haplotype associations with behavior (see Supplementary material). Analyses of each post-betrayal outcome controlled for the corresponding baseline outcomes. Initial level of anger displayed a severe departure from normality and was log-transformed to better approximate a normal distribution (however, post-betrayal anger was uncorrelated with both the raw scores and the log-transformed scores on initial anger; rs = 0.06 and 0.08, respectively).
Table 2 displays means and s.d. for major study variables. Initial defection in rounds 2–13 of the PDG was low, as indicated by low rates of defection (M = 1.7, SD = 2.38). Correspondingly, satisfaction ratings were high (M = 5.51, SD = 1.24) and anger ratings were low (M = 1.72, SD = 1.12). These data suggest that the program’s initial strategy successfully elicited participants’ trust.
Single-marker analyses
We conducted single-marker association tests with each of the 10 SNPs using the additive model (Hill et al., 2008), in which the effect of the genotype that is homozygous for the minor allele is equivalent to twice the effect of the genotype with one minor allele. As Table 3 shows, specific associations emerged for initial defection, post-betrayal satisfaction and post-betrayal anger. No significant single-marker associations emerged involving delay of retaliation post-betrayal.
Table 3.
Regression analyses with SNPs for the additive model
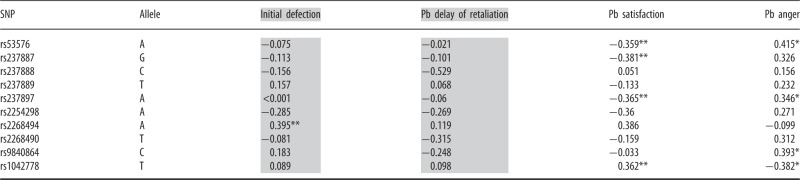 |
Gray represents unstandardized betas that need to be exponentiated to interpret on a rate ratio scale (40). n = 155–165.
Pb, post-betrayal controlling for baseline.
*P < 0.05; **P ≤ 0.01.
After Bonferroni correction for multiple testing, the association of rs2268494 and initial rates of cooperation remained significant (P = 0.008). However, the low number of individuals with the AA genotype on rs2268494 (n = 4) led us to re-run the analysis, grouping those with the AA and AT genotypes (n = 30) and comparing them with participants with the TT genotype (n = 135). Individuals with either the AA or AT genotype defected significantly more often (i.e. they were less cooperative) during the initial cooperative regimen than those with the TT genotype (b = 0.44, SE = 0.17, P = 0.008). Exponentiating b = 0.44 (see Atkins and Gallop, 2007) results in a rate ratio of 1.55, which indicates that compared with individuals with no copies of the A allele on rs2268494, those with one copy or two copies of the A allele defected 55% more frequently during the initial rounds of cooperative play, but this relationship became non-significant after Bonferroni correction (P = 0.084).
Several associations with individual SNPs and post-betrayal satisfaction were nearly statistically significant after Bonferroni correction: The addition of the G allele on rs237887 (P = 0.056), the A allele on rs237897 (P = 0.064) and the A allele on rs53576 (P = 0.1) was associated with lower levels of self-reported satisfaction after betrayal, whereas the addition of the T allele on rs1042778 (P = 0.069) was associated with higher levels of self-reported satisfaction after betrayal. However, none of these associations or single-marker associations with post-betrayal anger was statistically significant after multiple test correction.
Multi-marker analyses
Two haplotype blocks were identified: Block 1 contained rs237887 and rs2268490, and Block 2 contained rs9840864 and rs2268494. Haplotype phase (frequencies of specific haplotypes across the sample) was estimated using PLINK (Purcell et al., 2007; http://pngu.mgh.harvard.edu/purcell/plink). Block 1 included the following haplotype rates: AC (F = 0.618), GC (F = 0.248) and GT (F = 0.133) (no AT haplotype was estimated). Block 2 included the following haplotype rates: GT (F = 0.725), CT (F = 0.171) and CA (F = 0.104) (no GA haplotype was estimated). Thus, two specific haplotypes within each block had frequencies>0.15. This resulted in analysis of a total of four haplotypes (AC and GC from Block 1; CT and GT from Block 2). We conducted separate regressions for each dependent variable (initial defection, delay of retaliation, satisfaction and anger). As in single-marker analyses, we used the additive model, in which a person could have zero, one or two copies of the specific haplotype under consideration.
