Abstract
Acute peripheral inflammation with corresponding increases in peripheral cytokines affects neuropsychological functions and induces depression-like symptoms. However, possible effects of increased immune responses on social cognition remain unknown. Therefore, this study investigated the effects of experimentally induced acute inflammation on performance and neural responses during a social cognition task assessing Theory of Mind (ToM) ability. In this double-blind randomized crossover functional magnetic resonance imaging study, 18 healthy right-handed male volunteers received an injection of bacterial lipopolysaccharide (LPS; 0.4 ng/kg) or saline, respectively. Plasma levels of pro- and anti-inflammatory cytokines as well as mood ratings were analyzed together with brain activation during a validated ToM task (i.e. Reading the Mind in the Eyes Test). LPS administration induced pronounced transient increases in pro- (IL-6, TNF-α) and anti-inflammatory (IL-10, IL-1ra) cytokines as well as decreases in mood. Social cognition performance was not affected by acute inflammation. However, altered neural activity was observed during the ToM task after LPS administration, reflected by increased responses in the fusiform gyrus, temporo-parietal junction, superior temporal gyrus and precuneus. The increased task-related neural responses in the LPS condition may reflect a compensatory strategy or a greater social cognitive processing as a function of sickness.
Keywords: peripheral inflammation, social cognition, fMRI, cytokines, endotoxin
INTRODUCTION
Theory of Mind (ToM), a higher-order form of social cognition, comprises the ability to impute mental states of others such as thoughts, intentions, desires and beliefs (Premack and Woodruff, 1978). Owing to the fact that primates, including humans, live in hierarchically organized social groups, ToM ability is essential for the interaction in close relationships and communities to ensure support from others in different aspects of life (Brune, 2001). This capability for social cognition is demonstrably disturbed in patients with neuropsychiatric diseases such as depression and schizophrenia (Frith and Corcoran, 1996; Doody et al., 1998; Kerr et al., 2003; Inoue et al., 2004; Wang et al., 2008). Social processing including ToM ability is known to be mediated by several brain regions, including the precuneus, medial prefrontal and temporal cortical areas (Mar, 2011). However, the neural mechanisms mediating deficits in social cognition including ToM ability remain incompletely understood.
Alterations in pro-inflammatory peripheral cytokines have been shown to affect neuropsychological functions and are discussed to play a role in the pathophysiology of neuropsychiatric diseases such as schizophrenia (Meyer et al., 2009; Drexhage et al., 2010) or depression (Raison et al., 2006; Irwin and Miller, 2007; Miller et al., 2009). However, the putative link between peripheral immune activation and ToM ability has thus far not been investigated. Vaccination or administration of endotoxin (lipopolysaccharide, LPS), a complex glycolipid found in the outer membrane of gram-negative bacteria, has recently been used in humans as an experimental model to analyze the effects of acute immune activation on neuropsychological functions and brain activity (Brydon et al., 2008; Eisenberger et al., 2009; Harrison et al., 2009a,b; Eisenberger et al., 2010a; van den Boogaard et al., 2010; Kullmann et al., 2013). Intravenous injection of LPS induces a complex psycho-physiological response including the transient release of pro-inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Bahador and Cross, 2007) as well as a range of behavioral symptoms including depressed mood, social withdrawal, cognitive impairment, malaise and fatigue, collectively termed ‘sickness behavior’ (Reichenberg et al., 2001; Wright et al., 2005; Dantzer and Kelley, 2007). Whereas a number of studies in rodents observed social withdrawal and a reduction of exploratory behavior during acute inflammation (Larson and Dunn, 2001; Dantzer et al., 2008), social aspects of behavior in humans remain incompletely understood (Eisenberger et al., 2009; Eisenberger et al., 2010b). Furthermore, a study in rhesus monkeys even observed an increase in social interaction during acute inflammation (Willette et al., 2007). Data from experimental animal and human studies suggest that, in particular, pro-inflammatory peripheral cytokines (e.g. IL-1β, IL-6 and TNF-α) play a crucial role in mediating these symptoms by directly or indirectly communicating the peripheral inflammation to the brain (Konsman et al., 2002; Capuron and Miller, 2004; Dantzer, 2006; Ader, 2007).
We have previously shown that activation of right inferior orbitofrontal cortex was significantly increased in response to emotional stimuli during experimental endotoxemia, reflecting enhanced cognitive regulation of emotions as an adaptive response during an acute inflammation (Kullmann et al., 2013). However, effects of acute peripheral inflammation on social cognition, including ToM ability, remain unexplored. Therefore, in an extension of our previous reports, the present study analyzed the effects of endotoxin-induced inflammatory responses on mood and brain activity during a social cognition task (Reading the Mind in the Eyes Test), encompassing ToM mechanisms. We performed a double-blind randomized crossover functional magnetic resonance imaging (fMRI) study with healthy right-handed male volunteers who received an injection of LPS (0.4 ng/kg Escherichia coli) or saline to test the hypothesis that acute inflammation-induced alterations in ToM ability are mediated by activity changes in specific brain regions of interest (ROIs) known to mediate ToM ability, including medial prefrontal cortices, fusiform gyri, precuneus and temporal cortical areas (Baron-Cohen et al., 1999; Saxe et al., 2004; Mar, 2011).
METHODS AND MATERIALS
Subjects
Eighteen healthy right-handed male volunteers (mean age: 26.4 ± 3.1 years, mean body mass index: 25.2 ± 0.2) were recruited by public advertisement (i.e. flyers posted at the University Hospital Essen; Internet-posted advertisements), as previously reported (Kullmann et al., 2013). The majority were medical students (88%, n = 17), all were unmarried and rated their overall health as either good or very good. Mean trait anxiety scores were well within the normal range (32.6 ± 1.0) compared with published normative data (Laux, 1981).
Screening process
The in-depth screening process consisted of a physical examination; a personal semi-structured interview, performed by an experienced clinical psychologist; completion of standardized questionnaires and repeated laboratory analyses of blood samples (i.e. complete blood cell count, liver enzymes, renal parameters, electrolytes, coagulation factors and C-reactive protein) before and up to 1 week after completion of the study (see later in the text). Exclusion criteria included age <18 or >40 years; body mass index <17 and >30; any concurrent medical condition, including neurological, psychiatric, cardiovascular, immunological and endocrine conditions; any abnormality of blood laboratory analyses; any evidence of structural brain abnormality on structural magnetic resonance imaging (MRI) scan; MRI-specific exclusion criteria (i.e. phobic anxiety, claustrophobia or ferromagnetic implantations); history of allergies; current use of prescription and non-prescription medications; smoking and regular high alcohol use (>4 drinks per week).
