Abstract
Although much is known about the neural dynamics of maladaptive affective styles, the mechanisms of happiness and well-being are less clear. One possibility is that the neural processes of trait happiness are the opposite of those involved in depression/anxiety: ‘rose-colored glasses’ cause happy people to focus on positive cues while remaining oblivious to threats. Specifically, because negative affective styles have been associated with increased amygdala activation to negative stimuli, it may be happy people will not show this enhanced response, and may even show reduced amygdala activation to negative stimuli. Alternatively, if well-being entails appropriate sensitivity to information, happy people may process any relevant cues—positive or negative—to facilitate appropriate responding. This would mean that happiness is associated with increased amygdala activation to both positive and negative stimuli. Forty-two participants viewed affective stimuli during functional magnetic resonance imaging scanning. Happier participants showed greater amygdala responses to positive stimuli. Moreover, no significant relationships were found between happiness and responses to negative stimuli. In other words, for happy people, a tuning toward positive did not come at the cost of losing sensitivity to negativity. This work suggests that trait happiness is associated with a balanced amygdala response to positivity and negativity.
Keywords: positive emotions, amygdala, emotion, personality
People vary tremendously in their emotional reactivity to the environment, capacity for emotion regulation and dispositional levels of affect. These dispositional tendencies can be collectively described as affective style (Davidson, 2004). Affective style originates from genetic and/or early environmental influence and manifests in different expectations about the world and the most adaptive ways to interact with it. Whereas positive affective style is associated with low reactivity, high capacity for emotion regulation and positive affect (PA), negative affective style is associated with high reactivity, low capacity for emotion regulation and negative affect (NA; Davidson, 2004). Positive affective style may be associated with affectively desirable outcomes such as subjective happiness (Gross et al., 1998); negative affective style may be associated with affective disorders such as mania and depression (Meehl, 1975). Consequently, understanding affective style is crucial for predicting these different types of outcomes.
One critical brain region that is sensitive to affective style and affectively significant information is the amygdala. Although amygdala activation is enhanced following the presentation of negative cues, which implies a role for the amygdala as sensitive to negative information, recent work has uncovered a more general role for the amygdala as sensitive to motivationally relevant stimuli (Cunningham et al., 2008; Cunningham and Brosch, 2012; W.A.Cunningham, J.J.Van Bavel, and P.E.Stillman, in preparation). On this view, the amygdala is tuned to respond preferentially to the type of stimuli that are considered most relevant to an individual’s goals. Because the environmental events that are judged as relevant may differ as a function of affective style (Davidson, 1998), the amygdala may be differentially responsive to the environment depending on one’s affective style (Drevets, 2001; Mayberg, 2003). For example, depression, which involves increased sensitivity to negative information in the environment, is associated with increased and sustained amygdala activation to negative stimuli relative to healthy controls (e.g. Siegle et al., 2002; 2007; Victor et al., 2010). In contrast, positive affective style that manifests in happiness may result from tuning toward and greater activation to positive compared with negative information (Canli et al., 2002; Cunningham et al., 2005). These studies suggest the possibility of a hydraulic relationship between valenced stimuli and affective style whereby the ratio of activation to negative relative to positive cues determines one’s style.
Yet, although happy people may show an increased amygdala response to positive stimuli, a decreased activation to negative stimuli is only one possible pattern of results. Indeed, the most adaptive mode of amygdala function may not necessarily be insensitivity to negative stimuli (which often carries important information about the environment), but rather may be tuning toward both negative and positive stimuli, depending on the demands of the environment. The ability to recognize and respond appropriately to negative information may be necessary for a variety of reasons. First, lacking this ability is associated with affective disorders. For example, an overly positive response to the world without sensitivity to context is associated with the destructive outcomes of mania (Gruber et al., 2008; Gruber, 2011). Further, individuals who lack the ability to recognize and respond to distress in others, or are impaired in their ability to experience other socially informative negative emotions such as guilt, are prone to psychopathy (Hare, 1991) and may show decreased amygdala response to negative information (Kiehl et al., 2001; see Blair, 2003, for a review). Second, recent work has suggested that there may be an optimal ratio of positive to NA for psychological health (Fredrickson and Losada, 2005). NA can be appropriate in specific situations (Fredrickson, 2000) and can also serve as a useful contrast to PA, decreasing the tendency to adapt or habituate to a positive situation (Solomon, 1980). Additional sensitivity to positivity may also facilitate resilience and rapid recovery from these negative events (e.g. Fredrickson and Levenson, 1998; Lyubomirsky and Tucker, 1998). On this view, it is possible that differences between happier and less-happy people may emerge primarily for positive stimuli such that increased happiness is associated with increased amygdala responsivity to positive without a corresponding decrease in activation to negative. Indeed, to the extent that happy people respond adaptively to cues in the environment, they may even show an increased response to negative as well. Such findings would complement research suggesting that depressed individuals may sometimes experience affective blunting: an across-the-board decrease in both positive and NA (Andreasen, 1979; Berenbaum and Oltmanns, 1992).
