Abstract
Psychopathy is a personality disorder associated with callous and impulsive behavior and criminal recidivism. It has long been theorized that psychopaths have deficits in processing reward and punishment. Here, we use structural and functional magnetic resonance imaging to examine the neural correlates of reward and loss sensitivity in a group of criminal psychopaths. Forty-one adult male prison inmates (n = 18 psychopaths and n = 23 non-psychopaths) completed a functional magnetic resonance imaging task involving the gain or loss of money. Across the entire sample of participants, monetary gains elicited robust activation within the ventral striatum (VS). Although psychopaths and non-psychopaths did not significantly differ with respect to overall levels of VS response to reward vs loss, we observed significantly different correlations between VS responses and psychopathy severity within each group. Volumetric analyses of striatal subregions revealed a similar pattern of correlations, specifically for the right accumbens area within VS. In a separate sample of inmates (n = 93 psychopaths and n = 117 non-psychopaths) who completed a self-report measure of appetitive motivation, we again found that the correlation with psychopathy severity differed between groups. These convergent results offer novel insight into the neural substrates of reward and loss processing in psychopathy.
Keywords: psychopathy, reward, striatum, prison, fMRI
INTRODUCTION
Psychopathy is a mental health disorder characterized by callous and impulsive antisocial behavior. Present in roughly a quarter of adult prison inmates, psychopathy is associated with a disproportionately high incidence of violent crime, substance abuse and recidivism (Smith and Newman, 1990; Hare, 2003). Based on these personality and behavioral characteristics, it has long been postulated that psychopathy may be linked to abnormalities in processing reward and punishment (Cleckley, 1941; Lykken, 1957; Fowles, 1980; Gorenstein and Newman, 1980; Blair, 2008). Over several decades, a host of behavioral and psychophysiological studies have offered qualified support for this theory (Lykken, 1957; Schmauk, 1970; Newman et al., 1985; Arnett et al., 1997; Baskin-Sommers et al., 2010). More recently, functional brain imaging has been used to address this question at the neural-systems level (Buckholtz et al., 2010; Bjork et al., 2012). These functional magnetic resonance imaging (fMRI) studies have focused primarily on the ventral striatum (VS), a major subcortical target of mesolimbic dopamine neurons, which have been shown to signal the receipt and prediction of pleasurable rewarding stimuli (Schultz et al., 1997; Drevets et al., 2001; Schultz, 2010). Human functional imaging studies have reliably demonstrated VS activation in response to innately pleasurable stimuli, as well as to abstract stimuli predicting their occurrence (McClure et al., 2004; O'Doherty, 2004). Two recent fMRI studies of reward processing and psychopathy have associated certain psychopathic personality characteristics with heightened VS activity during the anticipation of monetary gain (Buckholtz et al., 2010; Bjork et al., 2012). These intriguing initial results raise a number of important questions. Although these findings associate psychopathy with hypersensitive neural responses in anticipation of monetary gain, to date, no study has examined the relationship between psychopathy and neural responses related to monetary loss. Moreover, both of the aforementioned reward-processing studies were conducted with non-forensic community participants, among whom few (if any) would meet criteria for the categorical diagnosis of psychopathy as defined for pathologically antisocial and criminal individuals (Hare, 2003). Although there are ample clinical and behavioral data suggesting that psychopathic traits fall along a continuum—with psychopaths representing a quantitatively greater manifestation of the traits rather than a qualitatively distinct category (Marcus et al., 2004; Edens et al., 2006; Walters et al., 2007, 2008)—there is not yet strong evidence to support the assumption that the neurobiological correlates of the disorder are similarly continuous (Koenigs et al., 2011). In other words, it may be the case that VS reward activity correlates with certain social and affective personality traits among individuals with overall low levels of psychopathy, as has been previously reported (Buckholtz et al., 2010; Bjork et al., 2012), but among actual psychopaths, the relationship between psychopathic trait severity and VS reward-related activity may be notably different.
This study will thus address two distinct but related questions on the neural basis of psychopathy: (i) Do psychopaths have significantly altered reward and/or loss sensitivity in VS? (ii) Is the relationship between psychopathy severity and VS reward/loss sensitivity consistent across the entire spectrum of psychopathy severity, or does the relationship differ depending on whether one exhibits low or high levels of psychopathic traits?
METHODS
Participants—Magnetic Resonance Imaging study
Participants were adult male inmates recruited from a medium-security Wisconsin correctional institution. Inmates were eligible if they met the following criteria: <45 years of age, IQ >70, no history of psychosis or bipolar disorder, no history of significant head injury or post-concussion symptoms and not currently taking psychotropic medications. Nine subjects (n = 6 non-psychopaths and n = 3 psychopaths) were excluded owing to a lack of button responses during the task (instruction non-compliance; see ‘fMRI task’ later in the text), leaving a final sample of 41 inmates (n = 18 psychopaths and n = 23 non-psychopaths).