Additional safety measures included a physical examination and normal blood cell counts 6 h post-injection as a pre-condition for subjects being allowed to leave the laboratory. Further, participants were not allowed to drive a vehicle on the days of the study, and underwent follow-up examinations including laboratory analysis of C-reactive protein levels 24 h after each session and 7 days after the final session. Subjects were informed about the study design and were only enrolled after written informed consent had been obtained. The study was conducted in accordance with the declaration of Helsinki and approved by the local ethics committee. Subjects were paid for their participation.
Study design
The study used a balanced, randomized, double-blind crossover design, which has previously been described in detail (Kullmann et al., 2013). It consisted of two identical study sessions (at least 7 days apart) during which blood samples and mood ratings were obtained at multiple time points. Subjects received either an intravenous injection of LPS (0.4 ng/kg of body weight) or an identical volume of endotoxin-free normal saline (placebo). We decide to choose a lower dose of LPS (0.4 ng/kg) in this study to avoid the typical more pronounced side effects of the higher LPS dose (0.8 ng/kg) (e.g. nausea, shivering and fever) because this would complicate the interpretation of the data. As previously shown in a dose-dependent comparison (Grigoleit et al., 2011), 0.4 ng/kg of LPS induced pronounced and significant changes in peripheral immune markers, which we believe are sufficiently pronounced to constitute a valid model of sickness behavior. Two hours post-injection, when pro-inflammatory cytokines have been shown to peak after LPS application (Reichenberg et al., 2001; Eisenberger et al., 2010b; Grigoleit et al., 2010; Benson et al., 2012), participants underwent structural MRI scanning followed by two fMRI sessions, consisting first of an emotional processing task (reported elsewhere, Kullmann et al., 2013) and second a social cognition task (Reading the Mind in the Eyes Test). Blood samples were drawn 0.25 h before and 1, 1.75, 3, 4, 6 and 24 h post-injection together with assessments of vital signs (blood pressure, pulse and temperature). Participants also completed mood questionnaires at baseline (−0.25 h) and twice post-injection (3, 6 h).
Procedures
Each study session lasted 7 h, starting at 12:00 pm. After arriving at the laboratory, an intravenous cannula was inserted into an antecubital forearm vein for repeated blood sampling and bolus drug injection (LPS or saline). LPS from E. coli (United States Pharmacopeia, Lot G3E069) was prepared for use in humans by dissolving the lyophilizate in sterile water, filtration through a 0.2-µm membrane and a microbial safety testing routine approved by the German Federal Agency for Sera and Vaccines (Paul Ehrlich Institute, Langen, Germany), as previously described (Grigoleit et al., 2010; Kullmann et al., 2013). Until use, the LPS solution was stored in endotoxin-free borosilicate tubes at −20°C. The injection of LPS or saline occurred 30 min after cannula insertion. Participants stayed at the laboratory until 6 h post-injection and returned 24 h after each session for a medical check-up.
fMRI paradigm
During stimulation, subjects were asked to lie relaxed inside the scanner and try to focus on the presented stimuli. The stimuli were presented visually by a notebook computer running the presentation software package (Neurobehavioral Systems, Davis, CA). The timing of the stimuli was controlled by the timing of the acquisition of magnetic resonance (MR) images, through pulses sent from the MR scanner to the parallel port of the stimulus presentation PC. All stimuli were presented using a screen inside the scanner room and video projection from outside. A mirror was fixed to the head coil to place the screen in the subject’s field of view.
As reported elsewhere (Kullmann et al., 2013), subjects underwent an emotional processing task during which subjects focused on alternating neutral and emotionally evocative visual stimuli drawn from the International Affective Picture System (Lang, 1997). Two different sets of 36 emotional pictures with aversive contents, such as facial mutilation, wounds and dead bodies, and 36 neutral pictures, such as furniture and appliances, which did not elicit strong emotions, were selected. Both sets were comparable with respect to the dimensions valence, arousal and dominance. To avoid any nonrandom version-dependent bias, the stimuli were presented in random order in each block for each subject. A total of six off blocks (each six neutral stimuli) and six on blocks (each six emotional stimuli) were presented. Each picture appeared for 5 s. Thus, each block lasted 30 s.
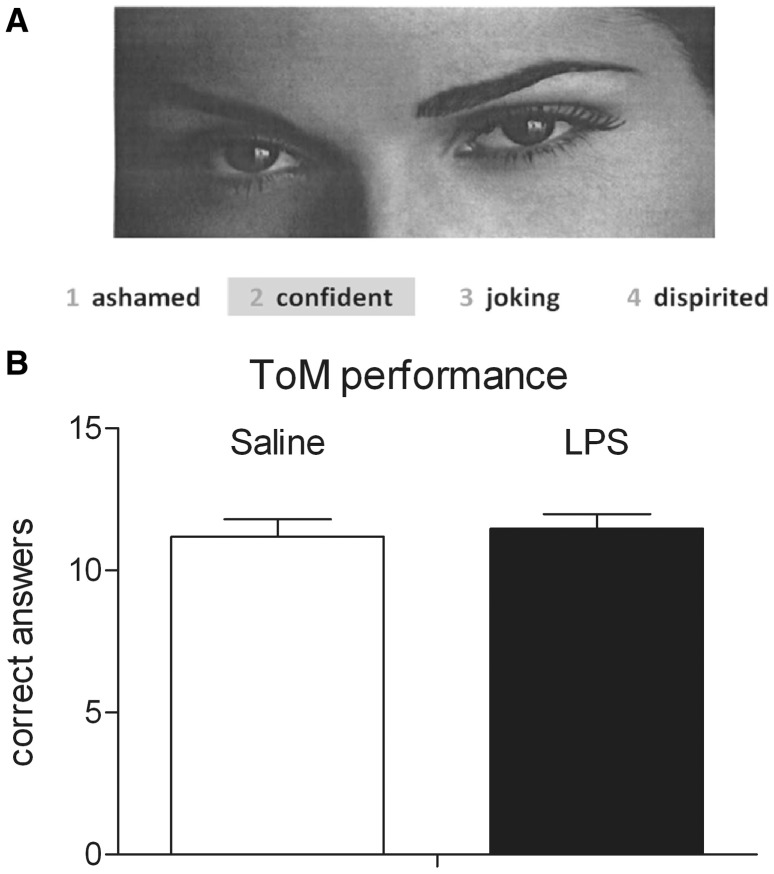
To assess social cognition performance, including ToM abilities, during the scanning session, we used the German adaptation of the Reading the Mind in the Eyes Test (‘Eyes’ test) (Bölte, 2005), which is based on the original English version devised by Baron-Cohen et al. (2001). In this task, subjects were asked to judge from the expression of another person’s eyes what that other person might be thinking or feeling, e.g. eyes could express panic, grief or desire (Figure 1A).