To examine individual differences in affective responding to valenced stimuli as a function of subjective happiness, participants viewed 10-s blocks of positive, negative and neutral photographs while in the scanner. Between blocks, participants rated their affective responses to the preceding block. Participants completed the Subjective Happiness Scale (SHS; Lyubomirsky and Lepper, 1999) to measure trait happiness. The SHS was used because it is a face-valid short scale that has been previously validated as a measure of happiness, demonstrating convergent validity through high correlations with other measures of happiness such as Bradburn’s (1969) Global Happiness Item, Andrews and Withey’s (1976) Delighted-Terrible Scale and satisfaction with Life Scale (see Lyubomirsky and Lepper, 1999). Moreover, the scale is distinct from measures of depression: it correlates only modestly with measures such as Beck’s (1967) Beck Depression Inventory (Lyubomirsky and Lepper, 1999), and, more importantly, it focuses on the range of subjective happiness in normal functioning rather than degrees of depression. That is, whereas depression scales can best differentiate among degrees of NA, while providing little information about degrees of PA, the SHS differentiates among participants on the high end of the happiness spectrum, and is thus best suited for testing questions specifically focused on degrees of happiness (W.A.Cunningham, J.P.Peterson, and Z.Lu, in preparation). Critically for our analyses, we examined separately the differences between positive/neutral and negative/neutral blocks to examine sensitivity to positive and negative stimuli separately. This distinction helped to differentiate between two possibilities: if happiness involves increased sensitivity to positive and decreased sensitivity to negative, we should find differences in amygdala activity for both positive and negative stimuli, whereas if happiness involves increased sensitivity to positive without decreased sensitivity to negative, we should find differences in amygdala activity only for positive stimuli. To control for the potential confound of hypomania (a manifestation of PA associated with negative outcomes), participants also completed a measure of hypomanic personality.
METHOD
Participants
Participants were 43 right-handed individuals with no history of neurological problems and normal or corrected-to-normal vision who completed both the functional magnetic resonance imaging (fMRI) and questionnaire portions of the study. All participants were native English speakers. One participant was dropped for having a phobic response to a specific stimulus category, leaving a final sample of 42 (21 male).1 Participants were compensated with $40.
Materials
A subset of the International Affective Picture System (IAPS; Lang et al., 1998) was used. Photos contained 87 positive and negative stimuli that were selected and matched on normed ratings of arousal (MPositive = 4.88, s.d.Positive = 0.96; MNegative = 5.16, s.d.Negative = 0.86) and valence extremity (MPositive = 2.07, s.d.Positive = 0.32; MNegative = −1.85, s.d.Negative = 0.34). An additional 77 neutral stimuli from the IAPS were included to allow for an investigation of stimulus extremity compared with stimulus valence (MArousal = 3.36, s.d.Arousal = 0.82; MValence = −0.21, s.d.Valence = 0.37).
Trait happiness was measured with the SHS (Lyubomirsky and Lepper, 1999), a four-item scale that measures self-reported trait happiness. SHS demonstrates excellent convergent validity from related constructs such as neuroticism and depression (e.g. the correlation between SHS and a common measure of depression is typically around r = −0.50; see Lyubomirsky and Lepper, 1999). Hypomania was measured with the Hypomanic Personality Scale (HPS; Eckblad and Chapman, 1986), which consists of 48 true–false self-report items capturing episodic shifts in emotion, behavior and energy. The HPS has excellent predictive validity for the onset of manic episodes, and prior work has demonstrated that high HPS scores are associated with intense PA across contexts (Gruber et al., 2008). Additionally, we collected measures of positive and negative affect using the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). The PANAS is a widely used measure of affect and consists of 20 affective words (10 positive and 10 negative).
Procedure
During six runs of fMRI scanning, participants were presented with pictures randomly selected from a subset of IAPS photographs (Lang et al., 1998) that varied on valence, extremity and arousal. To create a greater affective response, pictures were presented in 10-s blocks. In each block, 10 photos from one stimulus set (positive, negative or neutral) were presented for one second each. Following each block, participants indicated on a 1–4 scale the degree to which the block of photographs as a whole made them feel positive or negative. For half the participants, a key press of 1 represented most positive, and for the other half, a key press of 1 represented most negative. A fixation cross appeared between blocks for 2–6 s. Stimulus timing and order for the six runs was optimized using OptSeq (http://freesurfer.net/optseq/).
fMRI data acquisition and analysis
Scanning was conducted using a Siemens 3T Tim Trio Scanner at the Center for Cognitive and Behavioral Brain Imaging at The Ohio State University. Functional scanning was prescribed parallel to the AC/PC line, and nearly isotropic functional images were acquired from inferior to superior using a single-shot gradient echo planar pulse sequence (40 slices; 2.5 mm thick; TE = 25 ms; TR = 2500 ms; in-plane resolution = 2.5 × 2.5 mm; FOV = 250 mm). The first four volumes were discarded to allow for T1 equilibration effects. Following functional imaging, a high-resolution T1 anatomical image (160 sagittal slices; TE = 4.73 ms, TR = 1900 ms; resolution = 0.9 × 0.9 × 1.2 mm) was collected for normalization. fMRI data processing was carried out using fMRI Expert Analysis Tool Version 5.98, part of FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl. Before analysis, data were corrected for motion using MCFLIRT (Jenkinson et al., 2002) and were spatial smoothed using a Gaussian kernel of FWHM 6.0 mm. A 100 s highpass temporal filter was applied to remove drift components. ICA denoising was conducted using an automated toolbox (Tohka et al., 2008). Registration to high-resolution structural and/or standard space images was carried out using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
First-level time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al., 2001). For each run for each participant level, effects were modeled by convolving a double gamma hemodynamic response function against the preprocessed data for each of the three stimulus conditions. To remove variance associated with subject motion, motion parameters were included in the model as covariates of no interest. Participant-level estimates were then generated using a fixed-effects model combining estimates for each of the runs. Higher-level analysis of the whole brain data was carried out using FMRIB's Local Analysis of Mixed Effects stage 1 (Woolrich et al., 2004; Woolrich, 2008). Z (Gaussianised T/F) statistic images were thresholded using a corrected voxel significance threshold of P < 0.05 (Worsley, 2001). For all voxelwise correlational analyses, an anatomical amygdala mask was used to restrict the number of voxels considered in the analyses. Masks were generated and additional anatomical analyses were conducted using an explicit mask of amygdala voxels from the Harvard–Oxford Cortical/Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu). For all analyses, data were extracted for each condition for each participant separately for right and left amygdala.
