Abstract
Mindfulness—an attentive non-judgmental focus on present experiences—is increasingly incorporated in psychotherapeutic treatments as a skill fostering emotion regulation. Neurobiological mechanisms of actively induced emotion regulation are associated with prefrontally mediated down-regulation of, for instance, the amygdala. We were interested in neurobiological correlates of a short mindfulness instruction during emotional arousal. Using functional magnetic resonance imaging, we investigated effects of a short mindfulness intervention during the cued expectation and perception of negative and potentially negative pictures (50% probability) in 24 healthy individuals compared to 22 controls. The mindfulness intervention was associated with increased activations in prefrontal regions during the expectation of negative and potentially negative pictures compared to controls. During the perception of negative stimuli, reduced activation was identified in regions involved in emotion processing (amygdala, parahippocampal gyrus). Prefrontal and right insular activations when expecting negative pictures correlated negatively with trait mindfulness, suggesting that more mindful individuals required less regulatory resources to attenuate emotional arousal. Our findings suggest emotion regulatory effects of a short mindfulness intervention on a neurobiological level.
Keywords: mindfulness, emotion regulation, fMRI, insula, amygdale, prefrontal cortex
INTRODUCTION
Emotional dysregulation and maladaptive emotion regulation are major deficits in many psychiatric disorders, such as anxiety disorders or depression (Gross and Muñoz, 1995). Cognitive control strategies, such as the reappraisal of emotional situations or reality checking, are applied in psychotherapy to compensate these deficits (Beck, 2005; Ochsner and Gross, 2005; Disner et al., 2011). On the neurobiological level, cognitive emotion regulation studies find regulatory influences of prefrontal top–down inhibitory structures, such as the dorsolateral and ventrolateral prefrontal cortex (DLPFC, VLPFC) and the dorsomedial prefrontal cortex (DMPFC), on bottom–up emotion propagating structures, such as the amygdala (recent reviews: Kalisch, 2009; Diekhof et al., 2011; Kanske et al., 2011; selected studies: Beauregard et al., 2001; Ochsner et al., 2002; Urry et al., 2006; Banks et al., 2007; Herwig et al., 2007b). The notion of top–down regulatory influences of prefrontal regions on amygdala is further supported by tracing and stimulation studies in animals (Ghashghaei et al., 2007; Quirk, 2007).
Another strategy to deal with emotions is the practice of mindfulness, which can be described as an attentive non-judgmental focus on experiences in the here and now (Kabat-Zinn, 1994). Although mindfulness, rooted in ancient Eastern tradition, is typically related to meditation techniques, it is also increasingly implemented in western psychotherapy (Hofmann et al., 2010). Programs such as Mindfulness-Based Stress Reduction (MBSR; Kabat-Zinn, 1982) have shown beneficial effects on well-being in general (Baer, 2003; Brown and Ryan, 2003; Grossman et al., 2004) and on symptoms of various mental disorders (Hofmann et al., 2010; Chiesa et al., 2011). At the same time, the underlying neurobiological mechanisms of mindfulness remain elusive. Most studies have investigated extended mindful states in meditation practitioners (Cahn, 2006; Hölzel et al., 2011), where meditation was mostly associated with activation in attention and emotion-regulating areas, such as prefrontal cortex (PFC) and anterior cingulate cortex (ACC) (Chiesa and Serretti, 2009), whereas the amygdala representing an important region for emotion modulation and amplification showed decreased activation (Farb et al., 2007; Hölzel et al., 2011). Similarly, meditators showed structural brain changes in PFC areas as well as in viscerosensory and somatosensory areas—reflecting the focus on perception and interoception in meditation (Luders et al., 2009; Ott et al., 2011).
Beyond the context of meditation, mindfulness can be seen more generally as an attitude to face emotional situations with a focus on the current experience (Bishop et al., 2004). From this perspective, mindfulness could have an emotion-regulating effect in every-day emotional situations, similar to its implementation, for example, in psychotherapy for Borderline personality disorder (Linehan, 1993). However, few studies have looked at the induction of short states of mindfulness, which might reflect an aspect of such a general attitude.
Studies on affect labeling as a measure to increase experiential awareness found reduced activity in the amygdala and related structures and increases in prefrontal activity when labeling of negative facial stimuli (compared with gender labeling) (Lieberman et al., 2007). Further, subjects with higher trait mindfulness scores showed greater prefrontal and less amygdala activity during affect labeling (Creswell et al., 2007). Another study used the mindfulness-related construct ‘level of emotional awareness’ and found similar positive correlations between level of emotional awareness and activation in response to emotional stimuli in prefrontal, cingulate and insular cortex (McRae et al., 2008).
It is important to note that the above-mentioned studies contained a behavioral component, which could have interfered with generating a mindful state. The effects of a ‘pure’ mindfulness intervention during emotional stimulation have not been investigated so far. In a previous study, we investigated the mere direction of attention towards the current experience of emotions and bodily sensations without explicit behavioral control in comparison with cognitive self-reflection (Herwig et al., 2010). The experiential focus reduced amygdala activity and increased activation in frontal areas such as DMPFC and ACC and in brain regions related to somato- and viscerosensation (right insula). These findings point to a regulatory effect of mindfulness on brain regions related to emotion processing (Ochsner and Gross, 2005; Diekhof et al., 2011). However, in this paradigm, subjects did not face actual emotional stimuli.
To study the neural correlates of a short mindful state during expectation and perception of negative or potentially negative stimuli without a behavioral component, we used functional magnetic resonance imaging (fMRI) in a group of healthy subjects who were compared with a matched group not applying any emotion-regulation strategy (see Herwig et al., 2007a). Regions of interest (ROI), in particular the amygdala, and whole brain activations were analyzed. We hypothesized that when expecting and perceiving negative or potentially negative pictures, mindfulness would be associated with increased activations in regulatory structures (DLPFC, DMPFC) and with decreased activations in regions associated with emotional arousal, such as amygdala and insula.