Fig. 1.
(A) Example slide of persons’ eyes drawn from the ‘Eyes’ test by Baron-Cohen (2001). (B) Social cognition (ToM) performance assessed with the ‘Eyes’ test in healthy male subjects (n = 18) after administration of 0.4 ng/kg E. coli endotoxin (LPS) or placebo (saline). Data are shown as mean ± SEM.
The test started with a task instruction and two training items. Two different sets of 36 photographed eyes were subsequently presented in randomized order in each block. A total of six off blocks and six on blocks were presented in an alternated ABA … design. Each block lasted 30 s and consisted of three consecutive slides with photographs of persons’ eyes. During the control (off) block, subjects were asked to indicate whether each stimulus was a man or a woman (gender judgment). During the on block, subjects were asked to decide which of four simultaneously presented words best described the mental state of the photographed person (ToM judgment). Correct words were counterbalanced to each presentation position. Subjects were briefed to press a four-key controller (LUMItouch; Photon Control Inc., Burnaby, Canada) only with the right forefinger. Answers were recorded by the presentation software.
fMRI acquisition and analysis
All MR images were acquired using a 1.5-T MR (Sonata; Siemens, Erlangen, Germany) with a standard head coil. A three-dimensional FLASH sequence (TR 9.2 ms, TE 4.46 ms, flip angle 30°, FoV 240 mm, matrix 256 × 256 and slice-thickness 1.5 mm) was acquired. BOLD contrast fMRI images were acquired using an echo-planar technique (TR 3.1 s, TE 50 ms, flip angle 90°, FoV 240 mm and matrix 64 × 64) with 36 transverse slices with a thickness of 3 mm and 0.3-mm slice gap.
For data analysis, SPM 05 software (Wellcome Department of Cognitive Neurology, London, UK) was used. Before statistical analysis, three ‘dummy’ scans were eliminated to account for T1 relaxation effects, and images were realigned using sinc interpolation and normalized to the standard stereotactic space corresponding to the template from the Montreal Neurological Institute (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). Bilinear interpolation was applied for normalization. The images were smoothed with an isotropic Gaussian kernel of 9 mm. High-pass filtering with a cut-off period of 120 s and low-pass filtering with the hrf were applied. A voxel-by-voxel comparison according to the general linear model was used to calculate differences in activation between the active and resting conditions. For each subject, the first-level design matrix included a 2 × 2 factorial design with the factors ‘on and off condition’ for the social cognition (ToM) task assessed with the ‘Eyes’ test [ToM judgment (ToM); gender judgment (gender)] and ‘treatment condition’ (LPS, placebo). All regressors were obtained by convolving a box-car function of the event duration with the canonical hemodynamic response function implemented in SPM. Specific effects were tested with appropriate linear contrasts of the parameter estimates for the different regressors resulting in a t-statistic for each voxel. After model estimation, the ensuing first-level contrast images (LPSToM > LPSgender; placeboToM > placebogender) from each subject were used for second-level analysis, treating individual subjects as a random factor and including non-sphericity correction. Two separate analyses were conducted on the second (group) level: (i) as an initial step, we performed a one-sample t-test on data from the placebo condition to confirm activation in ROIs during the ToM task (ToM > gender). (ii) To directly compare ToM task-induced brain activation in the LPS condition and the placebo condition, paired t-tests were computed (LPSToM>gender > placeboToM>gender; placeboToM>gender > LPSToM>gender). (iii) To clarify if changes in state anxiety or alertness contribute to (or mediate) changes in activation in ROIs, peak state anxiety scores as well as alertness scores were included as covariates of no interest in the paired t-test within the LPS condition.
Small volume correction (SVC) with family-wise error (FWE) correction for multiple comparisons in specific ROIs at a level of P < 0.05 was performed. ROIs were chosen based on a meta-analysis of neuroimaging findings during non-story-based ToM studies (Mar, 2011), comprising the fMRI results during the ‘Eyes’ test by Baron-Cohen et al. (1999). ROIs included bilateral dorsomedial as well as medial prefrontal cortices (BA8/9 and BA10), superior temporal gyri, temporo-parietal junctions, precuneus and fusiform gyri. SVC was performed with templates constructed from the automated anatomical labelling toolbox in SPM (Tzourio-Mazoyer et al., 2002). All results are reported at P < 0.05 corrected for multiple comparisons, unless indicated otherwise. We additionally performed exploratory whole-brain analyses using a more liberal threshold of P < 0.001 (uncorrected), which are given in table legends. All results are given as Montreal Neurological Institute coordinates.
Questionnaires
Mood was assessed with a validated German questionnaire (‘Mehrdimensionaler Befindlichkeitsfragebogen’, MDBF), designed to estimate state emotions (Steyer et al., 1997). The MDBF has 12 items and three subscales quantifying current mood, alertness and calmness, similar to the Profile of Mood States (McNair et al., 1971). In addition, trait and state anxiety was assessed with the State-Trait-Anxiety Inventory (state version: STAI-S; trait version: STAI-T) (Laux, 1981). STAI-T was recorded during screening process. MDBF and STAI-S were assessed at baseline (−0.25 h) and after administration of LPS and saline (3, 6 h) outside the scanner.