RESULTS
Individual difference scores
An examination of the variability of the SHS suggests an ideal range of happiness scores. Specifically, participants’ scores ranged from 3 to 6.75 (out of a possible range of 1–7) and were normally distributed around a mean of 4.99 [standard deviation (s.d.) = 1.03]. Further, an examination of the HPS indicated that, on average, participants did not report a high degree of hypomanic traits [Mean (M) = 16.93, s.d. = 9.01, range = 3–45].2 PA and NA were also normally distributed (PA: M = 3.53, s.d. = 0.55, range = 2–4.4; NA: M = 1.96, s.d. = 0.62, range = 1.1–3.7; both scores out of a possible range of 1–5). Correlations among these scales and measures of internal consistency are presented in Table 1. With regard to their affective responses to blocks of stimuli during the fMRI task, participants rated (from 1 to 4) positive blocks most positively and negative blocks most negatively (F2,82 = 963.3, P < 0.0001; Mnegative = 1.28; Mneutral = 2.73; Mpositive = 3.78). SHS scores correlated with ratings of the positive stimuli (r40 = 0.49, P < 0.001), but did not correlate with the ratings of neutral (r40 = 0.04, P = 0.82) or negative stimuli (r40 = −0.22, P = 0.15).
Table 1.
Correlations and internal consistency of SHS, HPS and positive and negative affect schedule (PA and NA, respectively)
Indices (alpha) of internal consistency are presented along the diagonal.
Significant correlations are indicated as follows: *P < 0.05, **P < 0.01.
fMRI results
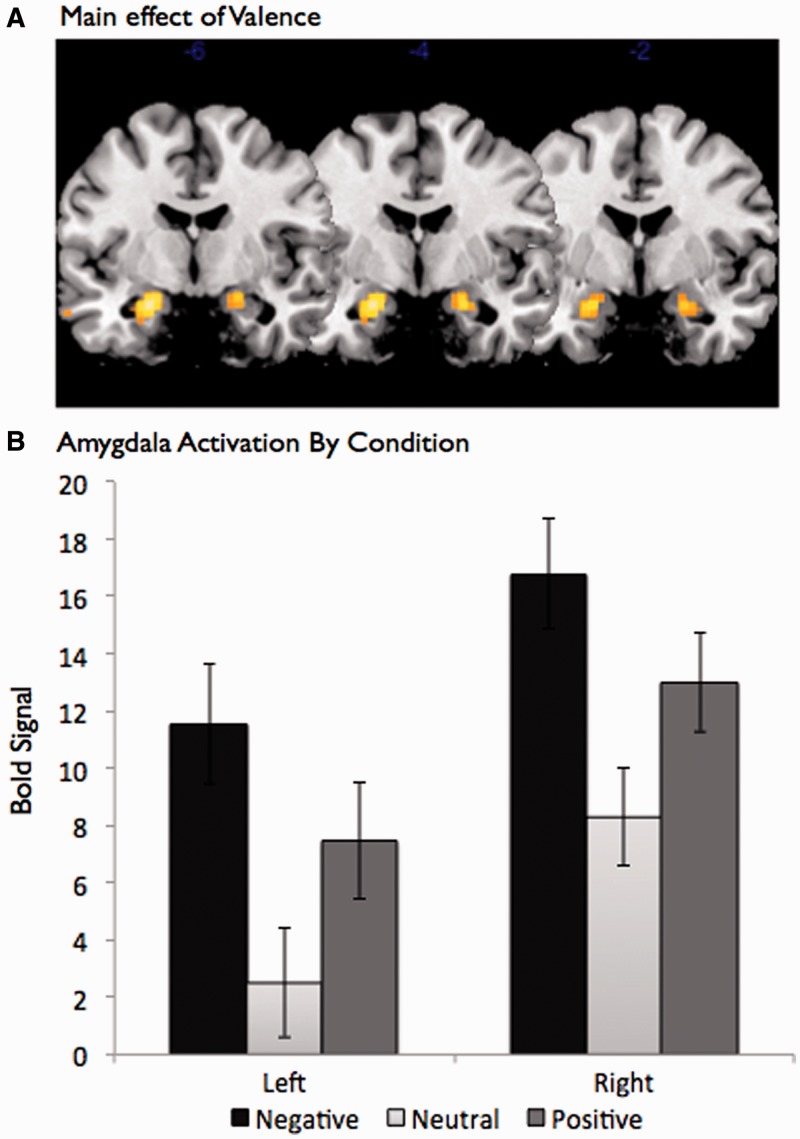
Previous work on affective valence and the amygdala has suggested a U-shaped function such that both positive and negative stimuli are associated with greater amygdala activation than neutral stimuli (see Cunningham and Brosch, 2012, for a review).3 These data have been taken as evidence that the amygdala is sensitive to the affective arousal or intensity of a stimulus, two features that are associated with both positive and negative stimuli, rather than specific valence (Anderson et al., 2003; Cunningham et al., 2004). Replicating this pattern of results, a significant main effect of valence on amygdala activation was found when conducting a whole brain analysis comparing the three valence conditions (left: F2,82 = 19.64, P < 0.0001; right: F2,82 = 29.45, P < 0.0001; see Figure 1A)4. Specifically, when interrogating voxels found to be significant in the overall analysis of variance (ANOVA), both positive stimuli [left: t(41) = 4.96, P < 0.001; right: t(41) = 4.73, P < 0.001] and negative stimuli [left: t(41) = 9.05, P < 0.0001; right: t(41) = 8.49, P < 0.0001] showed greater amygdala activation than neutral stimuli (see Figure 1B). Further, these results were replicated when extracting amygdala activation from anatomical masks [overall ANOVA: left: F(2, 82) = 24.9, P < 0.0001; right: F(2, 82) = 16.21, P < 0.0001; positive–neutral: left: t(41) = 4.40, P < 0.01; right: t(41) = 4.10, P < 0.01; negative–neutral: left: t(41) = 7.82, P < 0.0001; right: t(41) = 7.21, P < 0.0001]. The pattern of results suggests a standard U-shaped function whereby both negative and positive images elicit more amygdala activation than neutral images.