METHODS
Subjects
Fourty-nine healthy subjects [ages: 20–57, Mage = 29.98, s.d. = 7.96, 32 female, all right-handed according to a handedness questionnaire (Annett, 1970)] were included in the study. As assessed with semistructured interviews and checklists performed by an experienced psychiatrist (A.B.B.), the exclusion criteria were prior and current neurological and psychiatric illnesses; pregnancy; intake of any medication (except for oral contraceptives) or psychotropic drugs including excessive consumption of alcohol (regular intake of >7 U/week), cigarettes (>2 packs/day) and caffeine (>10 cups/day) and general contraindications against MRI examinations. Participants were recruited via mailing lists of the university of Zurich and personal contacts. The study was approved by the ethics committee of the canton of Zürich and conducted in compliance with the Declaration of Helsinki (World Medical Association, 2008). All participants gave written informed consent and received a financial compensation.
Twenty-three subjects participated in the trial without any emotion-regulation strategy (‘basic group’), and 26 subjects were given a mindfulness instruction (‘mindfulness group’). Two subjects of the mindfulness and one of the basic group were excluded from further analysis owing to excessive head movements (>3 mm in at least one direction) or reported drowsiness in the scanner, resulting in 24 analyzed subjects in the mindfulness group and 22 in the basic group (see details in Supplementary Table S1). The groups were assigned pseudo-randomly (matched for age and gender).
Meditation experience was assessed only in the mindfulness group. Experience with meditation or other mindfulness techniques was neither an inclusion nor an exclusion criterion for the mindfulness group. The goal was to obtain a naturalistic variation in this variable and to study overarching effects of a basic mindfulness instruction (overview of meditation experience: Supplementary Table S2).
Task
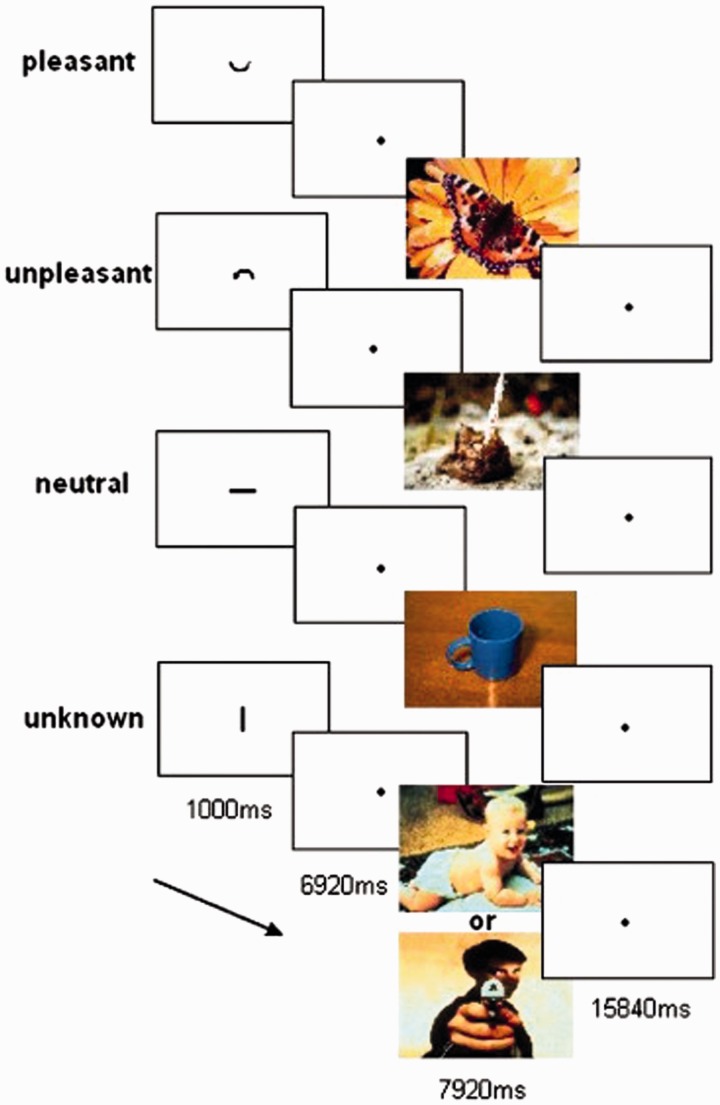
During fMRI scanning, the subjects performed an emotional expectation paradigm (described in detail in Herwig et al., 2007a, Figure 1), in which they expected and perceived cued emotional pictures of ‘known’ or ‘unknown’ valence. In ‘known’ trials, a cue was presented (duration 1000 ms), depicting either a ‘positive’ ‘∪’, a ‘negative’ ‘∩’ or a ‘neutral’ symbol ‘−’, announcing the emotional valence of the picture after the expectation period. In ‘unknown’ trials, the symbol ‘|’ announced an emotional picture of either pleasant or unpleasant content (50% probability). ‘Unknown’ therefore refers to an expectation period in which the valence of the subsequent picture is ambiguous. Expectation periods (6920 ms) followed the cue showing a blank screen with a small fixation point. Thereafter, the respective picture of positive, negative or neutral content was presented for 7920 ms [4 repetition times (TR)]. Trials ended with a baseline period of 15 840 ms (8 TR) allowing the BOLD signal to level off between trials.
Fig. 1.
Illustration of experimental task and durations. Cues are enlarged for presentation reasons. Their actual height in the experiment was about 1/40 screen size.
The task comprised one run consisting of 56 randomized trials, 14 for each condition: known positive (ps), known negative (ng), known neutral (nt) and ‘unknown’ (uk). The task was programmed with PresentationTM (Neurobehavioral Systems, USA) and presented via digital video goggles (Resonance Technologies, Northridge, CA). The symbols were intuitively understandable and required little cognitive resources to grasp their meaning. The task did not involve any motor reaction that could have interfered with the task.
Group instructions
Unlike most mindfulness studies that induce continuous mindful states, we restricted the mindfulness intervention to certain conditions: Subjects in the mindfulness group were instructed to apply mindful awareness only during unpleasant and ‘unknown’ trials. We chose this focus, because in the therapeutic setting, mindfulness strategies are commonly applied to deal with unpleasant emotional events. Positive conditions served primarily to balance the overall emotional valence of the stimuli and avoid negative mood induction.
The written instructions explicitly mentioned neither regulation/emotion regulation nor mindfulness but described common aspects of mindfulness definitions—i.e. non-judgmental awareness of the present moment, and openness to experience (Kabat-Zinn, 1994; Brown and Ryan, 2003): ‘Try to consciously be aware of yourself, of what happens to you at this moment. Do this while expecting the picture and while looking at it. Do not judge; remain conscious and attentive to your present state. You may focus on thoughts, on emotions or on bodily sensations.’