Blood cell counts and cytokine analyses
Total leukocyte numbers and a three-part white blood cell differential count were measured in ethylenediaminetetraacetic acid-treated blood samples using an automated hematology analyzer (KX-21N, Sysmex Deutschland GmbH, Norderstedt, Germany). Plasma for the measurement of cytokine levels was separated by centrifugation and stored at −80°C until analysis. Concentrations of plasma cytokines were analyzed using multiplexed bead-based assays (Bio-Plex Cytokine Assays, Bio-Rad Laboratories GmbH, Munich, Germany), an increasingly used technique alternative to the common sandwich enzyme-linked immunosorbent assay, which is based on the same principle of measurement but more suitable for screening of high numbers of samples with a wide concentration range (Khan et al., 2004; Elshal and McCoy, 2006; Ng et al., 2010). Briefly, plasma dilutions were incubated in duplicates with fluorescence-labelled beads that are coupled to monoclonal antibodies against human IL-6, IL-10, TNF-α and IL-1ra. On incubation with the detection antibodies against these cytokines, samples were incubated with streptavidin-PE (Becton Dickinson, Heidelberg, Germany). At least 100 beads per sample were analyzed on a flow cytometer (FACSCanto II, Becton Dickinson, Heidelberg, Germany). We have validated this technique in-house with commercially available high-sensitive enzyme-linked immunosorbent assay kits (R&D systems, Minneapolis, USA) and observed high linear correlations (r > 0.9; P < 0.001) between both methods. Absolute cytokine levels were calculated based on the mean fluorescence intensity of cytokine standard dilutions using a four-parameter logistic model (GraphPad Prism 5, La Jolla, CA, USA). Detection limits of the assays were 0.2 pg/ml (IL-6), 0.4 pg/ml (IL-10), 3 pg/ml (TNF-α) and 39.9 pg/ml (IL-1ra).
Statistical analysis (non-fMRI data)
Statistical analyses were performed using a standard statistical software program (SPSS 17; Inc., Chicago, IL). Absolute changes in immunological, physiological and psychological variables after endotoxin or placebo administration were evaluated by an analysis of variance for repeated measures designs. Only significant interactions (time × treatment) are presented, unless stated otherwise, and these were followed by paired t-tests comparing endotoxin vs saline at specific time points. The social cognition (ToM) performance assessed with the ‘Eyes’ test was calculated using a paired t-test. The alpha level was set at 0.05. All data are presented as mean and standard error of the mean (SEM), unless indicated otherwise.
RESULTS
Immunological, physiological and mood responses
Endotoxin administration induced a pronounced inflammatory response reflected by transient increases in pro- and anti-inflammatory cytokines, circulating neutrophils and body temperature (Table 1) (Kullmann et al., 2013). Plasma concentrations of the pro-inflammatory cytokines IL-6 (F = 30.7, P < 0.001) and TNF-α (F = 13.62, P < 0.001) significantly increased after LPS administration compared with saline. These concentrations are similar to previously reported studies (Reichenberg et al., 2001; Eisenberger et al., 2010b) using 0.8 ng/kg endotoxin. In addition, LPS injection resulted in significantly elevated plasma levels of the anti-inflammatory cytokine IL-10 (F = 25.65, P < 0.001) and the soluble IL-1 receptor antagonist IL-1ra (F = 21.01, P < 0.001).
Table 1.
Immunological, physiological and psychological responses to endotoxin
| Saline | LPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | −0.25 | 1.75 | 3 | 6 | 24 | −0.25 | 1.75 | 3 | 6 | 24 |
| IL-6 (pg/ml) | 2.13 | 2.71 | 5.74 | 6.35 | 2.77 | 2.29 | 144.25*** | 126.95*** | 9.70 | 2.72 |
| TNF-α (pg/ml) | 19.17 | 20.06 | 17.75 | 15.48 | 11.08 | 21.03 | 71.12** | 27.22 | 21.66 | 13.73 |
| IL-10 (pg/ml) | 0.79 | 0.90 | 0.81 | 0.95 | 0.96 | 0.89 | 23.94*** | 18.88*** | 1.85 | 0.98 |
| IL-1ra (ng/ml) | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 5.59*** | 0.70 | 0.07 |
| Neutrophil counts (×103/μl blood) | 3.78 | 4.58 | 4.38 | 4.24 | 3.54 | 3.66 | 5.38 | 10.49*** | 8.56*** | 2.91 |
| Body temperature (°C) | 36.70 | 36.68 | 36.86 | 36.73 | 36.88 | 36.78 | 37.22*** | 37.68*** | 37.03* | 36.79 |
| Positive mood index | 17.67 | 17.56 | 18.00 | 18.00 | 15.50** | 17.12* | ||||
| Alertness index | 15.61 | 15.33 | 14.83 | 15.83 | 12.50** | 14.00 | ||||
| Calmness index | 16.83 | 17.67 | 18.00 | 17.00 | 15.61** | 17.41 | ||||
| State anxiety index | 32.50 | 31.72 | 31.17 | 30.94 | 35.44* | 31.50 | ||||
Temporal changes of plasma cytokine levels, numbers of circulating neutrophils, body temperature and results of the modified German version of the Profile of Mood States (MDBF) and the State Anxiety (STAI-S) questionnaires in healthy male subjects (n = 18) after bolus administration of 0.4 ng/kg endotoxin (LPS) or placebo (saline). Data are shown as mean.
Significant differences between treatments: *P < 0.05, **P < 0.01, ***P < 0.001.
For more details see Kullmann et al. (2013).
The LPS condition was also characterized by a rapid and profound increase in the number of circulating neutrophils, peaking 3 h post-injection (F = 90.55, P < 0.001), and by a slight but significant increase in body temperature, with a maximum of 37.7 ± 0.08°C (vs 36.8 ± 0.07°C in the saline condition) at 3 h post-injection (F = 16, P < 0.001).
LPS application significantly affected mood 3 h post-injection; examination of the MDBF subscales showed impaired mood (F = 8.11, P < 0.01), alertness (F = 3.77, P < 0.05) and calmness (F = 9.56, P < 0.01) and increased state-anxiety 3 h after LPS administration (F = 4.73, P < 0.05) (Table 1).
Social cognition (ToM) performance
To test whether and to what extent LPS-induced immune activation would affect social cognition (ToM) performance, subjects performed the ‘Eyes’ test. Analysis revealed no significant differences between the LPS and saline conditions in the number of correct responses during ToM judgment (Figure 1B) as well as during control gender judgment (LPSToM: 11.47 ± 0.51 vs salineToM: 11.18 ± 0.61; LPSgender: 16.35 ± 0.31 vs salinegender: 16.71 ± 0.38; mean correct responses ± SEM).