Fig. 1.
Amygdala activation as a function of valence. (A) Main effect of valence. (B) The amygdala was more responsive to positive and negative images, relative to neutral images.
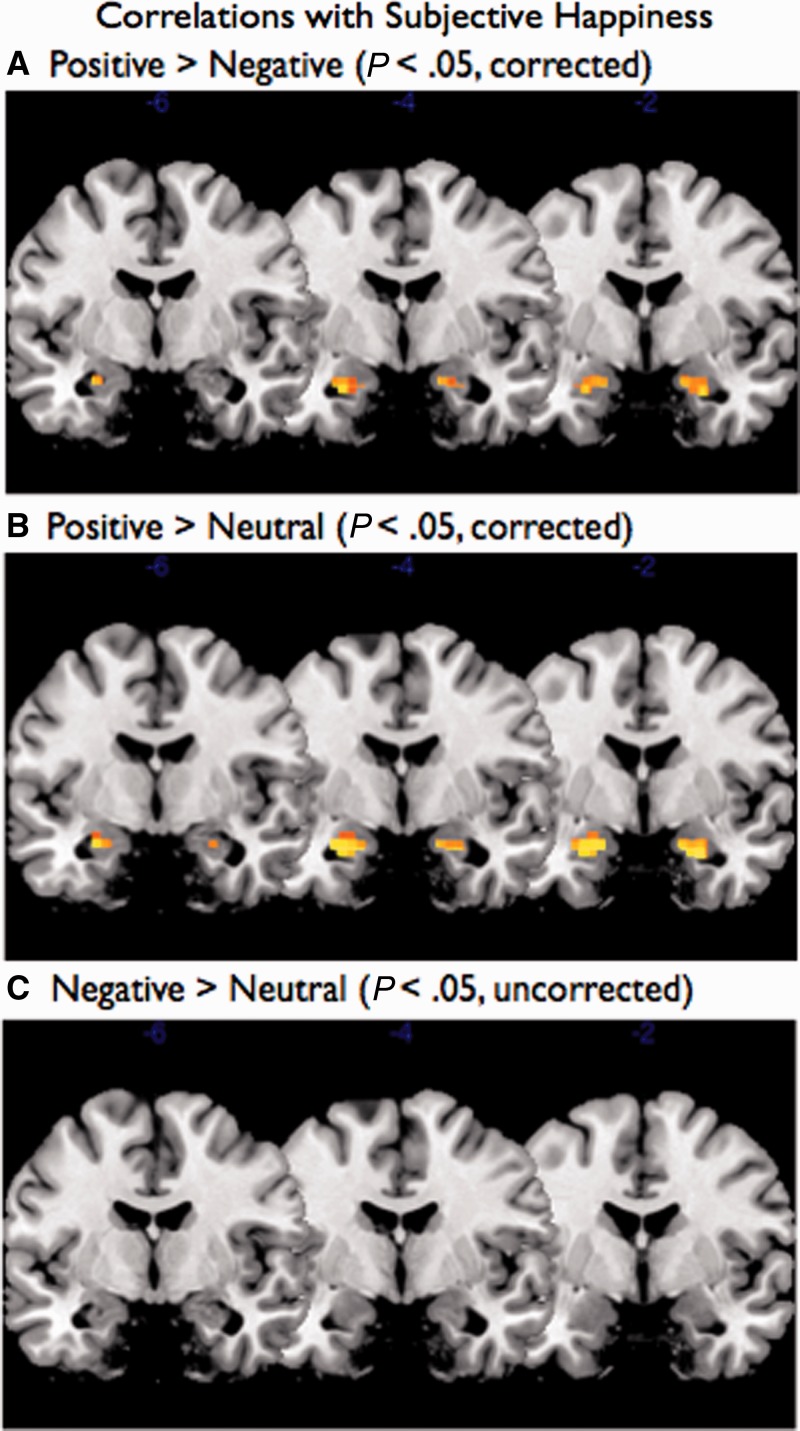
In a second set of analyses, we correlated the SHS scores with each of the amygdala voxels for the positive > negative, positive > neutral and negative > neutral contrasts. As can be seen in Figure 2, whereas the positive > negative and positive > neutral contrasts revealed voxels that were significant within the amygdala when correcting for multiple comparisons, no voxels were significant for the negative > neutral contrast even when dropping the threshold to P < 0.05 uncorrected. To quantify this variation in the amygdala response, we extracted the signal value for each participant and condition for the right and left amygdala voxels identified as significantly differentiating the valence conditions in the ANOVA analysis. Because these regions were identified from the ANOVA without consideration of subjective happiness, these voxels were selected independently from regression analyses using our individual differences of interest.5 As predicted, trait happiness was significantly associated with increased amygdala activation to positive compared with negative images [left: r(40) = 0.39, P < 0.05; right: r(40) = 0.33, P < 0.05]. More importantly, this effect was only observed for the positive stimuli: trait happiness was correlated with the positive > neutral contrast [left: r(40) = 0.49, P < 0.001; right: r(40) = 0.44, P < 0.01] but not the negative > neutral contrast [left: r(40) = 0.20, P = 0.19; right: r(40) = 0.06, P = 0.72]. Importantly, the correlation for the positive > neutral contrast was significantly larger than the correlation for the negative > neutral contrast (left: Z = 2.19, P < 0.05; right: Z = 2.12, P < 0.05; Steiger, 1980). That is, happiness is associated with increased response to positive (relative to neutral) but does not affect responses to negative (relative to neutral).