The attentional focus was formulated openly, as it is common to various mindfulness techniques. Also, we wanted to study general effects of mindfulness, independently of attentional focus.
The basic group was instructed to expect and perceive the emotional stimuli.
Prior to scanning, all participants completed a supervised training session to get accustomed to task and instruction. Training pictures did not re-appear in the main task. In a structured interview after scanning, participants were asked about their general experience of being in the scanner, of the task and of the respective instructions (subjective performance).
Stimuli
Pictures were taken from the International Affective Picture System (IAPS; Peter Lang, Miami, USA; Lang, 1995). They were matched for valence difference from neutral, complexity, contents and as far as possible for arousal (compare discussion in Herwig et al., 2007b). After scanning, subjects rated the emotional valence of the presented pictures (on printouts) on a 9-point Likert scale (1—very negative, 9—very positive).
FMRI acquisition
Imaging was performed with a 3.0 T GE SignaTM HD Scanner (GE Medical Systems, Milwaukee, USA, 8-channel head coil). Echo-planar imaging was performed for fMRI (TR / TE 1980 / 32 ms, 22 sequential axial slices, whole-brain, slice thickness 3.5 mm, 1 mm gap, resulting voxel size 3.125 × 3.125 × 4.5 mm, matrix 64 × 64, flip angle 70°). Nine hundred eight volumes were obtained per subject, 16 per trial. The first four volumes were discarded to allow for T2* equilibration effects. High-resolution 3D T1 weighted anatomical volumes were acquired (TR / TE 9.9 / 2.9 ms; matrix size 256 × 256; 1 × 1 × 1 mm3 resolution, axial orientation) for co-registration with the functional data. T2-weighted images in parallel to the EPI sequence were acquired to exclude T2-sensitive abnormalities
FMRI data analysis and statistics
FMRI data were analyzed using BrainVoyager QX 2.3 (Brain Innovation, The Netherlands, Goebel et al., 2006). Preprocessing of functional scans comprised motion correction, slice scan time correction, high frequency temporal filtering and removal of linear trends. Functional and 3-D structural measurements were then co-registered and transformed into Talairach space (Talairach and Tournoux, 1988). The resulting data sets (voxel size 3 × 3 × 3 mm3) were spatially smoothed with an 8 mm3 full width at half-maximum Gaussian kernel for group analysis. Single trials with fMRI signal artefacts of more than threefold the mean signal change resulting in outliers of beta weights (e.g. owing to head movements) were eliminated manually.
The model for statistical analysis contained eight predictors representing four expectation conditions (ng, ps, nt, uk), the respective presentation conditions and the factor group, resulting in totally nine predictors. The conditions were modeled as epochs using a two-gamma hemodynamic response function adapted to the applied period duration provided by BrainVoyager.
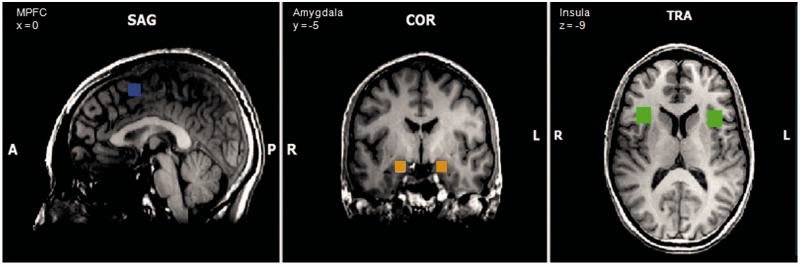
FMRI data analysis, based on the general linear model (GLM), comprised the following steps: On the single-subject level, fixed effects analyses were calculated for the expectation phase contrasts negative vs neutral (eng > ent) and unknown vs neutral (euk > ent) and for the perception phase contrast negative vs neutral (png > pnt). The neutral condition was subtracted to study emotion processing without general effects of expectation and perception of visual stimuli. Resulting summary images were subjected to second-level group analyses within predefined cubic ROIs: in our main ROI, the amygdala (Figure 2), and additionally in the DMPFC, DLPFC and anterior insula. ROIs were defined according to activation coordinates in fMRI studies on emotion regulation (amygdala; Ochsner et al., 2002; Ochsner et al., 2004; Goldin et al., 2008; Herwig et al., 2010) and studies on mindfulness (anterior insula, DMPFC, DLPFC; Creswell et al., 2007; Herwig et al., 2010) and anatomically validated using the Talairach client (Lancaster et al., 2000). MNI coordinates were non-linearly transformed into TAL space with Matlab’s mni2tal-function (Brett, 2009). Resulting ROI coordinates and sizes are given in Table 1. In each ROI, mean beta weights for our contrasts of interest (eng > ent, euk > ent, png > pnt) were compared between groups using student’s t-tests; effect sizes were calculated using Cohen’s d (Cohen, 1998). In the mindfulness group, mean beta weights were also correlated with trait mindfulness scores.
Fig. 2.
ROI of the DMPFC (blue), amygdala (orange box), insula (green).
Table 1.
ROI group analysis mindfulness group > basic group
| ROI (Coordinates x, y, z; BA) | Cluster size | eng > ent |
euk > ent |
png > pnt |
|||
|---|---|---|---|---|---|---|---|
| mm3 | t | P (d) | t | P (d) | t | P (d) | |
| Amygdala R (19, −8, −15; –) | 729 | −0.34 | 0.73 | 0.21 | 0.83 | −2.34 | 0.024 (0.71) |
| Amygdala L (−19, −8, −15; –) | 729 | 0.21 | 0.83 | 10.38 | 0.18 | −1.32 | 0.19 |
| Ant. Insula R (35, 15, 9; –) | 3375 | 0.82 | 0.42 | 0.91 | 0.37 | −0.61 | 0.95 |
| Ant. Insula L (−35, 15, 9; –) | 3375 | 2.31 | 0.03 (0.70) | 3.10 | 0.00 (0.93) | 0.11 | 0.91 |
| DMPFC R (5, 6, 50; 6/8) | 1000 | 2.26 | 0.03 (0.68) | 2.02 | 0.05 (0.61) | 0.10 | 0.92 |
| DMPFC L (−5, 6, 50; 6/8) | 1000 | 2.69 | 0.01 (0.81) | 2.68 | 0.01 (0.81) | 0.56 | 0.58 |
| DLPFC R (35, 20, 28; 9/46) | 3375 | 0.37 | 0.71 | 0.35 | 0.73 | 1.16 | 0.25 |
| DLPFC L (−35, 20, 28; 9/46) | 3375 | 2.80 | 0.01 (0.85) | 1.72 | 0.09 | 0.59 | 0.56 |
ROI analysis of emotion expectation negative vs neutral (eng > ent) and ‘unknown’ vs neutral (euk > ent) and emotion perception negative vs neutral (png > pnt) in the mindfulness group compared with the basic group. Mean beta weights of the contrasts were compared between groups using 2-tailed student’s t-tests with 44 df. Results rounded (two digits). Significant differences are given in bold (P < 0.05), differences with a trend in italics (P < 0.1). Effect sizes for the significant differences are indicated in brackets.