Neural responses during the social cognition (ToM) task
To confirm previous neuroimaging findings with regard to brain areas activated during non-story-based ToM tasks including the ‘Eyes’ test (Mar, 2011), we initially conducted a one-sample t-test within the placebo condition. As expected, the ToM > gender contrast activated the following ROIs: right and left dorsomedial prefrontal cortices (BA8/9), left fusiform gyrus, right and left superior temporal gyri (BA38/22/39/42) as well as the left temporo-parietal junction (BA22) and left precuneus (BA7) (P < 0.05 based on ROI analysis using SVC with FWE correction; Table 2). Medial prefrontal cortex (BA10) revealed no significant activation. Exploratory whole-brain analysis revealed greater activation of the right cingulate gyrus (BA32), right insula, left postcentral gyrus (BA2), left lingual gyrus (BA18), right and left inferior frontal gyri (BA46), right and left middle frontal gyri (BA6), right and left middle temporal gyri and right inferior occipital gyrus for ToM > gender during placebo condition (P < 0.001, uncorrected; Table 2).
Table 2.
Neural activation during the social cognition (ToM) task assessed with the ‘Eyes’ test within the placebo condition, one-sample t-test
| Brain regions | MNI coordinates for ToM > gender, placebo condition |
|||||
|---|---|---|---|---|---|---|
| H | x | y | z | BA | t-value | |
| Cingulate gyrus | R | 10 | 24 | 34 | 32 | 10.54 |
| Fusiform gyrus | L | −24 | −92 | −18 | 6.52* | |
| Insula | R | 34 | 24 | 2 | 9.09 | |
| Postcentral gyrus | L | −54 | −24 | 54 | 2 | 11.07 |
| Lingual gyrus | L | −20 | −102 | −12 | 18 | 13.27 |
| Dorsomedial prefrontal cortex | R | 10 | 26 | 34 | 9 | 9.75* |
| Dorsomedial prefrontal cortex | R | 6 | 16 | 48 | 8 | 6.49* |
| Dorsomedial prefrontal cortex | R | 4 | 26 | 50 | 8 | 6.38* |
| Dorsomedial prefrontal cortex | L | −6 | 16 | 48 | 8 | 7.45* |
| Dorsomedial prefrontal cortex | L | −6 | 28 | 44 | 8 | 6.41* |
| Dorsomedial prefrontal cortex | L | −10 | 24 | 44 | 8 | 5.61* |
| Inferior frontal gyrus | R | 30 | 22 | −12 | 6.73 | |
| Inferior frontal gyrus | R | 58 | 32 | 12 | 46 | 6.32 |
| Inferior frontal gyrus | L | −54 | 30 | 0 | 14.93 | |
| Middle frontal gyrus | R | 34 | −6 | 56 | 6 | 5.91 |
| Middle frontal gyrus | R | 30 | 0 | 48 | 5.31 | |
| Middle frontal gyrus | R | 44 | −4 | 60 | 3.96 | |
| Middle frontal gyrus | L | −44 | 20 | 32 | 11.82 | |
| Middle temporal gyrus | R | 50 | −36 | −2 | 5.72 | |
| Middle temporal gyrus | L | −54 | −46 | 4 | 21 | 10.66 |
| Superior temporal gyrus | R | 44 | 16 | −24 | 38 | 6.10* |
| Superior temporal gyrus | R | 50 | −30 | 0 | 22 | 5.12* |
| Temporo-parietal junction | L | −56 | −44 | 4 | 22 | 9.53* |
| Superior temporal gyrus | L | −60 | −42 | 20 | 22 | 5.57* |
| Superior temporal gyrus | L | −54 | −54 | 10 | 39 | 6.35* |
| Superior temporal gyrus | L | −60 | −36 | 20 | 42 | 4.07* |
| Inferior occipital gyrus | R | 30 | −92 | −10 | 8.19 | |
| Precuneus | L | −26 | −62 | 50 | 7 | 6.86* |
H, hemisphere with activation; R, right asymmetrical activation; L, left asymmetrical activation; BA, Brodmann areas.
One-sample t-test for ToM > gender during placebo condition (n = 18). All P < 0.05 based on ROI analysis using SVC with FWE correction (*) or whole-brain statistics at P < 0.001 uncorrected.
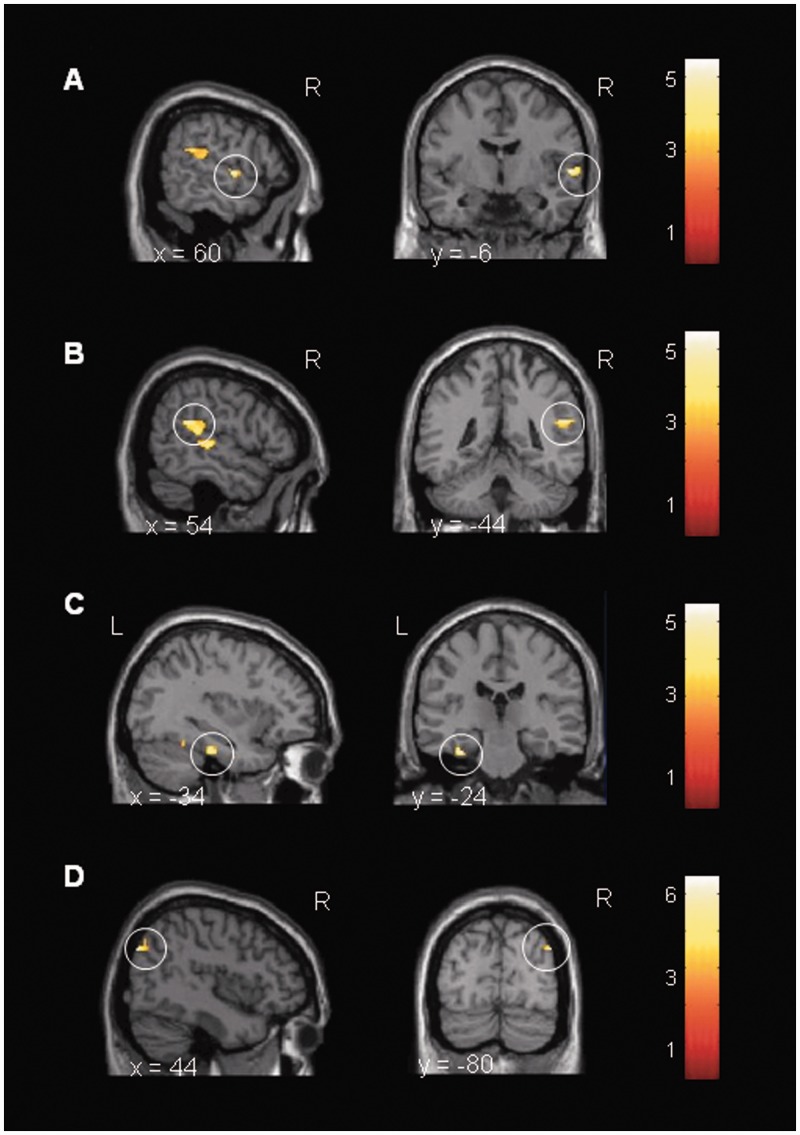
Subsequently, the effects of endotoxin on ToM task-related brain activation in specific ROIs were analyzed by the LPSToM>gender > placeboToM>gender contrast. Results demonstrated an enhanced activation of the right superior temporal gyri (BA22), right temporo-parietal junction, left fusiform gyrus and right precuneus (P < 0.05 based on ROI analysis using SVC with FWE correction, Figure 2, Table 3), regions known to be involved in the ability to understand the mental states of others. ROI analysis for medial prefrontal cortices did not reveal any significant activation. Moreover, exploratory whole-brain analysis revealed greater activation of the right lingual gyrus, right insula, right parahippocampal gyrus (BA27), left cingulate gyrus (BA24), right medial prefrontal cortex (BA10), right and left middle temporal gyri (BA19/39) and right superior temporal gyrus (BA41), right claustrum, right and left pre- and postcentral gyri (BA6, BA3/7), right inferior parietal lobule, right middle occipital gyrus and right and left cunei (BA18/30) in the LPSToM>gender > placeboToM>gender contrast (P < 0.001, uncorrected; Table 3). The placeboToM>gender > LPSToM>gender contrast within the ROIs and whole-brain analysis revealed no significant activations.