As shown in Table 4, we found haplotype associations with delay of retaliation post-betrayal as well as post-betrayal anger and satisfaction. None of the haplotypes was associated with initial defection in either haplotype block.
Table 4.
Regression analyses with haplotype blocks
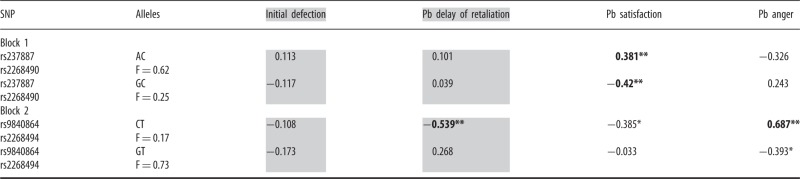 |
Gray represents unstandardized betas that need to be exponentiated to interpret on a rate ratio scale (40). Bold represents P < 0.05 after correction for multiple testing. n = 155–165.
F, frequency; Pb, post-betrayal controlling for baseline.
*P ≤ 0.05; **P < 0.01.
After Bonferroni correction, the CT haplotype was significantly associated with higher levels of anger post-betrayal (P = 0.012), the AC haplotype was significantly associated with higher levels of satisfaction post-betrayal (P = 0.022) and the GC haplotype was significantly associated with lower levels satisfaction post-betrayal (P = 0.037). Of particular interest was the finding that the CT haplotype was also significantly associated with fewer rounds of consecutive cooperation post-betrayal (i.e. faster retaliation), b = −0.539, P = 0.02. However, once again, because few individuals were homozygous for the CT haplotype (n = 2), the analysis was re-run, combining those who were heterozygous with those homozygous for this specific haplotype (n = 54). Individuals with one or two copies of the CT haplotype cooperated in significantly fewer rounds before defecting post-betrayal (b = −0.52, SE = 0.199, P < 0.01)—that is, they were faster to retaliate compared with individuals with no copies of this haplotype. Exponentiating this coefficient resulted in a rate ratio of .596. Thus, these individuals retaliated 1–0.596 = ∼40% more quickly post-betrayal. This association maintained statistical significance after Bonferroni correction (P = 0.038).
As noted, the CT haplotype was significantly associated with both higher levels of post-betrayal anger as well as faster retaliation post-betrayal. Post-betrayal anger was significantly associated with the number of rounds cooperated before defection (i.e. delay of retaliation) post-betrayal as well (b = −0.23, SE = 0.06, P < 0.001), raising the possibility that the CT hapotype’s association with delay of retaliation was due in part to its intermediate effect on the amount of anger experienced after betrayal. To test this possibility, we used the delta method (Oehlert, 1992; see Supplementary material) to determine the potential mediating role of post-betrayal anger on the effect of the OXTR CT haplotype on retaliation post-betrayal. The indirect effect was significant (Z = −12.13, P < 0.01), which suggests plausible mediation (Zhao et al., 2010; Rucker et al., 2011).
DISCUSSION
We report the first study associating variation on OXTR with individual differences in reactions to betrayals in trust in the iterated PDG. After the establishment of trust and high rates of cooperation, participants experienced a betrayal of trust. The CT haplotype (rs9840864, rs2268494) was significantly associated with higher levels of anger and faster retaliation after this betrayal. Evidence for a statistically significant indirect effect supported the view that the effect of the CT haplotype on retaliatory behavior was mediated by higher levels of anger post-betrayal. Thus, compared with persons without the CT haplotype, those with the CT haplotype were more angered by the betrayal, which appeared partially responsible for their faster tendency to retaliate.
Previous studies have yielded evidence suggesting that intranasal administration of oxytocin reduces betrayal aversion (Baumgartner et al., 2008) and concerns about being betrayed (i.e. for those high on attachment avoidance; De Dreu, 2012). Recently, Klackl et al. (2013) found that after a betrayal in trust, individuals given oxytocin were more likely to form non-personalistic attributions (i.e. the tendency to view one’s actions as a result of the situation as opposed to their character) toward their betrayers compared with placebo, which in turn mediated the association between angry rumination about being betrayed and trust. The present results add to this previous work involving intranasal administration of oxytocin by suggesting that genetic variation on OXTR might be associated with both emotional and behavioral reactions to betrayal.