ROI egde-length: 9 × 9 × 9 mm (amygdala), 10 × 10 × 10 mm (DMPFC), 15 × 15 × 15 mm (insula, DLPFC).
Ant., Anterior; DMPFC, Dorsomedial Prefrontal Cortex; DLPFC, Dorsolateral Prefrontal Cortex; R, right; L, left.
Furthermore, for the above-mentioned contrasts (eng > ent, euk > ent, png > pnt), we performed whole-brain random effects group comparisons. Results are reported on a voxel-wise threshold of P < 0.005 and a cluster threshold of five voxels (135 mm3), as suggested by Lieberman and Cunningham (2009), to avoid too many false negatives. Additionally, to correct for multiple comparisons, maps with a voxel-wise threshold of P < 0.005 were submitted to a Monte Carlo simulation (Goebel et al., 2006) for estimating cluster-level false-positive rates, yielding a corrected cluster-level of P < 0.05.
To verify the results of our main contrasts of interest, we also analysed the contrast negative vs positive (ng > ps, Supplementary Tables S4 and S5). We hypothesized activations similar to ng > nt, as mindfulness was applied in the negative, but not in the positive condition, although here valence constitutes a confounding factor.
To control for general differences between groups in emotion processing, we compared the contrast perception positive vs neutral. These conditions were not included in the mindfulness instruction; therefore, no group difference was hypothesized.
Finally, to control for general perceptual and attentional differences individually and between groups, we inspected activity in the primary visual cortex during stimulus perception, as closed eyes or diverted gaze would have resulted in decreased activity in V1 (ROI analysis, size = 729 mm3, x, y, z = 5/−5, −86, −3, data not shown).
Questionnaires and correlation statistics
Subjects completed German versions of questionnaires to assess levels of depression (Self-Rating Depression Scale, SDS; Zung, 2005), anxiety (State-Trait Anxiety-Inventory, STAI; Laux et al., 1981), as well as neuroticism and extraversion (Eysenck Personality Inventory, EPI; Eysenck and Eysenck, 1964).
The mindfulness group additionally completed one-dimensional trait mindfulness self-report questionnaires: The Freiburg Mindfulness Inventory (FMI, Walach et al., 2006) and the Mindful Attention and Awareness Scale (MAAS; Brown and Ryan, 2003). For more information consult Supplementary material (Supplementary Table S3).
RESULTS
Participant’s characteristics
The 46 subjects included in the analysis (ages: 20–57, Mage = 29.87, s.d. = 8.18, 15 female), 22 in the basic, 24 in the mindfulness group, did not differ between groups in terms of age [t(44) = −0.28, P = 0.78] or gender distribution [χ2(1, 46) = 0.12, P = 0.93]. For an overview of demographic data, see Supplementary Table S1.
Psychometric characteristics
There was no significant group difference regarding levels of depression, anxiety, neuroticism, extraversion or trait mindfulness (Supplementary Table S3).
The sample did not show clinically relevant degrees of depression or anxiety.
The mindfulness measures (MAAS/FMI) were highly intercorrelated [r(22) = 0.52, P < 0.01].
Interview and valence ratings after the scan
All subjects confirmed that they had been able to perform the general task. Subjects in the mindfulness group reported sufficient capability of following the mindfulness instruction in the scanner and experienced no abnormal emotional or meditative states (Supplementary Figure S1A). Subjects’ main focus of attention was distributed evenly over bodily sensations, thoughts and feelings (χ2 tests for categorical variables: P = 0.75, ns., see Supplementary Figure S1B).
The valence ratings of the pictures had good internal consistencies (Cronbach’s alpha α > 0.8), and groups did not differ in their ratings [tng(43) = 0.06, p = 0.95; tps(43) = 0.68, p = 0.50; tnt(43) = −0.55, p = 0.59].
FMRI analysis
ROI analysis and correlation results
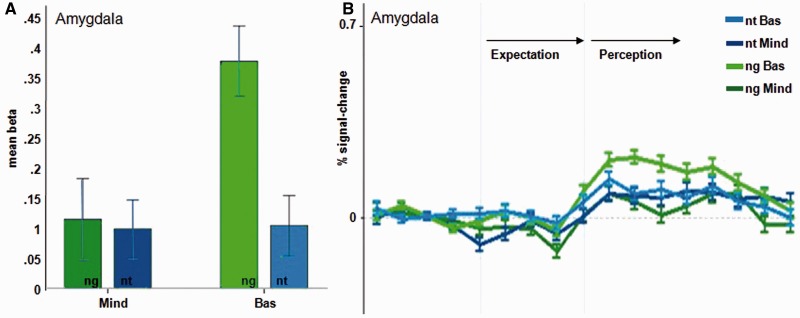
Our main ROI, the amygdala, showed decreased activation in the mindfulness group (right amygdala, d = 0.71, medium effect, Figure 3) during the perception of negative stimuli (Table 1).
Fig. 3.
(A) Significantly lower group mean beta weight of the mindfulness group (Mind) in the amygdala ROI (t(44) = − 2.91, P < .01) compared to the basic group (Basic),in the perception of negative pictures (ng), no group difference in the perception of neutral pictures (nt) (t(44) = − .08, P = .935). (B) Differing time courses for the amygdala ROI between groups in the perception of negative stimuli: Similarity of time courses of the perception of negative and neutral pictures only in the mindfulness group. Error bars indicate standard errors.