Fig. 2.
Cortical activation during the social cognition (ToM) task assessed with the ‘Eyes’ test. Paired t-test computed for the LPSToM>gender > placeboToM>gender condition. LPS-treated subjects displayed a stronger activation in (A) superior temporal gyrus [(60, −6, 2), t = 5.18], (B) temporo-parietal junction [(54, −44, 22), t = 5.26], (C) fusiform gyrus [(−34, −24, −26), t = 5.47] and (D) precuneus [(44, −80, 34), t = 6.57] after administration of 0.4 ng/kg E. coli endotoxin (n = 18). All data based on ROI analysis using SVC with FWE correction. The images are presented using a threshold at P < 0.01 uncorrected for visualization purposes.
Table 3.
Neural activation during the social cognition (ToM) task assessed with the ‘Eyes’ test within the LPS > placebo contrast, paired t-test
| Brain regions | MNI coordinates for LPSToM>gender > placeboToM>gender condition |
|||||
|---|---|---|---|---|---|---|
| H | x | y | z | BA | t-value | |
| Fusiform gyrus | L | −34 | −24 | −26 | 5.47* | |
| Lingual gyrus | R | 12 | −84 | 0 | 3.78 | |
| Insula | R | 40 | −4 | −6 | 4.15 | |
| Parahippocampal gyrus | R | 24 | −34 | −4 | 27 | 4.22 |
| Cingulate gyrus | L | −4 | 2 | 46 | 24 | 3.69 |
| Medial prefrontal cortex | R | 6 | 60 | −8 | 10 | 4.01 |
| Superior frontal gyrus | R | 30 | 40 | 38 | 3.90 | |
| Middle temporal gyrus | R | 44 | −84 | 20 | 19 | 4.62 |
| Middle temporal gyrus | L | −46 | −84 | 20 | 39 | 3.82 |
| Temporo-parietal junction | R | 54 | −44 | 22 | 5.26* | |
| Superior temporal gyrus | R | 60 | −6 | 2 | 22 | 5.18* |
| Superior temporal gyrus | R | 52 | −6 | 4 | 4.51 | |
| Superior temporal gyrus | R | 52 | −34 | 16 | 41 | 3.93 |
| Claustrum | R | 34 | −8 | −8 | 3.72 | |
| Precentral gyrus | R | 44 | −8 | 36 | 6 | 3.90 |
| Precentral gyrus | L | −50 | −5 | 53 | 6 | 3.69 |
| Postcentral gyrus | R | 8 | −52 | 72 | 7 | 3.86 |
| Postcentral gyrus | L | −38 | −24 | 46 | 3 | 4.27 |
| Inferior parietal lobule | R | 52 | −44 | 28 | 4.79 | |
| Middle occipital gyrus | R | 42 | −82 | 32 | 19 | 6.62 |
| Precuneus | R | 44 | −80 | 34 | 6.57* | |
| Cuneus | R | 10 | −60 | 6 | 30 | 3.89 |
| Cuneus | R | 2 | −84 | 16 | 18 | 4.06 |
| Cuneus | L | −2 | −82 | 16 | 18 | 3.78 |
| Cuneus | L | −12 | −100 | 2 | 3.79 | |
| Cuneus | L | −10 | −78 | 20 | 18 | 3.76 |
H, hemisphere with activation; R, right asymmetrical activation; L, left asymmetrical activation; BA, Brodmann areas.
Paired t-test for LPSToM>gender > placeboToM>gender (n = 18). The opposite contrast revealed no significant activations. All P < 0.05 based on ROI analysis using SVC with FWE correction (*) or whole-brain statistics at P < 0.001 uncorrected.
Changes in state anxiety did not affect brain activity in ROIs, including left fusiform gyrus [(−34, −24, −26), t = 5.37, P < 0.05], right superior temporal gyrus [(60, −6, 2), t = 5.63, P < 0.05) and right precuneus [(44, −80, 34), t = 6.58, P < 0.05), except for the right temporo-parietal junction [(54, −44, 22), t = 4.67, P > 0.05] (all based on ROI analysis using SVC with FWE correction). Further analysis revealed that changes in alertness did not affect activation of left fusiform gyrus [(−34, −26, −26), t = 5.91, P < 0.05] and right superior temporal gyrus [(60, −6, 2), t = 4.87, P < 0.05), whereas right precuneus [(44, −80, 34), t = 5.14, P > 0.05) and right temporo-parietal junction [(54, −44, 22), t = 4.29, P > 0.05] were affected by changes in alertness (all based on ROI analysis using SVC with FWE correction).
