Abstract
The dorsomedial prefrontal cortex (dmPFC) is consistently involved in tasks requiring the processing of mental states, and much rarer so by tasks that do not involve mental state inferences. We hypothesized that the dmPFC might be more generally involved in high construal of stimuli, defined as the formation of concepts or ideas by omitting non-essential features of stimuli, irrespective of their social or non-social nature. In an fMRI study, we presented pictures of a person engaged in everyday activities (social stimuli) or of objects (non-social stimuli) and induced a higher level of construal by instructing participants to generate personality traits of the person or categories to which the objects belonged. This was contrasted against a lower level task where participants had to describe these same pictures visually. As predicted, we found strong involvement of the dmPFC in high construal, with substantial overlap across social and non-social stimuli, including shared activation in the vmPFC/OFC, parahippocampal, fusiform and angular gyrus, precuneus, posterior cingulate and right cerebellum.
Keywords: mentalizing, theory of mind, mPFC, construal level
INTRODUCTION
Research on the neural underpinning of social cognition has revealed a distinction between two neural networks: a mirror network, involved in processing concrete observable behaviors, and a mentalizing network, involved in the inference of mental states or durable characteristics (see Frith and Frith, 2006; Van Overwalle and Baetens, 2009). One important region in the mentalizing network is the medial prefrontal cortex (mPFC).
The mentalizing system, and the mPFC in particular, is engaged by a broad range of tasks involving emotion, the self, theory of mind and evaluation (Mitchell, 2009; Van Overwalle, 2009). As noted by Mitchell (2009), the mPFC is rarely involved (or even deactivated) in most studies on semantic memory, perception and executive functioning. A recent meta-analysis by Van Overwalle (2011) supports this notion, showing that activation of the mPFC in a broad set of reasoning studies was proportional to the amount of mentalizing content in the stimulus material, defined broadly as stimuli containing goal-directed action, beliefs, moral (in)justice, personality traits, social categories or emotions. Nevertheless, in contrast to this domain-specific view, some studies exist in which activation of the mPFC was observed in absence of such content (see later in the text).
Several authors have suggested a common process underlying the recruitment of the mPFC, whether the task requires mental state inferences. Mitchell (2009) suggested that the mPFC might subserve fuzzy cognition, that is, cognition that is not aiming at a maximally accurate or direct representation of reality but is involved in representations, which are ‘inexact, probabilistic, internally generated and subject to revision’ (Mitchell, 2009, p. 249). That the mPFC is mainly recruited by social cognitive processes might then be due to the fact that they rely more often on fuzzy representations than non-social cognitive processes. However, this explanation leaves unspecified exactly what fuzzy representations and their constituent features are. According to Legrand and Ruby (2009), the mentalizing network, including the mPFC, is involved in evaluation, defined broadly as ‘inferential processes and memory recall’ (p. 264). As such, they assert that activity in the mentalizing system is not exclusively related to either self-related or mental state-related processes. However, this account does not explain why activation of the mentalizing network is proportional to the amount of mentalizing content in the stimulus material (Van Overwalle, 2011), and particularly why the network is often not activated by tasks that do seem to require inference processes and memory recall.
In the present study, we want to put forward an alternative hypothesis explaining mPFC activation more generally, both in tasks that do and do not involve the processing of mental states, particularly activation of its dorsal aspect [dorsomedial prefrontal cortex (dmPFC); centered at the Montreal Neurological Institute coordinates 0, 50, 35 with 15 mm radius as specified by Van Overwalle and Baetens, 2009]. Our hypothesis relies on construal level theory (for a review, see Trope and Liberman, 2010). Construal level theory posits that there are four dimensions of psychological distance toward a stimulus (spatial, temporal, social and hypothetical), with a positive correlation between psychological distance and construal level of the stimuli. A higher construal entails the preservation of central qualities while disregarding less essential features. To illustrate in the social domain, Trope and Liberman (2010, p. 442) claim that, ‘construing another person’s behavior in terms of a personality trait (a high-level construct) involves considering that person’s behavior in the past and future, in other places, and in hypothetical situations’. This process of mentally traversing psychological distance, away from the present, concrete, sensory here and now, might be a more general function subserved by the dmPFC.
Several studies using tasks involving mental state inferences suggest a correlation between dmPFC activation and construal level or psychological distance. The dmPFC has been shown to become more active in personality judgment, as the target is perceived to be more different from the self (social distance; Tamir and Mitchell, 2010). Higher dmPFC activation was observed when participants reflected on distal rather than proximal life goals (temporal distance; Packer and Cunningham, 2009). In an extensive meta-analysis, Van Overwalle and Baetens (2009) concluded that the dmPFC is involved in inferring enduring rather than temporary mental characteristics (e.g. personality traits vs goals), indicating a larger temporal distance in terms of construal level theory. Spunt and colleagues (2010, 2011) asked their participants to describe the how, what and why of another’s behavior. These questions prompt progressively higher levels of construal or levels of identification in terms of Action Identification Theory (Vallacher and Wegner, 1987), shifting focus from the concrete particulars of how the action is performed to its reasons, implications and effects. Spunt and colleagues (2010, 2011) found that engagement of the dmPFC progressively increased with the level of identification of an observed action (how < what < why). Results of one recent study seem less in keeping with our hypothesis: Tamir and Mitchell (2011) manipulated all four types of psychological distance in an affective forecasting task and found a positive correlation between psychological distance and dmPFC activation only for the social dimension.