Expecting negative stimuli was associated with increased activity in the mindfulness group in the left and right DMPFC (drDMPFC = 0.68, medium effect; dlDMPFC = 0.81, large effect), the left anterior insula (d = 0.70, medium effect) and the left DLPFC (d = 0.85, large effect) compared with the basic group. Expecting unknown stimuli revealed similar group differences, except for the group difference in the left DLPFC that did not reach significance. The amygdala was not differentially activated in any expectation phase.
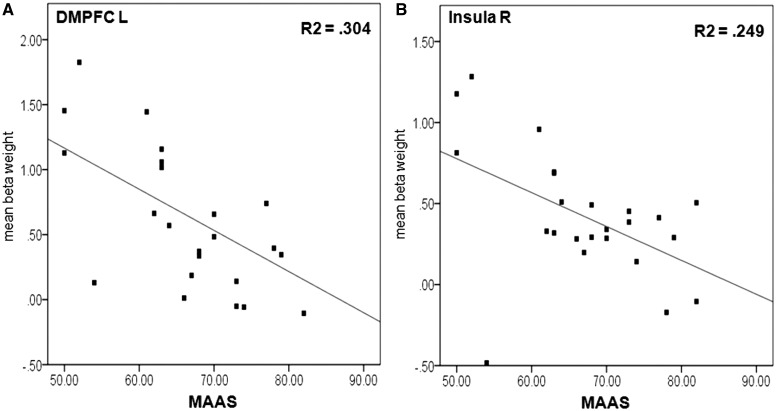
In the expectation phase, negative correlations between beta weights of the ROIs and trait mindfulness (MAAS) were found in the right DMPFC [eng > ent: r(22) = −0.51, P = 0.01; euk > ent: r(22) = −0.51, P = 0.01] and more strongly in the left DMPFC [eng > ent: r(22) = −0.59, P = 0.002; euk > ent: r(22) = −0.55, P = 0.005]. Further, the MAAS correlated negatively with activity in the right anterior insula [eng > ent: r(22) = −0.50, P = 0.01; euk > ent: r(22) = −0.49, P = 0.02, Figure 4].
Fig. 4.
Correlation of individual mean beta weights of the contrast expectation negative vs expectation neutral in the left DMPFC (A) and in the right anterior insula (B) with trait mindfulness scores (MAAS).
Whole-brain group comparison ‘mindfulness’ vs ‘no emotion regulation’
In the whole-brain analysis during the perception of negative stimuli (Table 2), the group comparison between mindfulness and basic group yielded no significant results after applying the cluster-wise corrected threshold according to the Monte Carlo simulation. When applying the empirical threshold of 135 mm3, we found activation clusters in left middle frontal gyrus and reduced activations in right hippocampus and left posterior insula.
Table 2.
Whole-brain group differences (mindfulness > basic) during the perception of negative stimuli
| Anatomic region | Brodmann area | Cluster size | Peak Talairach coordinates |
t-max | P-max | ||
|---|---|---|---|---|---|---|---|
| mm3 | X | Y | Z | ||||
| Perception negative > neutral, Clusters with Cluster-threshold: 135 mm3 | |||||||
| Middle frontal gyrus L | 46 | 323 | −47 | 39 | 23 | 3.9 | 2.85E-04 |
| Middle frontal gyrus L | 6 | 165 | −40 | 4 | 57 | 3.7 | 6.71E-04 |
| Middle frontal gyrus L | 6 | 270 | −49 | 10 | 48 | 4.0 | 2.76E-04 |
| Parahippocampal gyrus/hippocampus R | 252 | 25 | −15 | −17 | −3.7 | 6.80E-04 | |
| Insula L | 692 | −39 | −12 | 10 | −3.6 | 8.74E-04 | |
FMRI analysis of the perception of emotional stimuli in the mindfulness group vs the basic group. Activated areas in a random effects analysis (rfx) of the contrast negative > neutral for a minimum cluster size of 135 mm3 mean that the contrast difference (perception negative > expectation neutral) were greater in the mindfulness group compared with the basic group. Minimum cluster size for global error probability (Monte Carlo correction) of P < 0.05: 810 mm3 (30 functional voxels) showed no differences between the groups, but clusters exceed a threshold of 135 mm3 (five functional voxels).
R, right; L, left.
The expectation of negative stimuli (Table 3) revealed increased left-sided prefrontal activations (inferior DLPFC, middle and inferior frontal gyrus) in the mindfulness group compared with the basic group. Another left prefrontal activation located in the superior medial frontal gyrus (DMPFC) extended into the ACC. Two clusters in middle temporal gyrus were more active in the mindfulness group.
Table 3.
Whole-brain group differences (mindfulness > basic) during the expectation of emotional stimuli
| Anatomic region | Brodmann area | Cluster size | Peak Talairach coordinates |
t-max | P-max | ||
|---|---|---|---|---|---|---|---|
| mm3 | X | Y | Z | ||||
| (a) Expectation negative > expectation neutral | |||||||
| Inferior frontal gyrus (DLPFC) L | 44/45 | 3562 | −49 | 16 | 12 | 3.9 | 3.0E-04 |
| Middle/inferior frontal gyrus L | 45/47 | 2766 | −46 | 46 | −6 | 4.8 | 1.9E-05 |
| Middle frontal gyrus L | 6/9 | 8365 | −43 | 13 | 42 | 4.3 | 1.0E-04 |
| Superior/medial frontal gyrus (DMPFC) L (Figure 4) | 6 | 3094 | −7 | 7 | 54 | 4.7 | 2.6E-05 |
| Middle temporal gyrus L | 21 | 965 | −61 | −41 | −9 | 4.4 | 6.3E-05 |
| Middle temporal gyrus L | 39 | 3664 | −55 | −53 | 15 | 3.8 | 4.6E-04 |
| Supramarginal gyrus L | 40 | 694 | −58 | −47 | 30 | 3.8 | 4.1E-04 |
| (b) Expectation ‘unknown’ > expectation neutral | |||||||
| Inferior frontal gyrus L | 45/46/47 | 5354 | −52 | 25 | 12 | 4.3 | 1.06E-04 |
| Middle frontal/precentral gyrus (DLPFC) L | 6/9 | 6177 | −37 | −2 | 39 | 4.5 | 4.60E-05 |
| Superior/medial frontal gyrus (DMPFC) L | 6 | 2558 | −4 | 7 | 54 | 4.4 | 6.40E-05 |
| Precentral gyrus L | 6/44 | 928 | −58 | 4 | 12 | 3.8 | 4.99E-04 |
| Anterior insula R | 13 | 964 | 38 | 4 | 6 | 3.7 | 5.15E-04 |
| Insula anterior/posterior L | 13 | 3958 | −43 | 7 | 3 | 4.2 | 1.25E-04 |
| Cingulate anterior L | 32 | 1119 | −7 | 19 | 39 | 4.2 | 1.36E-04 |
| Inferior parietal lobule R | 40 | 1014 | 53 | −38 | 27 | 3.8 | 3.93E-04 |
| Caudate L | 1335 | −16 | 19 | 9 | 3.9 | 3.76E-04 | |
FMRI analysis of emotion expectation in the mindfulness group vs the basic group. Activated areas in a random effects analysis (rfx) with a voxel-wise threshold of P < 0.005 mean that the contrast difference (expectation emotional > expectation neutral) were greater in the mindfulness group compared with the basic group. (a) Expectation negative > expectation neutral. Minimum cluster size for global error probability of P < 0.05: 972 mm3 (34 functional voxel). (b) Expectation ‘unknown’ > expectation neutral. Minimum cluster size for global error probability of P < 0.05: 945 mm3 (35 functional voxel).