Fig. 2.
Correlations between subjective happiness and amygdala voxels for positive > negative, positive > neutral and negative > neutral contrasts. Whereas the positive > negative and positive > neutral contrasts revealed voxels that were significant within the amygdala when correcting for multiple comparisons, no voxels were significant for the negative > neutral contrast.
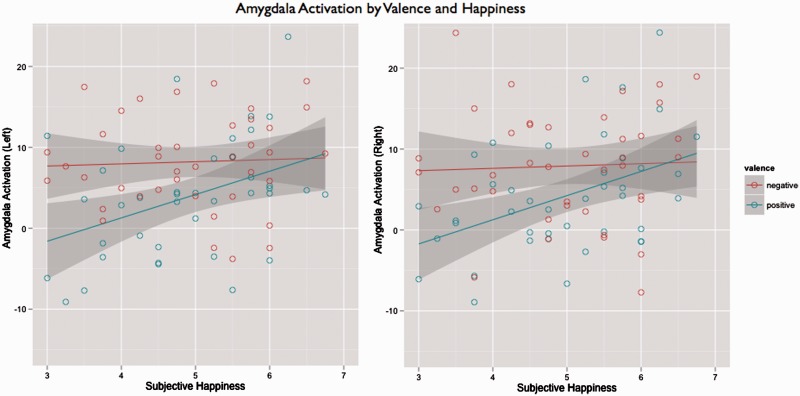
As can be seen in Figure 3, whereas activation to negative was unchanged with increasing levels of happiness, activation to positive increased with increasing levels of happiness. Further, the 95% confidence intervals indicate that for those participants reporting trait happiness of five or higher on the 7-point scale, amygdala activation to both positive and negative stimuli (relative to neutral) was statistically identical. In other words, amygdala activation among happier participants was equally high for positive and negative stimuli. These data suggest that happier people are not necessarily naïve or blind to negativity, but rather may respond adaptively to the world, recognizing both good and bad things in life. Indeed, although not significant, happier participants had a greater amygdala response to negative compared with neutral stimuli. Further, demonstrating that these effects are unlikely to be attributable to maladaptive aspects of PA, these correlations with trait happiness not only hold, but also become slightly stronger, when partialling out the effects of HPS scores [positive > negative: left: r(39) = 0.42, P < 0.05; right: r(39) = 0.34, P < 0.05; positive > neutral: left: r(39) = 0.50, P < 0.01; right: r(39) = 0.45, P < 0.05; negative > neutral: left: r(39) = 0.17, P = 0.19; right: r(39) = 0.05, P = 0.72]. See Table 2 for a full set of analyses using different criteria for amygdala regions of interest.
Fig. 3.
Amygdala activation as a function of subjective happiness score. Whereas activation to negative images was unchanged with increasing levels of happiness, activation to positive images increased with increasing levels of happiness, such that no difference in activity between negative and positive was observed for the happiest participants.
Table 2.
Amygdala effects and correlations with happiness
| Amygdala region | Contrast |
|||||||
|---|---|---|---|---|---|---|---|---|
| Positive > Neutral |
Negative > Neutral |
Negative > Positive |
PosNeg > Neutral |
|||||
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Amygdala (ANOVA) | ||||||||
| Mean | 4.73 | 4.96 | 8.49 | 9.05 | 3.76 | 4.09 | 13.22 | 14.01 |
| Standard error | 1.30 | 1.36 | 1.20 | 1.11 | 1.54 | 1.12 | 1.98 | 2.21 |
| t-value | 3.63 | 3.66 | 7.08 | 8.19 | 2.45 | 3.66 | 6.69 | 6.35 |
| SHS (r) | 0.44 | 0.49 | 0.06 | 0.21 | −0.33 | −0.39 | 0.33 | 0.40 |
| SHS (partial r) | 0.45 | 0.48 | 0.05 | 0.17 | −0.34 | −0.42 | 0.33 | 0.38 |
| HPS (r) | 0.01 | −0.10 | −0.03 | −0.23 | −0.03 | −0.11 | −0.02 | −0.18 |
| Difference (z) | 2.12 | 2.19 | ||||||
| Amygdala (subjective happiness correlated with negative > positive amygdala) | ||||||||
| Mean | 4.13 | 4.58 | 8.29 | 8.24 | 4.16 | 3.66 | 12.41 | 12.82 |
| Standard error | 1.47 | 1.53 | 1.03 | 1.39 | 1.21 | 1.78 | 2.23 | 2.31 |
| t-value | 2.81 | 3.00 | 8.04 | 5.94 | 3.42 | 2.06 | 5.57 | 5.54 |
| SHS (r) | 0.52 | 0.52 | 0.14 | −0.01 | −0.51 | −0.45 | 0.41 | 0.34 |
| SHS (partial r) | 0.51 | 0.51 | 0.10 | −0.01 | −0.53 | −0.45 | 0.39 | 0.33 |
| HPS (r) | −0.14 | −0.08 | −0.24 | −0.02 | −0.04 | 0.06 | −0.20 | −0.06 |
| Difference (z) | 2.86 | 3.01 | ||||||
| Amygdala (anatomical) | ||||||||
| Mean | 4.10 | 4.40 | 7.21 | 7.82 | 3.11 | 3.42 | 11.31 | 12.22 |
| Standard error | 1.24 | 1.27 | 1.09 | 1.03 | 1.45 | 1.07 | 1.83 | 2.05 |
| t-value | 3.31 | 3.46 | 6.61 | 7.62 | 2.14 | 3.19 | 6.19 | 5.97 |
| SHS (r) | 0.43 | 0.49 | 0.05 | 0.25 | −0.33 | −0.34 | 0.32 | 0.43 |
| SHS (partial r) | 0.43 | 0.48 | 0.04 | 0.22 | −0.34 | −0.37 | 0.32 | 0.41 |
| HPS (r) | −0.02 | −0.10 | −0.06 | −0.23 | −0.03 | −0.10 | −0.04 | −0.18 |
| Difference (z) | 2.06 | 1.84 | ||||||
Significant effects are bolded.