Although studies reporting dmPFC involvement without any mentalizing stimulus material are rather rare (Van Overwalle, 2011), some authors have found dmPFC activation in non-social reasoning. Crucially, the need for higher Construal Level (CL) may drive dmPFC activation in these cases too. For instance, increasing dmPFC recruitment has been found, given enhanced uncertainty and unconstrained choice, using a variety of paradigms and tasks (e.g. objective uncertainty in gambling studies, Xue et al., 2009; Stern et al., 2010; uncertainty in line length judgment, Grinband et al., 2006; free choice vs rule-governed choice, Rowe et al., 2008), which can be conceptualized as increasing distance on the hypothetical dimension toward the outcomes of the choices, leading to a higher CL. Furthermore, it has been shown that uncertainty by itself may lead to more global processing of stimuli (Förster, 2012). A number of studies demonstrated dmPFC involvement in non-social high construal reasoning, when tasks require the disregarding of inessential or unshared features of stimuli (e.g. judging abstract > concrete properties of animals, Goldberg et al., 2007; inductive > deductive reasoning, Goel et al., 1997; semantic distance in an analogy task, Green et al., 2010; constructing narrative or causal coherence, Ferstl and von Cramon, 2001, 2002; Siebörger et al., 2007; Kranjec et al., 2012; integrating premises, Fangmeier et al., 2006; sequences with task-irrelevant structure > random sequences, Turk-Browne et al., 2009).
The present research
In brief, we propose that the dmPFC might be crucially involved in higher construal of stimuli, independent of whether the task requires mental state inference. To test this hypothesis, we presented photos depicting either a woman performing routine activities (e.g. eating a salad) or manmade objects, animals and natural phenomena of neutral valence (Figure 1). Orthogonal to this, we manipulated level of construal, by asking participants to generate verbal descriptions of the visual characteristics of the image (low construal level—visual) or to make abstractions of what they saw (high construal level—category). More specifically, for person pictures, they were asked to generate personality traits that could possibly drive the depicted behavior. Such explicit trait inferences consistently engage the mentalizing system (e.g. Ma et al., 2012). For objects, they were asked to generate non-evaluative semantic categories by completing the sentence ‘This is an example of …’, a task used in the past to prime a high construal level (Wakslak and Trope, 2009). We expected that the high construal condition would activate the dmPFC more than the low construal condition, and that there would be an important overlap in the dmPFC for trait inference based on person pictures and semantic categorization based on object pictures.
Fig. 1.
Example pictures for person and object trials.
METHODS
Participants
Nine men and nine women, right-handed, aged between 18 and 31 years participated in exchange for €10. One additional participant was excluded because of excessive movement artifacts. None of the participants reported an abnormal neurological history, and all had normal or corrected-to-normal vision. Informed consent was obtained according to the guidelines of the Medical Ethics Committee at the Ghent University Hospital and the Brussels University Hospital.
Procedure
Participants practiced the tasks outside the scanner until they could execute them correctly and confidently. They were asked to perform the same tasks in the scanner, but in silence.
Each trial consisted of a picture presentation of 7 s. Participants were asked to generate responses during the entire presentation. The images either depicted a person engaged in everyday activities (person trials) or an animal, manmade object or natural phenomenon (object trials). Each picture was presented twice in the course of the experiment, once under low construal (visual-perceptual) and once under high construal (category) instructions. All trials were presented in a fully random order.
In low-construal trials, participants were requested to generate as many visual descriptions of the picture as possible during the entire 7 s presentation time. For person pictures, this entailed descriptions of the posture or position of the person, or descriptions of objects involved in the behavior. They were asked not to describe objects or aspects of the background that were not related to the behavior. For object pictures, this concerned color, texture, shape or structure of the object. For both picture types, participants were explicitly asked not to generate subjective (e.g. ‘big’) or evaluative (e.g. ‘ugly’, ‘concentrated’) descriptions or interpretations and to avoid descriptions based on what they knew but could not see (e.g. ‘it has a rectangular base’ for the Gizeh pyramid).
In high-construal person trials, participants were asked to generate personality traits that could drive the depicted behavior. As all person pictures displayed the same person, participants were asked to treat her as an actress. Furthermore, they were asked to base themselves on the behavior and not on her facial expression. Lastly, participants were asked not to infer temporary goals or motivations (e.g. ‘she wants to call somebody’). In high-construal object trials, participants were asked to generate non-evaluative categories that applied to the depicted object. As an aid, they were told to generate completions of the sentence ‘This is an example of …’, in line with Wakslak and Trope’s (2009) manipulation to prime a high construal mindset. They were explicitly informed that the name of the object, as well as subcategories of previously generated categories, was considered valid. They were asked not to categorize based on visual features (e.g. ‘yellow things’) or on subjective grounds (e.g. ‘ugly things’).
Each trial was preceded by a 2 s display of a fixation cross and a 2 s display of the instructions for that trial (‘Describe visually—person’, ‘Describe visually—object’, ‘Generate traits’ or ‘Generate categories’). After the presentation of each picture, participants were asked to rate how difficult they had found this particular task for this particular picture on a four-point scale (1 = very easy, 2 = rather easy, 3 = rather difficult, 4 = very difficult), by pressing one of four buttons on a response box positioned just below their non-dominant hand. The difficulty rating question remained on screen until participants responded.
Participants performed 10 practice trials inside the scanner before the start of the experiment. Immediately after scanning, they were presented with all the pictures and were asked to reproduce as much of the output they had generated during the experiment as possible. They were explicitly requested to include answers that were not in accordance with the instructions, as the main purpose of this procedure was to be able to exclude such trials from the analysis.