Note. Smaller clusters in the table result from manually splitting bigger clusters with several local maxima into anatomically separate subclusters.
R, right; L, left.
Similar left frontal activations were found in the mindfulness group during the expectation of ‘unknown’ stimuli (DLPFC, DMPFC, extending into left ACC, Figure 5). Further, the mindfulness group showed stronger activations in bilateral anterior insula, right inferior parietal lobulus and subcortically in the left caudate.
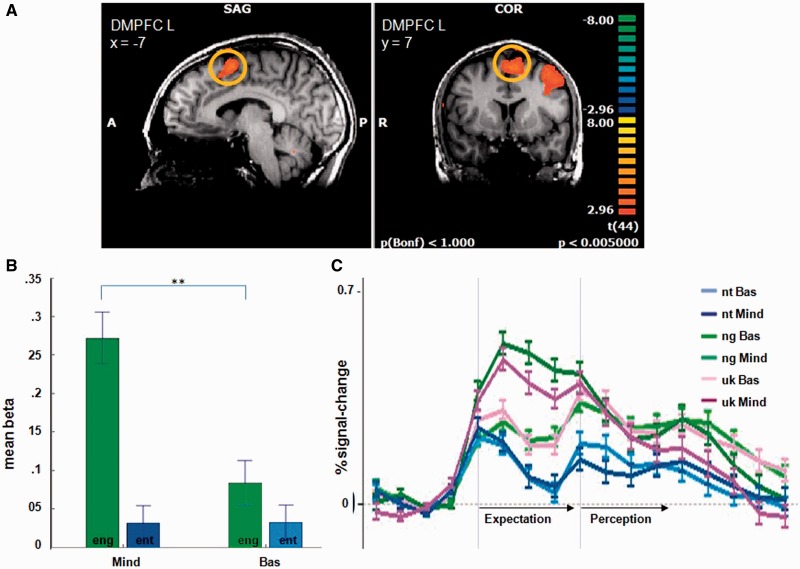
Fig. 5.
(A–C) Activations in the DMPFC in the group comparison ‘mindfulness’ vs ‘basic’ of the contrast expectation negative vs expectation neutral (eng > ent). (A) Higher DMPFC activity (yellow circle) in the mindfulness group in the expectation of negative stimuli. (B) Significantly higher mean beta weight of the peak DMPFC voxel (x = −7, y = 7, z = −54) in the mindfulness group (Mind) [t(44) = 4.30, P < 0.0001] in the expectation of negative stimuli (eng) compared with the basic group (Bas), no group difference in the expectation of neutral stimuli (ent). (C) Time courses of the conditions for this region. Error bars indicate standard error.
The contrast expectation negative vs expectation positive revealed similar increased mindfulness-related activations in left prefrontal areas (Supplementary Table S5). Group comparisons for the ‘positive vs neutral’ contrasts revealed no significant differential brain activity (data not shown).
DISCUSSION
In the present study, a brief mindfulness intervention showed evidence of emotion-regulating effects on the neural level during an emotional expectation task. We found increased activation in brain regions associated with emotion regulation, along with reduced activation in brain regions involved in the processing of emotional valence and arousal.
Specifically, during the expectation of potentially negative (‘unknown’) or certainly negative stimuli, the mindfulness group showed increased activation in DMPFC and other prefrontal regions as compared with the basic group—both in the ROI and the whole-brain analysis. Further, the mindfulness instruction reduced activity in regions involved in emotion processing during the perception of negative stimuli in the right amygdala, parahippocampal and insular regions.
During the expectation of negative or potentially negative pictures, activity in DMPFC and insula correlated negatively with trait mindfulness.
Activations in structures involved in interoceptive processing and attention
During the perception of negative stimuli, the posterior insula showed less activation in the mindfulness group in comparison with the basic group. A comparable deactivation has been reported in a study on verbal affect labeling (Lieberman et al., 2007). Arousal has been found to activate the insula (Lewis et al., 2007). Therefore, the mindful instruction could have resulted in less arousal/autonomic activation during negative-picture viewing compared with the basic group. Also, the negative correlation between right insula activation with trait mindfulness in the expectation phase could indicate that more mindful individuals experienced less emotional arousal.
In the ROI analysis, in the expectation phase, activity in the left anterior insula was increased in the mindfulness group. The insula is also a key structure for interoceptive awareness (Critchley et al., 2004) and the awareness of emotions (Craig, 2009), and it has been found to undergo structural changes in meditators (Lazar et al., 2005; Hölzel et al., 2010). Increased activity in this region in the mindfulness group could therefore reflect the instructed focus on bodily sensations and emotions. There was no group difference in the ROI analysis in the right anterior insula in the expectation phase. However, in our design, insula activation remains difficult to interpret because reduced arousal and focus on body awareness may have opposite effects on this region. Additionally, depending on the size of these two effects and on the specific area within the insula, group difference will become apparent or not. Besides the involvement of the insula in the processing of internal information, particularly emotional arousal, the insula is activated in regulatory processes (e.g. Diekhof et al., 2011). Again, this adds complexity to the interpretation of our findings regarding the insula.