DISCUSSION
To analyze the effects of acute endotoxin-induced peripheral inflammation on social cognition (ToM) task performance and corresponding neural responses, healthy male subjects received an injection of endotoxin (LPS, E. coli) or saline in a balanced, randomized, double-blind crossover design. LPS administration expectedly induced a transient systemic inflammation along with mood impairment, consistent with previous findings (Reichenberg et al., 2001; Eisenberger et al., 2010b; Grigoleit et al., 2011). The behavioral analysis revealed that the inflammatory response had no discernable effect on ToM task performance, indicating that the ability to infer others’ states of mind and to predict how others feel, think and behave is not impaired during an acute inflammatory response in healthy male subjects. However, in the LPS condition, BOLD responses in regions mediating ToM ability (i.e. superior temporal gyrus, temporo-parietal junction) were significantly enhanced. This could be indicative either of a centrally mediated compensatory strategy or of enhanced social cognitive processing as a function of sickness, consistent with previous evidence (Eisenberger et al., 2009).
From an evolutionary viewpoint, our finding that ToM task performance was not affected during an inflammatory response makes sense. The ability to reliably and consistently perceive complex social information expressed through the eyes of others facilitates the identification of potential danger on the one hand and ensures adequate social communication and prediction of others’ behaviors on the other hand. In fact, one could have also expected increased ToM ability during low levels of sickness. Either way, the interpretation of our negative finding with regard to ToM task performance remains speculative. Alternative explanations, including suboptimal sensitivity of the ‘Eyes’ test, should also be considered, and future studies may consider the need to find more sensitive methods to assess different aspects of social cognition performance during endotoxemia.
In contrast to the behavioral results, analysis of BOLD responses with respect to ToM task-related neural activation within specific ROIs revealed several significant differences between LPS and placebo. ToM task-related neural activation was significantly enhanced in the LPS condition in left fusiform gyrus, right temporo-parietal junction, right superior temporal gyrus and right precuneus, as well as (albeit at a more liberal statistical threshold) medial prefrontal cortex (BA10). These effects are interesting, given previous evidence that the superior temporal gyrus and temporo-parietal junction mediate ToM ability (Abu-Akel, 2003; Saxe and Kanwisher, 2003; Carrington and Bailey, 2009; Mar, 2011). Especially the superior temporal gyrus is reportedly involved in perceiving eyes and their emotional expressions to extract information about goals and outcomes of future behavior of others (Allison et al., 2000; Narumoto et al., 2001; Carrington and Bailey, 2009). Furthermore, the medial prefrontal cortex is known to be involved in ‘mentalizing’, a concept to describe the understanding of others’ mental states (Frith and Frith, 2003, 1999). In addition, the fusiform gyrus is relevant for the processing of facial information related to the social significance of direct gaze (George and Conty, 2008), and the precuneus reportedly mediates imagination processes required to infer the mental states of others (Mar, 2011). In the absence of LPS effects on ToM task performance, the enhanced BOLD response in ToM-relevant brain regions may reflect an enhanced processing effort during the social cognition (ToM) task, which may constitute a compensatory strategy of the central nervous system (CNS) to maintain normal social cognition performance during states of inflammation. Alternatively, a greater task-related neural response in the LPS condition may reflect a greater social cognitive processing as a function of (or adaptation to) sickness. The interpretation of a compensatory strategy of the brain is supported by a study that discussed the results of increased brain activity in IFN-α-treated patients as a compensatory CNS effect to maintain task performance (Capuron et al., 2005). Unfortunately, to the best of our knowledge, there exists no direct test of our ‘compensatory’ interpretation. The alternative idea of a greater social cognition-related neural processing could demonstrate an adaptive response to low-level sickness. This is also supported by findings of a study by Eisenberger et al. (2009) that found that larger increases in IL-6 levels were correlated with greater neural activity in ToM-associated brain regions during a social pain-related task after LPS application. Both possibilities of interpretation are also consistent with an evolutionary perspective of improved chances of survival in the event of own illness or injury involving inflammation-induced changes in mood (Hart, 1988; Dantzer and Kelley, 2007).
Furthermore, it is possible that the observed neural alterations during the social cognition task are not specific for social cognition, which would also fit with the absence of behavioral results. Indeed, the processing of social stimuli also includes emotional aspects of social cognition. Thus, alterations in neural activation during LPS could reflect a more generalized effect of the acute inflammation on different aspects of neural processing including not only social but also emotional and cognitive functions. This notion is supported by another finding from our group documenting that endotoxin-induced immune activation increased sensitivity to visceral pain stimuli (Benson et al., 2012) a response that encompasses cognitive as well as emotional components.
Clearly, further studies are needed to elucidate the effects of potential sex differences on social cognition tasks during an acute inflammation. Therefore, female subjects should be included in future investigations. Finally, as previously shown by our group (Grigoleit et al., 2011), higher doses of LPS lead to greater physiological responses, including increased pro-inflammatory cytokine release, and dose-dependent effects on mood and neuropsychological functions such as learning and memory. Therefore, future studies should also elucidate putative dose-dependent effects of LPS on social cognition performance.
The rationale and putative clinical background for our study was derived from previous evidence that neuropsychiatric patients demonstrate impaired ToM task performance (Frith and Corcoran, 1996; Kerr et al., 2003; Inoue et al., 2004; Wang et al., 2008) on the one hand and alterations in pro-inflammatory peripheral cytokines (Irwin and Miller, 2007; Drexhage et al., 2010; Meyer et al., 2009; Miller et al., 2009) on the other hand. Additionally, treatment with cytokines such as IFN-α induces depressive-like symptoms (Raison et al., 2005, 2009). However, the functional link between peripheral immune activation and social behavior is largely unknown. So far, only one previous study in healthy subjects revealed increased self-reported feelings of social disconnection along with the well-known effects on mood after administration of a higher endotoxin dose (i.e. 0.8 ng/kg E. coli endotoxin) (Eisenberger et al., 2010b). However, no aspects of social behavior and/or social cognition task performance were assessed in this study (Eisenberger et al., 2010b).