Stimulus material
The 30 person images were stills taken from video clips kindly provided by Spunt and Lieberman (2012). The object pictures depicted 15 manmade objects or structures (e.g. a lawn mower, a pyramid), 15 non-manmade objects, such as animals (e.g. a heron), plants or parts thereof (e.g. an acorn) and natural phenomena (e.g. lightning), all on a neutral background.
In a pilot study, three groups of 86 participants in total rated the valence of half of the object pictures (68 in total) or all the person pictures (51) on a five-point scale (1 = very unpleasant, 2 = rather unpleasant, 3 = neutral, 4 = rather pleasant and 5 = very pleasant) and generated visual characteristics and categories under the same instructions as applied in the experiment. Additionally, they rated how difficult they found this task (1 = very easy, 2 = rather easy, 3 = average, 4 = rather difficult and 5 = very difficult). All groups were instructed to complete the task in 90 min.
To minimize the influence of valence effects, all pictures with a mean valence rating <2 or >4 were excluded. To ensure a maximal variation of difficulty over trials, we made alternating selections of the most and least difficult stimuli for the visual and non-visual task out of the remaining pool of pictures. As such, we obtained 30 person pictures, 15 manmade object pictures and 15 non-manmade object pictures. All pictures were cropped to 717 × 461 pixels and were centrally presented.
Imaging procedure
Images were collected with a 3 T Magnetom Trio MRI scanner system (Siemens Medical Systems, Erlangen, Germany), using an 8-channel radiofrequency head coil. The monitor display with a resolution of 1024 × 768 pixels was projected onto a screen at the end of the magnet bore, visible by means of a mirror mounted on the head coil. We used E-Prime 2.0 software (www.pstnet.com/eprime; Psychology Software Tools) for the stimulus presentation. To minimize head movements, foam cushions were placed inside the head coil. First, we collected a high resolution T1-weighted structural scan (MP-RAGE), followed by a functional run of 922 volume acquisitions (30 axial slices; 4 mm thick; 1 mm skip) using a gradient-echo echoplanar pulse sequence (TR = 2 s; TE = 33 ms; 3.5 × 3.5 × 4.0 mm in-plane resolution).
Image processing
The fMRI data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London). To remove noise and artifacts, data were preprocessed for each functional run. Functional data were corrected for differences in acquisition time between slices per whole-brain volume, realigned within and across runs to correct for head movement and coregistered with each participant’s anatomical data. Functional data were then transformed into a standard anatomical space (2 mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute). These normalized data were spatially smoothed (6 mm full-width-at-half-maximum) using a Gaussian kernel. Subsequently, to exclude excessive motion artifacts and correlations between motion and experimental design or between global mean signal and the experimental design, we examined the realigned data using the Artifact Detection Tool software package (http://web.mit.edu/swg/art/art.pdf; http://www.nitrc.org/projects/artifact_detect). Outliers were identified in temporal difference series by assessing between-scan differences (Z-threshold: 3.0, scan to scan movement threshold 0.45 mm; rotation threshold: 0.02 rad). No correlations between motion and experimental design or global signal and experimental design were identified. One female participant was excluded from further analysis owing to excessive motion artifacts, leaving a total of nine male and nine female participants. Finally, trials in which participants reported incorrect answers in the post-scan questionnaires (4.2% of the total, rated by two independent judges) were omitted by including them in the error term. Six directions of motion parameters from the realignment step and outlier time points (defined by Artifact Detection Tool) were included as nuisance regressors. We used a default high-pass filter of 128 s, and serial correlations were accounted for by the default auto-regressive AR(1) model.
Statistical analyses
The statistical analysis involved first-level single participant analyses (specifying each condition of interest) all time-locked at the presentation of the picture, using a canonical hemodynamic response function with event duration set to 7 s (given the instruction to generate responses during the entire stimulus presentation), followed by second-level group analyses. Comparisons of interest were performed at the second-level using the general linear model of SPM8 in which the event-related design was modeled including a participant’s factor to account for between-participant variance. To account for variance explained by differences in difficulty between conditions, we included a regressor of no interest with the mean difficulty rating per participant per condition (overall mean centered, no interactions specified). To statistically compare the experimental conditions, we conducted a repeated measures analysis of variance on the parameter estimates associated with each trial type, with Construal Level (high vs low) and Picture Type (person vs object) as within-participant factors. Comparisons of interest were implemented as linear contrasts using a random-effects model. As a main effect of either Construal Level or Picture Type could be potentially be driven by one of both comprising individual conditions, we conducted conjunction analyses (conjunction null) to identify regions more active in high than low construal tasks across both types of pictures. A voxel-based statistical threshold of P ≤ 0.05 (FWE-corrected), as well as a minimum cluster size of 10 voxels, was used for all comparisons.
Lastly, we identified brain regions in which activation was a linear function of the difficulty rating over all experimental conditions per participant. For this analysis, on the first level, all trials were treated as belonging to a single condition, and difficulty rating per trial per participant was included as a parametric modulator. The obtained t-statistics were analyzed on the group level by means of one sample t-tests (testing for significantly positive and negative slopes), treating participant as a random effect, again using a voxel-based statistical threshold of P ≤ 0.05 (FWE-corrected) and a minimum cluster size of 10 voxels.
RESULTS
Difficulty ratings
A repeated measures analysis of variance with Construal Level (high vs low) and Picture Type (person vs object) as within-participant factors revealed a significant main effect of Construal Level, F(1, 17) = 42.26, P < 0.001 and a significant interaction between Construal Level and Picture Type, F(1, 17) = 10.30, P < 0.01. The mean difficulty per condition and the results of the post hoc comparisons can be found in Table 1. Given the significant differences between conditions, we included difficult rating as a covariate of no interest in the fMRI analysis to limit its influence as a potential confounding variable.