Increased frontal activations in the mindfulness group
Increased DMPFC activation during the expectation of ‘unknown’ and negative stimuli in the mindfulness group is in line with findings on cognitive emotion regulation (Ochsner et al., 2002; Herwig et al., 2007b; meta-analysis: Kalisch, 2009; Diekhof et al., 2011). Further, the DMPFC was activated in self-experiential states such as affect labeling (Taylor et al., 2003; Creswell et al., 2007; Lieberman et al., 2007) and mindful self-awareness without any external stimulation (Herwig et al., 2010). Further, this cortical structure has been found to be thicker in meditators (Lazar et al., 2005) and to be active during meditation (Hölzel et al., 2007; Brewer et al., 2011; Ott et al., 2011). However in comparison with meditation novices, experienced meditators showed decreased DMPFC activations in a mindful state (Farb et al., 2007) or when viewing emotional pictures during meditation (Taylor et al., 2011). These findings parallel the negative correlation between DMPFC activity in the expectation of negative stimuli and trait mindfulness (MAAS). The correlation indicates that more mindful individuals allocated less frontal resources, possibly reflecting a more efficient use of these structures (Brefczynski-Lewis et al., 2007), or a less cognitive evaluation of emotional situations (Farb et al. 2007; Taylor et al., 2011).
Differential activation of the DLPFC between groups in the expectation phase was identified in the ROI and whole-brain analyses. The DLPFC is a core structure for executive functions (Smith and Jonides, 1999) that has been associated with reappraisal of negative stimuli (Ochsner et al., 2002; Herwig et al., 2010) and with state-mindfulness in meditation-naïve subjects (Creswell et al., 2007; Farb et al., 2007). Further, a meta-analysis identified less (left) DLPFC activation in depression (Fitzgerald et al., 2008). With regards to our instruction, the activation could also reflect the subject’s attempt to hold experiences of the ‘present’ in working memory (Farb et al., 2007).
The whole-brain analysis revealed mindfulness-related prefrontal activation in bilateral inferior frontal gyrus (IFG) extending into the anterior insula. This region was activated in previous studies on cognitive control (Beauregard et al., 2001; Herwig et al., 2007b; Diekhof et al., 2011), as well as in in self-awareness tasks (Morin and Michaud, 2007).
Taken together, we interpret the prefrontal activation in the mindfulness group as activation of regulatory structures owing to the mindfulness instruction, although regulation was not explicitly mentioned to the subjects. These structures may also be activated owing to other conscious regulation strategies (i.e. reappraisal). However, owing to the rather low level of stress induced by the task, we have no reason to believe that participants in the mindfulness group or low in trait mindfulness used such conscious regulation. Also, all participants reported in the structured interview that they had been able to follow the instructions, and none mentioned the use of other regulatory strategies.
Did mindfulness attenuate negative emotions?
The mindfulness group showed reduced activation in the right amygdala ROI during the perception of negative pictures, compared with the basic group. The amygdala—initially related to fear processing (e.g. Phan et al., 2004)—has been found to be activated in expectation of ‘unknown’ or negative pictures (Phelps et al., 2001; Bermpohl et al., 2006; Herwig et al., 2007a). Rather than valence-specific processing, amygdala activation supposedely reflects more general emotional arousal or salience (Anderson and Phelps, 2001; Fusar-Poli et al., 2009; Morrison and Salzman, 2010). The mindfulness group also displayed decreased activity in the parahippocampal area and insula during the perception of negative stimuli, and more mindful subjects had a reduced insula activity in the expectation phase. This reduced activity in brain regions associated with emotional arousal supports the interpretation that our short mindfulness intervention had emotion-regulatory effects on the neural level.
Only an effect of attention?
Frontal activations in the mindfulness group could reflect general networks for task execution and attention (Hölzel et al., 2011). Attention influences the processing of emotional stimuli (Pessoa et al., 2005), dampens emotional reactivity and decreases amygdala activation (Lutz et al., 2008; McRae et al., 2010). Thus, one could conclude that a main effect of the mindfulness instruction lies in a modified attentional focus.
However, some reduction of attention supposedly is a general aspect of emotion regulation, be it reappraisal (additional cognitive processes added to the perception) or distraction (decreasing attention to the stimuli), which resulted in comparable activation patterns (McRae et al., 2010; Kanske et al., 2011). Furthermore, mindful breathing in novices similarly involved neural structures of attention (Dickenson et al., 2013). However, the influence of regulatory strategies on attention does not weaken their usefulness for therapeutic purposes. And in contrast to other emotion-regulation strategies, mindfulness deliberately draws the attention to the present moment experience and to feelings, thus not away from but to the trigger of arousal.
If our results were only an effect of attention withdrawal, this would have resulted in reduced general activation of visual areas in comparison with the basic group (Pessoa et al., 2002). However, activations differed neither on the whole-brain level [even at low statistical thresholds (P = 0.05)] nor in an ROI analysis of V1. Considering activations in the expectation and perception phases, we rather suggest that increased awareness of bodily sensations, thoughts and emotions before the emotional event [reflected in PFC activations in the expectation phase (compare Barrett et al., 2007)] influenced emotion generation such that emotional reactivity was dampened when the emotional stimulus appeared. Possibly, this represents the core mechanism of mindfulness and the way it allows for a detached, metacognitive experience of emotions (Arch and Craske, 2006).
Therapeutic implications and future research
In our study, trait mindfulness correlated negatively with neuroticism, depression and anxiety, concurring with the repeatedly reported positive relation between mindfulness and well-being (Brown and Ryan, 2003; Giluk, 2009).
Focusing on present sensations, feelings and thoughts—as induced in this task—is a minimal readily applicable aspect of mindfulness, which has not yet been extensively studied in the context of mental health. The current study indicates that already such a short and simple mindfulness instruction holds the potential of regulating emotion processing. The identified neural correlates of increased top–down prefrontal control over bottom–up emotion-generating processes are disrupted in psychiatric diseases such as depression (DeRubeis et al., 2008; Drevets et al., 2008). Thus, fostering mindfulness skills might hold the potential for strengthening emotion regulation and add support to the use of integrative approaches in psychotherapy that incorporate mindfulness practice (Segal et al., 2002; Hayes et al., 2004). We tentatively interpret our results in this manner. However, further studies investigating short mindful states in healthy subjects and psychiatric populations will provide deeper insights into these mechanisms. Also, studies on the effect of mindfulness on positive emotional events are desirable. This would broaden our understanding of mindfulness and could offer clinically relevant implications for disturbed positive emotion regulation in disorders such as manic episodes or depressive anhedonia. On the neural level, future studies could clarify the interaction of brain regions in short mindful states and further elucidate the role of the insula in emotion regulation.