With respect to brain imaging findings in neuropsychiatric patients, altered neural responses in schizophrenic patients during ToM tasks have been reported (Benedetti et al., 2009; Brune et al., 2011; Walter et al., 2011), whereas no such study exists in patients with depression. The only other existing report in healthy subjects found that larger increases in IL-6 levels were correlated with greater neural activity in ToM-associated brain regions during a social pain-related task after LPS application, which is broadly consistent with our findings (Eisenberger et al., 2009). Based on our results, it could be hypothesized that the observed deficits in higher-order social cognition including ToM ability in neuropsychiatric patients are not caused by pro-inflammatory cytokines. However, endotoxin-induced inflammation in healthy subjects is of acute and transient nature. In contrast, depressed patients have less pronounced but chronic elevations in pro-inflammatory cytokines (Raison et al., 2006; Himmerich et al., 2008; Dinan et al., 2009). Thus, it may well be that much less pronounced but chronically increased cytokine levels affect social cognition. However, this has never been directly investigated thus far. Despite these obvious differences in nature, extent and duration of inflammatory responses, it has been proposed that endotoxin administration constitutes a model for certain aspects of human depression. This notion is primarily based on the significant mood changes that are reliably induced with endotoxin models, and that correlate with pro-inflammatory cytokine response (Reichenberg et al., 2001; Wright et al., 2005). However, the validity of this experimental model for human depression has also been prominently questioned (DellaGioia and Hannestad, 2010), which is further supported by our findings.
In summary, our findings document that the social ability to predict how others feel, think and behave is not impaired during an acute inflammatory response in healthy male subjects. At the same time, ToM performance during endotoxemia is associated with increased activation in relevant brain regions, which could indicate an enhanced processing effort and/or a compensatory strategy at the level of the CNS. Clearly, further studies are required to elucidate the effects of peripheral immune activation on social cognition in both healthy subjects and patients with neuropsychiatric diseases.
Acknowledgments
The authors thank Christina Banner, Anne Winkelhaus, Christina Rosenberger, Philipp Lichte, Philipp Kobbe and Armin de Greiff for their technical assistance and friendly help. This work was supported by a grant from the German Research Foundation [Sche 341/14-1].
REFERENCES
- Abu-Akel A. A neurobiological mapping of theory of mind. Brain Research Reviews. 2003;43:29–40. doi: 10.1016/s0165-0173(03)00190-5. [DOI] [PubMed] [Google Scholar]
- Ader R. Psychoneuroimmunology. 4th edn. Amsterdam; Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Bahador M, Cross AS. From therapy to experimental model: a hundred years of endotoxin administration to human subjects. Journal of Endotoxin Research. 2007;13:251–79. doi: 10.1177/0968051907085986. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:241–51. [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research. 2009;114:154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Benson S, Kattoor J, Wegner A, et al. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153:794–9. doi: 10.1016/j.pain.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Bölte S. Reading Mind in the Eyes Test für Erwachsene. Frankfurt am Main; 2005. http://www.klinik.uni-frankfurt.de/zpsy/kinderpsychiatrie/Downloads/Eyes_test_erw.pdf. [Google Scholar]
- Brune M. Social cognition and psychopathology in an evolutionary perspective. Current status and proposals for research. Psychopathology. 2001;34:85–94. doi: 10.1159/000049286. [DOI] [PubMed] [Google Scholar]
- Brune M, Ozgurdal S, Ansorge N, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011;55:329–37. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. 2008;63:1022–9. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biological Psychiatry. 2004;56:819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological Psychiatry. 2005;58:190–6. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30:2313–35. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurologic Clinics. 2006;24:441–60. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior and Immunity. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neuroscience and Biobehavioral Reviews. 2010;34:130–43. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T, Siggins L, Scully P, O'Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. Journal of Psychiatric Research. 2009;43:471–6. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Doody GA, Gotz M, Johnstone EC, Frith CD, Owens DG. Theory of mind and psychoses. Psychological Medicine. 1998;28:397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Review of Neurotherapeutics. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010a;68:748–54. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior and Immunity. 2010b;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Corcoran R. Exploring ‘theory of mind' in people with schizophrenia. Psychological Medicine. 1996;26:521–30. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Conty L. Facing the gaze of others. Neurophysiologie Clinique. 2008;38:197–207. doi: 10.1016/j.neucli.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Grigoleit J-S, Kullmann JS, Wolf OT, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE. 2011;6:e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit JS, Oberbeck JR, Lichte P, et al. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiology of Learning and Memory. 2010;94:561–7. doi: 10.1016/j.nlm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009a;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biological Psychiatry. 2009b;66:415–22. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews. 1988;12:123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, et al. Depression, comorbidities and the TNF-alpha system. European Psychiatry. 2008;23:421–9. doi: 10.1016/j.eurpsy.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Tonooka Y, Yamada K, Kanba S. Deficiency of theory of mind in patients with remitted mood disorder. Journal of Affective Disorders. 2004;82:403–9. doi: 10.1016/j.jad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior and Immunity. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kerr N, Dunbar RI, Bentall RP. Theory of mind deficits in bipolar affective disorder. Journal of Affective Disorders. 2003;73:253–9. doi: 10.1016/s0165-0327(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry. Part B, Clinical Cytometry. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in Neurosciences. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Lichte P, et al. Neural response to emotional stimuli during experimental human endotoxemia. Human Brain Mapping. 2013;34(9):2217–27. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, editors. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain, Behavior and Immunity. 2001;15:371–87. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD. State-Trait-Angstinventar (STAI) Weinheim: Beltz; 1981. [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annual Review of Psychology. 2011;62:103–34. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Doppelman LF. EITS Manual for Profile of Mood States. San Diego, CA: EdITS; 1971. [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophrenia Bulletin. 2009;35:959–72. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Research. Cognitive Brain Research. 2001;12:225–31. doi: 10.1016/s0926-6410(01)00053-2. [DOI] [PubMed] [Google Scholar]
- Ng AH, Uddayasankar U, Wheeler AR. Immunoassays in microfluidic systems. Analytical and Bioanalytical Chemistry. 2010;397:991–1007. doi: 10.1007/s00216-010-3678-8. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Chimpanzee problem-solving: a test for comprehension. Science. 1978;202:532–5. doi: 10.1126/science.705342. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biological Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annual Review of Psychology. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind". Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M, editors. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF) Göttingen: Hogrefe-Verlag; 1997. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Boogaard M, Ramakers BP, van Alfen N, et al. Endotoxemia-induced inflammation and the effect on the human brain. Critical Care. 2010;14:R81. doi: 10.1186/cc9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Schnell K, Erk S, et al. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Molecular Psychiatry. 2011;16:462–70. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
- Wang YG, Wang YQ, Chen SL, Zhu CY, Wang K. Theory of mind disability in major depression with or without psychotic symptoms: a componential view. Psychiatry Research. 2008;161:153–61. doi: 10.1016/j.psychres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain, Behavior and Immunity. 2007;21:807–15. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain, Behavior, and Immunity. 2005;19:345–50. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]