Table 1.
Descriptive statistics for difficulty ratings and post scan responses (n = 18)
| Person—Visual | Person—Category | Object—Visual | Object—Category | |
|---|---|---|---|---|
| Mean difficulty rating | 2.08a | 2.50b | 2.15a | 2.28c |
| Standard deviation | 0.48 | 0.63 | 0.56 | 0.65 |
| Average number of correct responses | 2.53d | 1.58e | 3.38f | 2.54d |
| Standard deviation | 0.64 | 0.48 | 0.61 | 0.64 |
Means with a different subscript differed significantly according to the post hoc tests (Bonferroni corrected, P < 0.05).
Post-scanning responses
Based on the post-scan questionnaire, we also tallied the number of correct responses per condition (Table 1). For all but one participant, there was a significant negative correlation between this measure and the difficulty ratings (mean Pearson r = −0.32, all P-values < 0.05), indicating that less correct responses were given in trials judged to be difficult. Given that these measures were gathered at different points in time (during vs post-scan) and entail quantifications of different types of responses (subjective rating vs number of verbal responses), this reliable negative correlation seems to support the validity of these measurements at least to some extent.
To make sure that the high construal conditions for persons and objects were clearly separated, we verified how many mental state responses were made in the high construal object condition. For animal and natural phenomenon pictures (Table 2), mental state/social responses were rare (<3%), and when they occurred, they uniformly concerned mental states the participants experienced themselves (e.g. describing a tiger as ‘an animal many people are afraid of’). Trials containing such mental state responses were rejected from the fMRI analysis. None of the manmade object pictures elicited mental state responses; therefore, none of these trials were rejected from the analysis on this basis. Interestingly, most manmade objects elicited functional categorizations (e.g. ‘gardening equipment’), which do imply human intervention to a limited extent. However, setting a clear criterion to distinguish between functional and non-functional responses was difficult (e.g. is ‘lawn mower’ a functional categorization? Is ‘shoe’?), as was any other further meaningful subcategorization of these responses. As motivated later in the text, functional categorizations were not excluded from the fMRI analysis.
Table 2.
Categorization and representative examples for high construal responses for animal pictures (399 responses in total) and natural phenomenon pictures (282 responses in total)
| Category | Occurence | Examples |
|---|---|---|
| Animals | ||
| Biological | 48.87% | mammal, bird, reptile |
| ‘Animal’ | 20.30% | |
| Habitat | 11.78% | sea animal, airborne animal, forest creature |
| Other | 7.02% | living organism |
| ‘Predator’/‘Prey’ | 4.51% | |
| Diet | 3.51% | insect/fish eater, carnivore |
| Social/Mental state | 2.26% | danger (tiger, eagle), poison (ladybug) |
| Function | 1.00% | food |
| Visual (low CL) | 0.71% | long legs, small, hairy |
| Natural phenomena | ||
| Other (name) | 52.84% | weather, nest, wood |
| Botanical term | 30.85% | tree, fruit, plant |
| Function | 14.18% | fuel, energy source, food |
| Social/Mental state | 2.13% | danger, bad weather (lightning), sour tasting things (lemon) |
Manmade object pictures did not elicit mental state responses. Further categorization was difficult (see text).
Imaging results
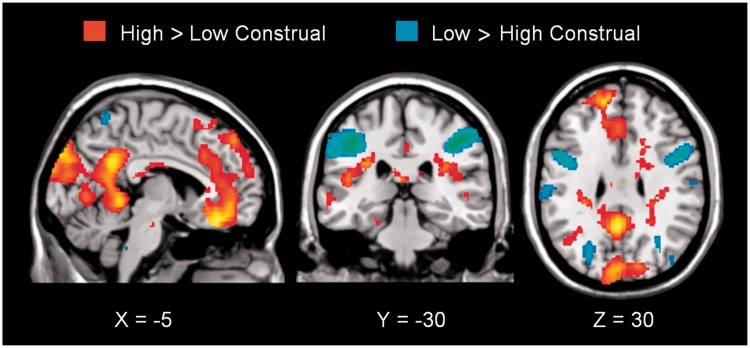
To verify our hypothesis that semantic categorizing is subserved by areas that are also recruited by a task requiring mental state inferences, we computed the conjunction of the high construal level (category) > low construal level (visual) contrast across the person and object conditions. This conjunction analysis revealed activations in the dmPFC, as well as in the ventral medial prefrontal cortex (vmPFC), extending to the orbitofrontal cortex (OFC), and the precuneus (also regions of the mentalizing network), the cuneus, fusiform, parahippocampal, posterior cingulate and angular gyrus and the right cerebellum (all after controlling for difficulty rating and applying an FWE-corrected threshold of P < 0.05; Table 3 and Figure 2).
Table 3.