The amygdala and positive and negative affect
Trait happiness is not only associated with high levels of PA, but also with low levels of NA (Diener et al., 1999). Because of this, it is unclear from the previous analyses whether the observed effects are attributable to the presence of PA or the absence of NA. To examine this question, we performed a series of regressions predicting amygdala activation to the positive > neutral and the negative > neutral contrasts from individual differences in positive and NA as measured by the PANAS. For the positive > neutral contrast, these analyses indicated that amygdala activation was significantly associated with the PA subscale of the PANAS [left: b = 6.08, t(39) = 2.64, P < 0.05; right: b = 6.22, t(39) = 2.82, P < 0.01]. The relationships with the NA subscales were not significant for either the left [b = −2.682, t(39) = −1.30, P = 0.20] or the right amygdala [b = −1.84, t(39) = −0.933, P = 0.36].6
Predicting subjective happiness from amygdala activation
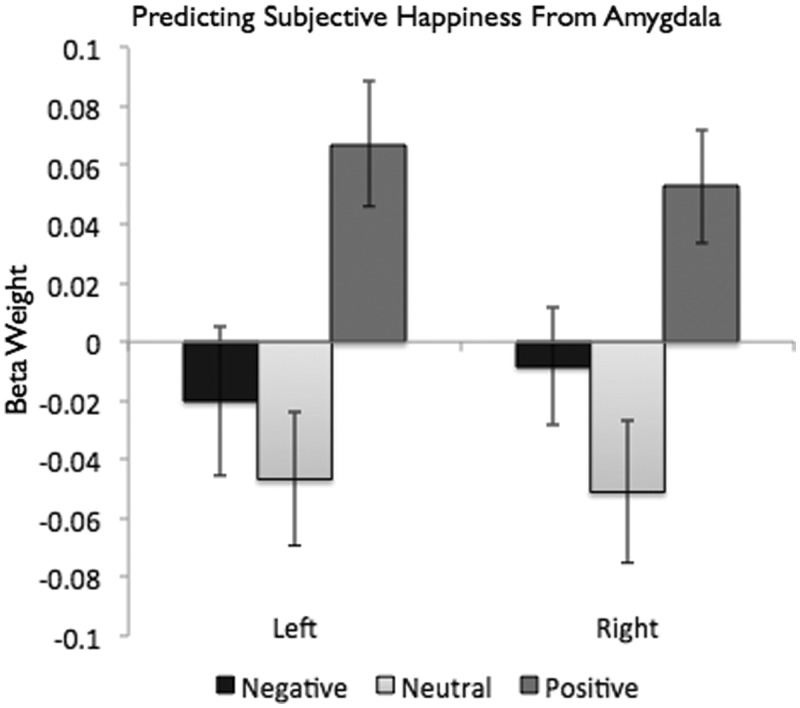
In each of the previous analyses, it was necessary to contrast activation to positive and negative with a baseline condition (neutral images). To examine the independent contributions of each type of stimulus, we regressed activation to the positive, negative and neutral images against the SHS scores. This analysis allowed us to estimate the effect of each of the amygdala scores while simultaneously controlling for the others. For these analyses, we found that the positive [left: b = 0.067, t(38) = 3.169, P < 0.01; right: b = 0.053, t(38) = 2.736; P < 0.01], but not the negative [left: b = −0.020, t(38) = −0.793, P = .43; right: b = 0.008, t(38) = −0.418, P = 0.67], blocks significantly predicted SHS scores (see Figure 4). Consistent with the idea that both positive and negative stimuli are associated with amygdala activation for happy people, we found that amygdala activation to neutral stimuli was negatively associated with subjective happiness, meaning that the amygdalae of happier people activate less to neutral stimuli than those of less-happy people [left: b = −0.046, t(38) = −2.065, P < 0.05; right: b = −0.051, t(38) = −2.117, P < 0.05]. That is, for happier people, there was a greater difference between the valenced (both positive and negative) and neutral stimuli.
Fig. 4.