Limitations
The subjects’ experience with meditation and trait mindfulness were assessed only in the mindfulness group, and the prior meditation experience was diverse in this sample. This naturalistic approach was chosen to study neural correlates of the initialization of mindfulness in general, which is considered a small, but fundamental, part of the complex process of mindfulness and meditative states. However, the heterogeneity of the sample with regard to this factor might have blurred some mindfulness-related effects on the neural level.
The expectation of negative or possibly negative pictures in the whole-brain analysis revealed no group differences in the amygdala. Possibly, the threat of upcoming negative pictures might have been too weak to elicit prominent arousal in the sample of healthy individuals in the basic group, which showed no amygdala activation on the whole-brain level in the expectation phase. Therefore, the present data cannot comprehensively answer the question of emotion regulation through mindfulness in the expectation phase. Furthermore, mindful regulation and the certainly and possibly negative conditions are completely overlapping. Therefore, the study cannot separate effects of trait mindfulness on emotion processing from the implementation of the mindful regulation instruction. In this study, as in comparable previous studies (e.g. Herwig et al., 2007b), we deliberately decided against a behavioral control task, which would induce preparatory and executive processes and may cause distraction from the mental task and emotional involvement. However, attentional presence was systematically inquired in post-scanning interviews and by monitoring individual brain activation in visual areas. Pictures in this study were not rated with respect to arousal. Valence ratings after the task showed no group differences. A previous study on cognitive control showed the same effect (Herwig et al., 2007b). However, this measurement after scanning cannot give exact evidence of the emotional experience in the scanner.
CONCLUSION
The present study examined neural correlates of a short and simple mindfulness induction when expecting and facing negative or potentially negative emotional events. Mindfulness was associated with marked recruitment of brain structures involved in top–down emotion regulation, mainly in the expectation of negative or potentially negative stimuli. During the perception of the negative stimuli, mindfulness attenuated activations in brain regions associated with emotion processing. These results are reminiscent of findings of cognitive control instructions (Ochsner et al., 2002), of mindfulness without emotional stimulation (Herwig et al., 2010) and of attention and emotion regulation network activations in meditators (Chiesa and Serretti, 2009). It seems that at least some components of mindful states that may have an attenuating effect on emotional arousal can be elicited without intensive training and their neural correlates become visible. Further studies are desirable to clarify the neurobiological mechanisms of short mindful states and mindful emotion regulation.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by Swiss National Science Funds (SNF) grant No. 3200B0_12120.
REFERENCES
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–21. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001:6993–7000. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The Current State of Cognitive Therapy: A 40-Year Retrospective. Archives of General Psychiatry. 2005;62:953–59. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, et al. Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. NeuroImage. 2006;30:588–600. doi: 10.1016/j.neuroimage.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, et al. Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–41. [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences. 2007;104:11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. Cambridge, UK: MRC CBSU WIKI; 2009. http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach#Approach_2:_a_non-linear_transform_of_MNI_to_Talairach (5 April 2013, date last accessed) [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Cahn BR. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Brambilla P, Serretti A. Neuro-imaging of mindfulness meditations: implications for clinical practice. Epidemiology and Psychiatric Sciences. 2011;20:205–10. doi: 10.1017/s204579601100028x. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine. 2009:1–14. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates; 1998. [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medecine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9:788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J, Berkman ET, Arch J, Lieberman MD. Neural correlates of focused attention during a brief mindfulness induction. Social Cognitive and Affective Neuroscience. 2013;8:40–7. doi: 10.1093/scan/nss030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58:275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–77. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SB. Manual of the Eysenck Personality Inventory. 4th edn. London: University of London Press; 1964. [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giluk TL. Mindfulness, big five personality, and affect: a meta-analysis. Personality and Individual Differences. 2009;47:805–11. [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–64. [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: a meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hayes AM, Feldman G. Clarifying the construct of mindfulness in the context of emotion regulation and the process of change in therapy. Clinical Psychology: Science and Practice. 2004;11:255–62. [Google Scholar]
- Herwig U, Kaffenberger T, Baumgartner T, Jäncke L. Neural correlates of a ‘pessimistic' attitude when anticipating events of unknown emotional valence. NeuroImage. 2007a;34:848–58. doi: 10.1016/j.neuroimage.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Jäncke L, Brühl AB. Self-related awareness and emotion regulation. NeuroImage. 2010;50:734–41. doi: 10.1016/j.neuroimage.2009.12.089. [DOI] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, et al. Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage. 2007b;37:652–62. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6:537. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go, There you are: Mindfulness Meditation in Everyday Life. New York: Hyperion; 1994. [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience & Biobehavioral Reviews. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21:1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe. Studies of motivation and attention. American Psychologist. 1995;50:372–85. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar. Weinheim: Beltz; 1981. [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005:1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17:742–8. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Linehan M. Skills Training Manual for Treating Borderline Personality Disorder. New York: Guilford Press; 1993. [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. NeuroImage. 2009;45:672–8. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41:648–55. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Michaud J. Self-awareness and the left inferior frontal gyrus: inner speech use during self-related processing. Brain Research Bulletin. 2007;74:387–96. doi: 10.1016/j.brainresbull.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Current Opinion in Neurobiology. 2010;20:221–30. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ott U, Hölzel BK, Vaitl D. Brain structure and meditation: how spiritual practice shapes the brain. In: Walach H, Schmidt S, editors. Neuroscience, Consciousness and Spirituality. Proceedings of the Expert Meeting in Freiburg/Breisgau 2008. Berlin: Springer; 2011. pp. 119–28. [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28:249–55. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004:258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001:437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Prefrontal-amygdala interactions in the regulation of fear. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 27–46. [Google Scholar]
- Segal ZV, Williams JM, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press; 2002. [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: Three-dimensional Proportional System. New York: Thieme Medical; 1988. [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S. Measuring mindfulness – the Freiburg Mindfulness Inventory (FMI) Personality and Individual Differences. 2006;40:1543–55. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire, France: World Medical Association; 2008. [Google Scholar]
- Zung WW. Self-rating depression scale. In: Scalarum CIP, editor. Internationale Skalen für Psychiatrie. Göttingen: Beltz; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.