Whole-brain analysis of conjunctions of high vs low construal across person and object conditions, all at threshold <0.05 (FWE-corrected, number of voxels ≥ 10)
| High construal level: Person (Category—Visual) ∩ Object (Category—Visual) |
|||||||
|---|---|---|---|---|---|---|---|
| MNI coordinates | |||||||
| BA | x | y | z | t | k | ||
| dmPFC | 10 | −10 | 58 | 24 | 5.97* | 86a | |
| 9 | −12 | 52 | 32 | 7.10** | 86a | ||
| 8 | −10 | 36 | 48 | 6.49* | 24 | ||
| Ventromedial prefrontal cortex/orbitofrontal cortex | 10 | −2 | 44 | −12 | 6.51* | 272b | |
| 11 | −4 | 34 | −22 | 8.76** | 272b | ||
| 11 | −12 | 30 | −8 | 5.85 | 272b | ||
| Fusiform gyrus | 20 | L | −58 | −12 | −28 | 5.89 | 20 |
| Posterior cingulate | 29 | −12 | −42 | 4 | 5.73 | 103c | |
| 29 | −12 | −52 | 4 | 5.95* | 103c | ||
| Parahippocampal gyrus | 30 | −6 | −46 | −4 | 6.56* | 103c | |
| Precuneus (inferior) | 31 | −4 | −52 | 30 | 7.92** | 201 | |
| Angular gyrus | 39 | L | −40 | −62 | 36 | 6.52* | 35 |
| Cerebellum | R | 28 | −74 | −36 | 6.26* | 42d | |
| R | 40 | −76 | −36 | 5.74 | 42d | ||
| R | 32 | −84 | −36 | 5.80 | 42d | ||
| Cuneus | 18 | −12 | −86 | 18 | 6.03* | 19 | |
| 19 | −6 | −92 | 36 | 6.48* | 67 | ||
BA = Brodmann’s Area; L and R = left and right hemispheres; x, y and z = Montreal Neurological Institute coordinates in the left–right, anterior–posterior and inferior–superior dimensions, respectively; t = t-score at those coordinates (peak value); k = cluster size (in voxels). Regions with ks that share a subscript originate from the same cluster.
*P < 0.01; **P < 0.001 (FWE-corrected).
Fig. 2.
Conjunction of High > Low Construal Level [i.e. Person (Category > Visual) ∩ Object (Category > Visual)] and Low > High Construal Level [i.e. Person (Visual > Category) ∩ Object (Visual > Category)] (P < 0.001; uncorrected).
Further, we determined which brain regions were differentially engaged in person and object conditions, given high vs low construal (Table 4). Small clusters in the dmPFC and posterior mFC were more active during person as opposed to object conditions, as well as the fusiform and parahippocampal gyri. Conversely, a large cluster in the superior precuneus and the right lateral PFC were more active in object than person conditions. Supplementary tables provide the results of high > low and low > high construal contrasts separately for person and object pictures (Supplementary Table 1) and a conjunction analysis of both low > high construal contrasts (Supplementary Table 2, see also Figure 2). Given the significant difference in difficulty between the conditions, we also conducted a parametric analysis over all conditions to verify whether mPFC activation was correlated with difficulty. We found no such correlation (see Supplementary Table 3). Lastly, for the three most prominent activation peaks (dmPFC, vmPFC and precuneus), we calculated percentage signal change for all four conditions and the 2 s fixation period preceding each experimental trial (Supplementary Table 4).
Table 4.
Whole-brain analysis of differences between high construal of persons and objects, all at threshold <0.05 (FWE-corrected, number of voxels ≥ 10)
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| BA | x | y | z | t | k | |
| Category: Person > Object Person(Category—Visual) − Object(Category—Visual) | ||||||
| dmPFC | 9 | −2 | 50 | 38 | 5.78 | 16 |
| Posterior medial frontal cortex | 6 | −6 | 32 | 60 | 6.51* | 30 |
| Parahippocampal gyrus | 37 R | 30 | −42 | −12 | 5.73 | 52a |
| 19 R | 28 | −56 | −12 | 6.17* | 19 | |
| Fusiform gyrus | 37 R | 22 | −40 | −18 | 6.18* | 52a |
| Category: Object > Person Object(Category—Visual) − Person(Category—Visual) | ||||||
| Lateral prefrontal cortex | 9 L | −44 | 26 | 34 | 5.82 | 15b |
| 9 L | −42 | 34 | 34 | 5.57 | 15b | |
| 9 R | 42 | 28 | 34 | 6.37* | 23 | |
| Precuneus (superior) | 7 | −2 | −64 | 52 | 7.47** | 327 |
BA = Brodmann’s Area; L and R = left and right hemispheres; x, y and z = Montreal Neurological Institute coordinates in the left–right, anterior–posterior and inferior–superior dimensions, respectively; t = t-score at those coordinates (peak value); k = cluster size (in voxels). Regions with ks that share a subscript originate from the same cluster.
*P < 0.01; **P < 0.001 (FWE-corrected).
DISCUSSION
High vs low construal
We hypothesized that the dmPFC is crucially involved in higher construal, whether the processing of mental states is required or not. Consequently, we predicted common greater dmPFC involvement in person categorization (trait inference) and object categorization than in matched, visual, low construal tasks. Our results confirmed this prediction. Additionally, this overlap encompassed other parts of the mentalizing network (vmPFC/OFC and precuneus) and the semantic system, as well as the right cerebellum (see Figure 2 and Table 3).
Although the present results confirm that the dmPFC is involved in construing both behavior and objects at a higher level, they are open to several interpretations. First, as high construals require a mental departure from the immediate here and now (Trope and Liberman, 2010), the high construal tasks required more internally oriented processes than low construal tasks. Second, in the context of the present study, the high construal conditions may have appealed more to self-guided unconstrained processes.