Subjective happiness predicted by activation to each valenced image type (controlling for the others). Positive, but not negative, image blocks significantly predicted subjective happiness scores; neutral blocks were negatively associated with subjective happiness scores.
DISCUSSION
For some time, the amygdala has been considered to be the key region associated with NA (Whalen, 1998). Lesions to the amygdala result in deficits in the ability to learn about threatening cues (Anderson and Phelps, 2001), and individual differences in amygdala activation and anatomical size have been associated with psychiatric disorders (Abercrombie et al., 1998; Strakowski et al., 1999; Drevets, 2001; Siegle et al., 2002; Mayberg, 2003; Lange and Irle, 2004; Siegle et al., 2007; see Sheline, 2003, for a review) and their underlying personality dispositions (e.g. neuroticism; Haas et al., 2007; Cunningham et al., 2010). Yet, more recent research has demonstrated a role for the amygdala in PA. These findings have shifted the conceptualization of the amygdala from a threat detector to one associated with a more general function in accessing motivational relevance (for reviews, see Sander et al., 2003; Cunningham and Brosch, 2012). On this view, although survival is of critical importance, the ability to tune attention and cognitive processing to the most relevant aspects of the environment brings adaptive value.
In previous work linking positive stimuli to amygdala activation, greater amygdala activation has been shown for extraverts viewing faces with happy expressions (relative to fearful; Canli et al., 2002) and for people who are more promotion focused (gain sensitive; Higgins, 1997) to positive words (relative to negative; Cunningham et al., 2005). Further, greater amygdala activation was observed to liked famous names when participants were asked to attend to the positive aspects of the individuals (Cunningham et al., 2008). Although these findings have suggested a more general role in the processing of valence for the amygdala, it has been unclear from this work whether the differences in amygdala tuning are the result of greater attention to the positive features alone, or from a hydraulic function in which a tuning toward positive necessitates a tuning away from negative.
In the present work, we demonstrate that enhanced amygdala activation can be linked to a positive outcome: subjective happiness. Unlike participants lower in happiness who showed a greater amygdala response primarily to negative stimuli, participants higher in happiness showed amygdala activation to both positive and negative stimuli. Critically, this pattern of results demonstrates that the enhanced amygdala response observed for happy people to positive stimuli need not come at the cost of sensitivity to negative stimuli. Indeed, if anything, the happier participants showed a greater response to negative than neutral stimuli than the less-happy participants. These data suggest that happiness does not reflect neural naiveté. That is, happy people are not insensitive to negative cues in the environment; rather, they may be tuned toward the most important aspects of the environment. This tuning may reflect a higher degree of affective flexibility, allowing happier people to respond adaptively to both environmental challenges and opportunities.
This pattern of results is consistent with the idea that goals and needs are hierarchically organized and that security and survival goals are primary for most people. Negative emotions may be a useful signal of important environmental events—for example, in a life-threatening situation, it is adaptive to recognize and respond immediately. Indeed, according to Maslow’s (1943) classic hierarchy of needs, people must first meet physiological and safety needs before seeking additional opportunities. More recent research has supported this idea, suggesting that although other needs can be fulfilled while basic needs remain unmet (e.g. people can enjoy socializing with friends even while hungry), the more primary needs still receive the most attention (Tay and Diener, 2011). As such, threats to security must always be monitored for and attended to, exploring additional opportunities are a second priority. Consistent with this hypothesis for amygdala function, although focusing on negative aspects of ambivalent stimuli simultaneously eliminates activation to positive features and enhances activation to negative features, focusing on positive aspects of these stimuli enhances activation to positive features without affecting activation to negative features (Cunningham et al., 2008). In other words, amygdala activation to positive stimuli may be more malleable than activation to negative, and the ability to recognize and respond appropriately to negative information may be a necessary component of happiness and well-being.
These findings naturally raise questions regarding their implications for depression. As noted in the introduction, previous studies on individual differences in affective style have focused primarily on the negative end of the continuum, either by using scales that are sensitive primarily to increasing dysfunction or by comparing clinical groups with control groups. Comparing the results of those studies to the current findings provides some evidence that happiness is not simply the inverse of depression. Most notably, previous work on depression and amygdala activation has shown hyperactivation to negative stimuli for those participants who are most depressed. To the extent that happiness is simply the inverse of depression, one might predict decreased amygdala activation to negative stimuli in the present study. However, we did not find that pattern of results; indeed, if anything, happier people had a (non-significantly) greater amygdala response to negative images. That said, of course happiness and depression are, by definition, mutually exclusive. As such, it will be necessary in future research to explore the affective space between depression and happiness and note the shifts in amygdala responsivity. Because the relationship between the amygdala response to positive and negative is not negatively coupled, this suggests that multiple forms of affective style may exist, ranging from the highly threat sensitive (high amygdala response to negative, low response to positive) to emotional blunting (low amygdala response to both positive and negative).
Although these data suggest that happier people may be tuned toward positive stimuli in the environment, it is unclear from this study the degree to which this is an automatic process. Previous work has shown that direct attention to one evaluative dimension (e.g. positive, negative or both) can shape the amygdala response toward the attended dimension (Cunningham et al., 2008). However, when participants are asked to attend to one of two presented stimuli, a U-shaped amygdala response to valence is observed to attended stimuli (such that responses are greatest for negative and positive stimuli, and least for neutral), but an amygdala response is also observed to negative unattended stimuli (W.A.Cunningham, J.J.Van Bavel, and P.E.Stillman, in preparation). Because participants were asked to evaluate the stimuli following each block, and in doing so may have increased attention to the valenced aspects of the stimuli, future research should examine whether attending to the valenced nature of the stimuli is necessary to have a heightened amygdala response to positive stimuli for happier people.