To date, most studies investigating the relationship between OXTR and social phenotypes have focused primarily on rs53576 (e.g. Chen et al., 2011), rs2254298 (Tost et al., 2010) and rs1042778 (e.g. Israel et al., 2009). For example, in a pioneering study, Israel et al. (2009) found that variation on rs1042778 as well as several three- to five-locus haplotypes was associated with generosity in the Dictator Game and selflessness in the Social Value Orientations task (Van Lange et al., 1997). Results maintained significance after permutation-based correction for multiple testing. In another research effort, Apicella et al. (2010) examined associations of nine OXTR polymorphisms and an index of prosociality created from a modified Dictator Game (the Recipient was a charity rather than another person) and the Trust game. In contrast to Israel et al. (2009), after correction for multiple testing, no association of any OXTR SNP genotype with this index was significant.
In the present study, after multiple test correction, there were no significant associations with rs53576, rs2254298 or rs1042778 and any of the measured outcome variables in single- or multi-locus tests (as in Apicella et al., 2010). In addition, no significant associations were found with any OXTR SNPs and our measure of initial defection, which represented baseline cooperative or prosocial tendencies. However, it is difficult to know how closely to compare the present results with previous findings because: (i) few researchers have examined OXTR SNPs rs9840864 and rs2268494, or haplotypes containing these SNPs; (ii) the differences between our phenotypes of interest (i.e. post-betrayal anger and retaliation post-betrayal) and those that other groups have examined (e.g. dictator giving in Israel et al., 2009, and autism spectrum disorders in Lerer et al., 2008) are substantial and (iii) the haplotypes examined in previous studies also included other OXTR SNPs in addition to rs9840864 and rs2268494. Nonetheless, the main findings in the present study are in general agreement with Israel et al. (2009), who found a significant association in a five-locus haplotype including the C allele on rs9840864 and the T allele on rs2268494 and less giving in the dictator game among several significant specific haplotype associations (S. Israel, personal communication).
In addition to the association between the CT (rs9840864, rs2268494) haplotype and higher levels of anger post-betrayal, other haplotype–emotion associatons emerged as well. Specifically, the AC (rs237887, rs2268490) haplotype was associated with higher levels of satisfaction post-betrayal, and the GC (rs237887, rs2268490) haplotype was associated with lower levels of satisfaction post-betrayal. This pattern of associations suggests that the A or G allele on rs237887 may contribute differentially to emotional reactions post-betrayal in conjunction with the common C allele on rs2268490. This is plausible because rs237887 and rs2268490 are in moderate LD (r2 = 0.24; Lin et al., 2007).
Previous research examining associations of rs237887 and/or rs2268490 and aspects of social cognition and behavior has found mixed results. In both single- and multi-marker analyses, Israel et al. (2009; S. Israel, personal communication) found that the A allele in rs237887 and the C allele in rs2268490 were associated with dictator giving and selfless social value orientation. Wu et al. (2012) found nominal associations in a Chinese population in trait perspective-taking (an aspect of cognitive empathy) and individuals with the AA genotype on rs237887, whereas individuals with the GG genotype demonstrated nominal associations with both cognitive (i.e. engagement in fantasy) and emotional aspects of empathy (i.e. personal distress).
Although previous studies examining OXTR–affect associations either did not include the same SNPs associated with emotional reactions in the present study, or did not find them when they did include some of the same SNPs, the present results add to research suggesting that variation on OXTR may be associated with individual differences in affect (Lucht et al., 2009; Kawamura et al., 2010). In addition, recent research has shown that intranasal oxytocin can increase defensive aggression (De Dreu et al., 2010), and both oxytocin in cerebrospinal fluid (Lee et al., 2009) and variation on several OXTR SNPs (Malik et al., 2012) have been correlated with a history of aggression. Therefore, the present findings also add to a growing body of research suggesting that variation in the oxytocin system may influence approach-related emotion and behavior such as anger and aggression (Kemp and Guastella, 2011).