First, regarding the distinction between internally and externally oriented modes of information processing, previous research has associated the lateral prefrontal cortex with internally oriented processes and the mPFC with externally oriented processes. For example, according to Burgess and colleagues’ gateway hypothesis (2007), the lateral rostral PFC is associated with attending to internal sources of information, whereas the medial PFC (BA10) is associated with attending to externally generated or maintained information (with experimental support from, e.g. Henseler et al., 2011). This seems difficult to reconcile with the present results, showing greater dmPFC involvement in those that are allegedly more internally oriented, as well as a large number of studies from social neuroscience, demonstrating a robust association between mPFC activation and self-reflection (e.g. Jenkins and Mitchell, 2011; for a review, see Van Overwalle, 2009). This contradiction has been pointed out before (Burgess et al., 2007; Henseler et al., 2011) and resolved by distinguishing between more rostral mPFC activations (at the convexity to the lateral cortex) in studies involving internally oriented (as opposed to externally oriented) processes and more caudal mPFC activations in the social neuroscience literature. Although this account may resolve the apparent contradiction between the findings, it leaves entirely unclear why internally oriented processes result in greater activation in the (antero)lateral cortex in some cases (e.g. Henseler et al., 2011) and more (posterior) medial activation in others (e.g. many social neuroscience studies and the present results).
Second, another difference between high and low construal tasks might be the degree to which they appealed to self-guided unconstrained processes. The dmPFC is part of the default network (Gusnard et al., 2001), which is often deactivated during cognitively demanding tasks, but which has a relatively high metabolic activity in absence of a specific task-directed context. Perhaps, the dmPFC was more involved in the high construal tasks because they delimited the range and variability of correct answers less than the low construal tasks. The precise function of the dmPFC within the default network remains poorly understood. According to Bar and colleagues (2007), the mPFC is crucially involved in contextual association, generating predictions about the immediate relevant future. Other researchers have proposed that this region plays a key role in specific meta-cognitive demands pertaining to the processing of self-generated information (e.g. Turner et al., 2008). Therefore, if the high construal tasks rely more on self-guided processes, the involvement of the dmPFC may be due to relatively higher dependence on contextual association or the particular meta-cognitive demands imposed by self-generated information.
Unexpectedly, we also found a prominent activation of the vmPFC, extending to the orbitofrontal cortex, in the conjunction of high vs low construal conditions. The vmPFC has been associated with emotion processing and regulation (for reviews, see Davidson and Irwin, 1999; Phan et al., 2002; Quirk and Beer, 2006), and more importantly, with processing the affective significance of concepts (Binder et al., 2009). We selected pictures with a neutral valence in the pilot study. As we presented the same pictures in high and low construal level conditions, differences in the degree of affective processing can only be explained by task differences. Given the strong affective connotation of most personality traits (e.g. Baetens et al., 2011), the involvement of the vmPFC in our trait inference task is perhaps self-evident. For the object categorization task, this seems more surprising.
Interestingly, recent research suggests a direct link between affect and abstraction. Critcher and Ferguson (2011) reported that the affective meaning of stimuli may be processed automatically, and that this process is facilitated by an abstract mindset. Pexman and colleagues (2007) found that the vmPFC was more involved in processing abstract than concrete concepts. Further, a recent meta-analysis suggests that the vmPFC may function as a hub between conceptual information and affective responses (Roy et al., 2012). According to this view, the vmPFC is crucially involved in the abstraction of situations or stimuli in terms of their affective meaning. Taken together, the role of the vmPFC in affective processing and the notion that an abstract mindset can facilitate the extraction of affective significance may offer a tentative explanation of the vmPFC involvement in the present study.
Apart from the mPFC, we found several significant activations in the conjunction of high vs low construal conditions in other brain regions, about which we had no a priori hypotheses. The activation of the right cerebellum may seem surprising, considering the traditional view on this structure as subserving autonomic and motor functions. However, there is increasing acknowledgement of the cerebellum’s role in higher cognitive tasks (for a review, see Stoodley and Schmahmann, 2009). More specifically, the right cerebellum plays an important role in a variety of linguistic processes (De Smet et al., 2007; Stoodley and Schmahmann, 2009) and in particular, in semantic relatedness judgment of concepts (Xiang et al., 2003). A recent review (Carrington and Bailey, 2009) reported involvement of the cerebellum in 25% of the reviewed theory of mind studies (e.g. Völlm et al., 2006), a ratio comparable with that of the temporal poles and precuneus, regions often considered central to the mentalizing network.
Other activations in this conjunction included the parahippocampal, fusiform, angular and posterior cingulate gyrus, and the precuneus. These areas have been classified as core components of the semantic system (for reviews and more specific descriptions of the functional contribution of these areas, see Price, 2000; Binder et al., 2009). A recent review (Binder et al., 2009) concluded that the left dmPFC and vmPFC too are core components of the human semantic system (although in this study, ‘dmPFC’ may refer to a region extending more laterally and caudally than defined by Van Overwalle and Baetens, 2009). As these authors defined the semantic system by comparing tasks concerning the meaning of words with tasks that focused on lower-level characteristics (e.g. phonology, orthography) or required only shallower processing of their meaning, their results could also be interpreted in terms of the higher construal required in these semantic tasks.
The present results seem at odds with one previous study (Tamir and Mitchell, 2011), which explicitly investigated the impact of all four dimensions of psychological distance according to construal level theory in an opinion/preference judgment task. In proximal trials, participants had to indicate to which extent they themselves held certain opinions or preferences in the present here and now. In distal trials, they had to rate how much they anticipated holding the same opinions or preferences somewhere else (spatial distance), in the future (temporal distance), how much they believed somebody else to hold this opinion or preference (i.e. ‘Obama’, social distance) or how much they themselves would if they were of the opposite sex (hypothetical distance). According to construal level theory, greater psychological distance is associated with higher construal. Therefore, if dmPFC activation is associated with high construal, one would expect involvement of this region in the comparison of distal vs proximal judgments. However, this comparison yielded little significant activation: none for the temporal and spatial dimension and clusters in the precuneus and cingulate gyrus for the hypothetical dimension. Only the results for the social dimension were in accordance with the results of the present study, with significant activations in the dmPFC, orbitofrontal cortex and precuneus for distal vs proximal judgments. How can this discrepancy be explained?