In summary, happier people may be better equipped to notice and respond appropriately to both opportunity and threat in the environment. Rather than being driven by psychological insensitivity to negativity, the present work suggests that happiness is fostered by an affective response that is flexible and appropriate to the demands of the situation. We conclude by suggesting that happy people are joyful, yet balanced: they notice and seek positive experiences while also being sensitive to potential costs to well-being. This strategy helps them to function optimally across the variety of situations that can arise in the social world.
Conflict of Interest
None declared.
Acknowledgments
This project was supported by the National Science Foundation (BCS-1122352) and Templeton Foundation.
Footnotes
1The participant reported worrying about the phobic category in all blocks, making the comparison between positive, negative and neutral blocks of stimuli impossible.
2Given the low degree of hypomanic personality scores in this sample, this scale is used only as a covariate in analyses to ensure that amygdala effects can be attributed to the adaptive forms of positive affect. Greater variation in these scores would be necessary to make inferences about hypomania per se.
3Although the amygdala typically shows greater activation for positive and negative stimuli relative to neutral, some studies show a J-shaped function such that the activation to negative stimuli is larger than to positive stimuli.
4Results for right and left amygdala were nearly identical for all results and are therefore collapsed for all analyses.
5To further ensure independence, all analyses were reanalyzed in a series of analyses in which differences in amygdala activation were extracted for the positive, negative and neutral conditions using an independent anatomical mask (the Oxford-Harvard Atlas). In addition, a third set of analyses were conducted using amygdala voxels determined to be significant by correlating the amygdala contrast to negative and positive blocks. These analyses replicated the main results reported in the article, and are presented in Table 1 for completeness.
6Additional models were run predicting amygdala activation from SHS as well as the positive and negative subscales of the PANAS. In each of these models, SHS remained a significant predictor of the positive > neutral contrast (left: b = 3.87, t(38) = 2.43, P < .05; right: b = 2.91, t(38) = 2.14, P < .01). Once SHS was entered into the model, neither the positive or negative subscales of the PANAS predicted amygdala activation.
REFERENCES
- Abercrombie HC, Schaefer SM, Larson CL, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–7. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Affective flattening and the criteria for schizophrenia. American Journal of Psychiatry. 1979;136:944–7. doi: 10.1176/ajp.136.7.944. [DOI] [PubMed] [Google Scholar]
- Andrews FM, Withey SB. Social Indicators of Well-Being: America’s Perception of Life Quality. New York: Plenum Press; 1976. [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Neurobiological basis of psychopathy. The British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Bradburn NM. The Structure of Psychological Well-Being. Oxford: Aldine; 1969. [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Arbuckle NL, Jahn A, Mowrer SM, Abduljalil AM. Aspects of neuroticism and the amygdala: chronic tuning from motivational styles. Neuropsychologia. 2010;48:3399–404. doi: 10.1016/j.neuropsychologia.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–9. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of Black and White faces. Psychological Science. 2004;15:806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:202–11. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science. 2008;19:152–60. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–30. [Google Scholar]
- Davidson RJ. Affective style: causes and consequences. In: Cacioppo JT, Berntson GG, editors. Essays in Social Neuroscience. Cambridge, MA: MIT Press; 2004. pp. 77–91. [Google Scholar]
- Diener E, Suh EM, Lucas RE, Smith HL. Subjective well-being: three decades of progress. Psychological Bulletin. 1999;125:276–302. [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive–emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. Journal of Abnormal Personality. 1986;95:217–33. doi: 10.1037//0021-843x.95.3.214. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. Cultivating positive emotions to optimize health and well-being. Prevention and Treatment. 2000;3 Article 1. http://psycnet.apa.org/journals/pre/3/1/1a/ [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60:678–86. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Sutton SK, Ketelaar T. Relations between affect and personality: support for the affect-level and affective-reactivity views. Personality and Social Psychology Bulletin. 1998;24:279–88. [Google Scholar]
- Gruber J. Can feeling too good be bad? Positive emotion persistence (PEP) in bipolar disorder. Current Directions in Psychological Science. 2011;20:217–21. [Google Scholar]
- Gruber J, Johnson SL, Oveis C, Keltner D. Risk for mania and positive emotional responding: too much of a good thing? Emotion. 2008;8:23–33. doi: 10.1037/1528-3542.8.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121:249–56. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist — Revised. Toronto, Ontario, Canada: Multi-Health Systems; 1991. [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SA. Global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1998. [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychological Medicine. 2004;34:1059–64. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Lepper H. A measure of subjective happiness: preliminary reliability and construct validation. Social Indicators Research. 1999;46:137–55. [Google Scholar]
- Lyubomirsky S, Tucker KL. Implications of individual differences in subjective happiness for perceiving, interpreting, and thinking about life events. Motivation and Emotion. 1998;22:155–86. [Google Scholar]
- Maslow AJ. A theory of human motivation. Psychological Review. 1943;50:1–21. [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bulletin of the Menninger Clinic. 1975;39:295–307. [PubMed] [Google Scholar]
- Sander D, Grafman J, Zella T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauser SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauser SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. American Psychologist. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–51. [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Tay L, Diener E. Needs and subjective well-being around the world. Journal of Personality and Social Psychology. 2011;101:354–65. doi: 10.1037/a0023779. [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. NeuroImage. 2008;39:1227–45. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–88. [Google Scholar]
- Woolrich MW. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; 2001. [Google Scholar]