This study has several limitations. First, the sample size was small for a population-based association study of unrelated individuals. Given this constraint, only haplotypes with frequencies >0.15 were examined (as in Kawamura et al., 2010). The sample size in the present study also prevented us from examining potential differences in variation on OXTR and the phenotypes of interest among individuals who might be viewed as entirely self-interested (i.e. four individuals were removed because they did not establish trust), or among a subsample of individuals who might have defected during the breach in trust, and then attempted to coerce their ‘partner’ into cooperating. Future studies with a larger sample size are needed to further elucidate the genetic basis of such behaviors. Nevertheless, the sample size is similar to those of other recent OXTR association studies examining aspects of social cognition and behavior (Rodrigues et al., 2009; Chen et al., 2011), and our results appear somewhat less likely to have resulted from type I error due to our use of Bonferroni correction for multiple testing. In any case, researchers have recently documented that most molecular genetic studies have lacked sufficient statistical power for detecing all but the largest relationships between allelic variation and behavioral phenotypes (Benjamin et al., 2012), so we hasten to acknolwedge the possibility that our results are false-positives. We strongly recommend replication with a larger sample size.
Second, although research on OXTR is rapidly progressing, and several studies have found associations between variation on OXTR and brain structure and function (Tost et al., 2010), the functionalities of the polymorphisms examined here are not yet known. It has been suggested that intron 3 on OXTR (the location of all significantly associated SNPs in the present study) may be involved in transcriptional suppression (Mizumoto et al., 1997). However, the associations observed here may result from LD associations with other functional markers on OXTR (Lin et al., 2007) or in other genes (e.g. Montag et al., 2011). Again, we note that these results are in need of replication and should be viewed as preliminary.
Third, restricting our sample to non-Hispanic White undergraduates limits the generalizability of our findings to people from other groups. However, racial homogeneity reduces the likelihood that the findings resulted from type I error caused by population stratification (Risch et al., 2002; Cardon and Palmer, 2003). In addition, examining individuals from a single age-group prevented the potentially confounding influence of age-related differences in social decision-making (Nielsen and Mather, 2011). Nonetheless, some of the lack of agreement in our results with those of Israel et al. (2009), who examined an Israeli sample, and those of Apicella et al. (2010), who examined a Swedish sample, may be attributed to differences in the phenotypes examined, sample size and power, methods used to correct for multiple testing and allele frequency distributions among Northern Europeans, Southern Europeans and European Americans (Price et al., 2008; Tian et al., 2008).
The present study revealed that three specific haplotypes on OXTR were associated with emotional reactions after an experimental betrayal of trust in an iterated PDG—one of the first and most venerated economic games in the social sciences. Moreover, one haplotype was associated with variation in the speed at which individuals retaliated after betrayal, apparently due in part to its intermediate effects on how angry participants became after they had been betrayed. These results, therefore, set out some targets for future work that marries genetics with neural, cognitive and behavioral methods (Baumgartner et al., 2008; Rilling et al., 2012) to identify the specific neurocognitive systems that are responsible for the emotional and behavioral variation observed herein. Future studies that involve measurement of genetic variation as well as the exogenous administration and endogenous measurement of oxytocin (Rilling et al., 2012), or perhaps the measurement of differences in OXTR DNA methylation (Unternaehrer et al., 2012), may also help to develop a more comprehensive understanding of the role of the oxytocin system in social cognition and behavior.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This project was supported by seed funds associated with a University of Miami Distinguished Professorship (to C.S.C.); the National Science Foundation [BCS0544617 to C.S.C.]; the National Institute of Mental Health [R01MH071258 to M.E.M.]; and the Air Force Office of Scientific Research [FA9550-12-1-0179 to M.E.M]. Support was also provided during a postdoctoral fellowship [MH15750] [B.A.T.].