First, it must be noted that a preference or opinion by itself may constitute a relatively high level of construal so that a rather strong manipulation of psychological distance may be required to overcome potential ceiling effects. By contrast, the tasks in the present study were selected to directly elicit differences in construal level, without manipulation of social distance. More critically, stimuli for the spatial, temporal and hypothetical dimensions in the study by Tamir and Mitchell (2011) were selected based on the criterion that the distance manipulation did not influence preference/opinion ratings (e.g. ‘How much would you like eating a banana here vs. in Oxford?’). As a result, the manipulation created an artificial context in the distal conditions and may therefore have been insufficient to bring about a higher construal of the activity. Interestingly, the same stimulus selection criterion was not applied for the social dimension (another person always has at least some different opinions/preference), and the results for this dimension (i.e. greater dmPFC activation for distal than proximal trials) was in accordance with the present study. Obviously, further research using more ecologically valid manipulations is needed to shed light on the precise impact of psychological distance and to reconcile the results of Tamir and Mitchell (2011) with the present study.
High construal of persons vs objects
Our results revealed only limited clusters that were more activated in high construal of persons than of objects. This comparison yielded small but significant activations in the dmPFC, as well as in the parahippocampal, fusiform and posterior medial frontal cortex (see Table 4). The slightly stronger activation of the dmPFC in the person condition parallels the more frequent occurrence of dmPFC activation in social than in non-social reasoning studies (Van Overwalle, 2011). One tentative interpretation of this preferential but non-exclusive relation may be that the need for higher construal evolved primarily to subserve our social functioning. High construals such as intentions, alliances and personality traits must have been important for our survival, even in a primitive stage of human development.
Conversely, apart from a small cluster in the lateral prefrontal cortex, an extensive area in the superior precuneus was more active in high vs low construal of objects than of persons. This part of the medial parietal lobe subserves diverse functions (for a review, see Cavanna and Trimble, 2006). Possibly, it was more active in object trials because they engaged more mental imagery, by virtue of the fact that the objects were presented on a neutral background, isolated from any context, whereas person trials depicted an actor in a real setting.
Limitations
As participants judged the high construal conditions as more difficult, it cannot be excluded that the greater involvement of the mPFC in these conditions was due to differences in experienced task difficulty. This possibility is especially relevant, given that previous research has argued for a general role of the dmPFC in attention processes (Walter et al., 2009). We tried to exclude this alternative explanation by including the reported difficulty as a covariate in the main analyses. Second, we verified in which voxels activation was a linear function of difficulty, revealing no such relations in the mPFC. Nevertheless, one might argue that the difficulty reported by the participants is an inadequate measure for these purposes. Therefore, it would be useful to compare high and low level construal of stimuli while keeping the objective difficulty of both tasks equal. However, owing to the qualitatively different nature of the person and object tasks, this seems challenging.
As a second alternative account for higher dmPFC activation in high vs low construal conditions for objects, one could claim that the higher construal of objects somehow provoked more processing of mental states or other social content, i.e. because animals can also be social agents. To allow for maximal generalization of our findings, we included both living (i.e. animals), non-living, manmade and non-manmade objects in the stimulus set. The post-scanning responses (Table 2) indicate that mental state responses were rare and uniformly concerned mental states of the perceiver to animal pictures or natural phenomena (these trials were rejected from the analysis). Further, previous studies comparing the neural representation of animals and manmade objects did not report greater dmPFC activation associated with either stimulus type (see meta-analysis by Binder et al., 2009). A related alternative account of increased mPFC activation is that the high construal task may have activated the goals associated with depicted objects, thereby activating intentions or action schemata, especially for manmade objects. However, the vast majority of the pictures did not depict manipulable objects. Moreover, according to Van Overwalle’s meta-analysis of >200 fMRI studies (2009), dmPFC activation is not reliably recruited during processing of action goals or goal-directed movement. Therefore, it seems unlikely that the activation of goals or actions associated with the subset of objects that did have strong functional associations would explain the involvement of the dmPFC in the present study. Nonetheless, future research is needed to explore these alternative explanations more thoroughly.
General conclusions
We posited that involvement of the dmPFC, both in tasks that do and do not require mental state inferences, might be driven by the need to represent stimuli at a higher level of construal. In line with this hypothesis, high as opposed to low construal conditions, both for pictures involving persons and objects, shared activations in the dmPFC, vmPFC and precuneus (among other regions). Further research is required to gain insight in the precise characteristics of construal level processes that drive the activation of the dmPFC. Speculatively, the so-called mentalizing network might be essentially involved in deriving the meaning of stimuli, as opposed to their individual properties. This could entail both definable (semantic) meaning and experiential meaning, and different parts of the mentalizing network may subserve different roles in this respect. That activation of the mentalizing system is much more common in the social neuroscience literature than in other disciplines could then be simply due to the more frequent employment of tasks aimed at the meaning of stimuli.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN Online.
Acknowledgments
The authors are deeply indebted to Dr Bob Spunt and co-authors for kindly sharing stimulus material, and to Dr Marcel Brass for his invaluable input. Kris Baetens is a PhD fellow of the Research Foundation–Flanders (FWO).
REFERENCES
- Baetens K, Van der Cruyssen L, Achtziger A, Vandekerckhove M, Van Overwalle F. N400 and LPP in spontaneous trait inferences. Brain Research. 2011;1418:83–92. doi: 10.1016/j.brainres.2011.08.067. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;428:420–8. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11(7):290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30(8):2313–35. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Critcher CR, Ferguson MJ. Affect in the abstract: abstract mindsets promote sensitivity to affect. Journal of Experimental Social Psychology. 2011;47(6):1185–91. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- De Smet HJ, Baillieux H, De Deyn PP, Mariën P, Paquier P. The cerebellum and language: the story so far. Folia Phoniatrica et Logopaedica. 2007;59(4):165–70. doi: 10.1159/000102927. [DOI] [PubMed] [Google Scholar]
- Fangmeier T, Knauff M, Ruff CC, Sloutsky V. FMRI evidence for a three-stage model of deductive reasoning. Journal of Cognitive Neuroscience. 2006;18(3):320–34. doi: 10.1162/089892906775990651. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. The role of coherence and cohesion in text comprehension: an event-related fMRI study. Cognitive Brain Research. 2001;11(3):325–40. doi: 10.1016/s0926-6410(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: coherence or theory of mind? NeuroImage. 2002;17(3):1599–612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Förster J. GLOMOsys: the how and why of global and local processing. Current Directions in Psychological Science. 2012;21(1):15–19. [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. Neuroreport. 1997;8(5):1305–10. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Fiez JA, Schneider W. Selective retrieval of abstract semantic knowledge in left prefrontal cortex. The Journal of Neuroscience. 2007;27(14):3790–8. doi: 10.1523/JNEUROSCI.2381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, Dunbar KN. Connecting long distance: semantic distance in analogical reasoning modulates frontopolar cortex activity. Cerebral Cortex. 2010;20(1):70–6. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–63. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henseler I, Krüger S, Dechent P, Gruber O. A gateway system in rostral PFC? Evidence from biasing attention to perceptual information and internal representations. NeuroImage. 2011;56(3):1666–76. doi: 10.1016/j.neuroimage.2011.02.056. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. 2011;6(3):211–18. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Kranjec A, Cardillo ER, Schmidt GL, Lehet M, Chatterjee A. Deconstructing events: the neural bases for space, time, and causality. Journal of Cognitive Neuroscience. 2012;24(1):1–16. doi: 10.1162/jocn_a_00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116(1):252–82. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Ma N, Vandekerckhove M, Baetens K, Van Overwalle F, Seurinck R, Fias W. Inconsistencies in spontaneous and intentional trait inferences. Social Cognitive and Affective Neuroscience. 2012;7(8):937–50. doi: 10.1093/scan/nsr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends in Cognitive Sciences. 2009;13(6):246–51. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA. Neural correlates of reflection on goal states: the role of regulatory focus and temporal distance. Social Neuroscience. 2009;4(5):412–25. doi: 10.1080/17470910902750186. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG. Neural correlates of concreteness in semantic categorization. Journal of Cognitive Neuroscience. 2007;19(8):1407–19. doi: 10.1162/jocn.2007.19.8.1407. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–59. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rowe J, Hughes L, Eckstein D, Owen AM. Rule-selection and action-selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cerebral Cortex. 2008;18(10):2275–85. doi: 10.1093/cercor/bhm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebörger FT, Ferstl EC, von Cramon DY. Making sense of nonsense: an fMRI study of task induced inference processes during discourse comprehension. Brain Research. 2007;1166:77–91. doi: 10.1016/j.brainres.2007.05.079. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Falk EB, Lieberman MD. Dissociable neural systems support retrieval of how and why action knowledge. Psychological Science. 2010;21(11):1593–8. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Satpute AB, Lieberman MD. Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience. 2011;23(1):63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience. 2012;32(10):3575–83. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Gonzalez R, Welsh RC, Taylor SF. Updating beliefs for a decision: neural correlates of uncertainty and underconfidence. Journal of Neuroscience. 2010;30(23):8032–41. doi: 10.1523/JNEUROSCI.4729-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):10827–32. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. The default network distinguishes construals of proximal versus distal events. Journal of Cognitive Neuroscience. 2011;23(10):2945–55. doi: 10.1162/jocn_a_00009. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Construal-level theory of psychological distance. Psychological Review. 2010;117(2):440–63. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Scholl BJ, Chun MM, Johnson MK. Neural evidence of statistical learning: efficient detection of visual regularities without awareness. Journal of Cognitive Neuroscience. 2009;21(10):1934–45. doi: 10.1162/jocn.2009.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MS, Simons JS, Gilbert SJ, Frith CD, Burgess PW. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46(5):1442–53. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallacher RR, Wegner DM. What do people think they’re doing? Action identification and human behavior. Psychological Review. 1987;94(1):3–15. [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. NeuroImage. 2011;54(2):1589–99. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. NeuroImage. 2009;48(3):564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29(1):90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wakslak C, Trope Y. The effect of construal level on subjective probability estimates. Psychological Science. 2009;20(1):52–8. doi: 10.1111/j.1467-9280.2008.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Matthiä C, Wiebking C, et al. Preceding attention and the dorsomedial prefrontal cortex: process specificity versus domain dependence. Human Brain Mapping. 2009;30(1):312–26. doi: 10.1002/hbm.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, et al. Involvement of the cerebellum in semantic discrimination: an fMRI study. Human Brain Mapping. 2003;18(3):208–14. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–27. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.