REFERENCES
- Apicella CL, Cesarini D, Johannesson M, et al. No association between oxytocin receptor (OXTR) gene polymorphisms and experimentally elicited social preferences. PLoS One. 2010;5:e11153. doi: 10.1371/journal.pone.0011153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins DC, Gallop RJ. Rethinking how family researchers model infrequent outcomes: a tutorial on count regression and zero-inflated models. Journal of Family Psychology. 2007;21:726–35. doi: 10.1037/0893-3200.21.4.726. [DOI] [PubMed] [Google Scholar]
- Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–6. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Balding DJ. A tutorial on statistical methods for population association studies. Nature Reviews Genetics. 2006;7:781–91. doi: 10.1038/nrg1916. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bartz J, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Benjamin DJ, Cesarini D, van der Loos MJ, et al. The genetic architecture of economic and political preferences. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8026–31. doi: 10.1073/pnas.1120666109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottom WP, Gibson K, Daniels SE, Murnighan JK. When talk is not cheap: substantive penance and expressions of intent in rebuilding cooperation. Organization Science. 2002;13:497–513. [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. The Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. American Journal of Human Genetics. 2004;74:106–20. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein R, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19937–42. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Nickerson DA. Definition and clinical importance of haplotypes. Annual Review of Medicine. 2005;56:303–20. doi: 10.1146/annurev.med.56.082103.104540. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nature Genetics. 2005;37:1217–23. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012;37:871–80. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society. Series B (Methological) 1977;39:1–38. [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, et al. Arginine vasopressin and oxytocin modulate human social behavior. Annals of the New York Academy of Sciences. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hill WG, Goddard ME, Visscher PM. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genetics. 2008;4:e10000008. doi: 10.1371/journal.pgen.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Liu X, Akiyama T, et al. The association between oxytocin receptor gene (OXTR) polymorphisms and affective temperaments, as measured by TEMPS-A. Journal of Affective Disorders. 2010;127:31–7. doi: 10.1016/j.jad.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: a novel hypothesis. Current Directions in Psychological Science. 2011;20:222–31. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates nueral circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klackl J, Pfundmair M, Agroskin D, Jonas E. Who is to blame? Oxytocin promotes nonpersonalistic attributions in response to a trust betrayal. Biological Psychology. 2013;92:387–94. doi: 10.1016/j.biopsycho.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009;34:1567–73. doi: 10.1016/j.psyneuen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry. 2008;13:980–8. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. American Journal of Human Genetics. 2007;80:531–8. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, et al. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:860–6. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, Beitchman JH. The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes Brain and Behavior. 2012;11:545–51. doi: 10.1111/j.1601-183X.2012.00776.x. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Pedersen EJ, Schroder J, Tabak BA, Carver CS. Harsh childhood environmental characteristics predict exploitation and retaliation in humans. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2013;280:20122104. doi: 10.1098/rspb.2012.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mizumoto Y, Kimura T, Iveil R. A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Molecular and Cellular Endocrinology. 1997;135:129–38. doi: 10.1016/s0303-7207(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Montag C, Fiebach CJ, Kirsch P, Reuter M. Interaction of 5-HTTLPR and a variation on the oxytocin receptor gene influences negative emotionality. Biological Psychiatry. 2011;69:601–3. doi: 10.1016/j.biopsych.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Mather M. Emerging perspectives in social neuroscience and neuroeconomics of aging. Social Cognitive and Affective Neuroscience. 2011;6:149–64. doi: 10.1093/scan/nsr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Tit for tat in heterogenous populations. Nature. 1992;355:250–3. [Google Scholar]
- Oehlert GW. A note on the delta method. American Statistician. 1992;46:27–9. [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Butler J, Patterson N, et al. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet. 2008;4:e236. doi: 10.1371/journal.pgen.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–61. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–71. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race, and disease. Genome Biology. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: current practices and new recommendations. Social and Personality Psychology Compass. 2011;5:359–71. [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therpauetic potential. Frontiers in Neuroendocrinology. 2011;32:426–50. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Luna LR, Bono G, Berry JW. Conciliatory gestures facilitate forgiveness and feelings of friendship by making transgressors appear more agreeable. Journal of Personality. 2012;80:503–36. doi: 10.1111/j.1467-6494.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthniess and attractiveness. Hormones and Behavior. 2009;56:128–32. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Tian C, Plenge RM, Ransom M, et al. Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet. 2008;4:e4. doi: 10.1371/journal.pgen.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13936–41. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Translational Psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lange PAM, De Bruin EMN, Otten W, Joireman JA. Development of prosocial, individualistic, and competitive orientations: theory and preliminary evidence. Journal of Personality and Social Psychology. 1997;73:733–46. doi: 10.1037//0022-3514.73.4.733. [DOI] [PubMed] [Google Scholar]
- West SA, El Mouden C, Gardner A. Sixteen common misconceptions about the evolution of cooperation in humans. Evolution and Human Behavior. 2011;32:231–62. [Google Scholar]
- Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. Journal of Affective Disorders. 2012;138:468–72. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Jr, Chen Q. Reconsidering baron and kenny: myths and truths about mediation analysis. Journal of Consumer Research. 2010;37:197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