The Psychopathy Checklist-Revised (PCL-R) (Hare, 2003) was used to assess psychopathy. The PCL-R assessment involves a 60- to 90-min interview and file review to obtain information used to rate 20 psychopathy-related items as 0, 1 or 2. Participants were assessed for substance use disorder with the Structured Clinical Interview for DSM-IV Disorders (First et al., 2002) (Table 1).
Table 1.
Participant group characteristics
| Variable | Non-psychopaths (n = 23) | Psychopaths (n = 18) | P |
|---|---|---|---|
| Demographic | |||
| Age | 32.4 (8.0) | 32.2 (6.5) | 0.94 |
| Race (Caucasian/African American) | 21/2 | 13/5 | 0.21 |
| Neuropsychological | |||
| IQa | 100.7 (11.9) | 100.1 (11.2) | 0.87 |
| Digit span back | 6.9 (2.7) | 6.5 (3.3) | 0.69 |
| Anxiety/Negative affectb | 10.7 (8.1) | 13.3 (9.0) | 0.33 |
| Psychopathy | |||
| PCL-R total | 14.1 (3.5) | 31.7 (1.7) | <0.001 |
| Factor 1 | 4.8 (2.2) | 11.7 (1.8) | <0.001 |
| Factor 2 | 7.3 (3.3) | 17.2 (1.4) | <0.001 |
| Substance abusec | |||
| Alcohol | |||
| Prevalence | 10/23 | 9/18 | 0.76 |
| Age of onset | 21.5 (3.3) | 18.6 (2.0) | |
| Cannabis | |||
| Prevalence | 6/23 | 8/18 | 0.32 |
| Age of onset | 19.3 (4.5) | 19.5 (8.0) | |
| Cocaine | |||
| Prevalence | 4/23 | 6/18 | 0.29 |
| Age of onset | 20.5 (2.9) | 20.5 (5.3) | |
| Stimulants | |||
| Prevalence | 1/23 | 2/18 | 0.57 |
| Age of onset | 18 | 15,23 | |
| Opioids | |||
| Prevalence | 3/23 | 5/18 | 0.27 |
| Age of onset | 20.7 (5.1) | 18.8 (3.3) | |
| Sedatives | |||
| Prevalence | 1/23 | 2/18 | 0.57 |
| Age of onset | 27 | 20/22 | |
| Hallucinogens | |||
| Prevalence | 1/23 | 4/18 | 0.15 |
| Age of onset | 20 | 18.3 (2.8) |
abased on Shipley Institute of Living Scale (Zachary, 1986), bbased on Welsh Anxiety Scale, cbased on diagnosis of abuse or dependence in the Structured Clinical Interview for DSM-IV Disorders (SCID) (First, 2002).
P-values for race distribution and substance abuse prevalence were computed with Fisher exact test.
All other P-values are based on t-tests (means presented followed by standard deviations in parentheses).
P-values were not calculated for substance abuse age of onset owing to relatively small sample sizes of abusers for most substances.
Participant groups
Participants were recruited based on their PCL-R scores. Psychopathic inmates had PCL-R scores of ≥30, whereas non-psychopathic inmates had PCL-R scores of ≤20 (Hare, 2003). Group characteristics for the magnetic resonance imaging (MRI) study are presented in Table 1. The psychopathic and non-psychopathic groups did not significantly differ with respect to age, race or intelligence. Importantly, the groups also did not differ with respect to lifetime diagnosis of substance use disorder (abuse or dependence) for any of the following substances: alcohol, cannabis, cocaine, opioids, stimulants, sedatives or hallucinogens.
Participants—Follow-up Behavioral Activation System study
As a follow-up to the MRI results, we analyzed data from a separate group of inmates. These inmates are a subset of individuals who had all previously completed a self-report measure of Behavioral Inhibition System (BIS) and Behavioral Activation System (BAS) traits (Newman et al., 2005; Wallace et al., 2009). To mirror the group analysis scheme for the functional and structural MRI data, we analyzed BIS/BAS data from only those inmates who were classified as psychopaths (PCL-R ≥30; n = 93) or non-psychopaths (PCL-R ≤20; n = 117) in the previous studies. These adult Caucasian male inmates met the same eligibility criteria as the participants in the MRI study (<45 years of age, IQ >70, no history of psychosis or bipolar disorder, no history of significant head injury or post-concussion symptoms and not currently taking psychotropic medications). The BIS/BAS scale (Carver and White, 1994) is a 20-item questionnaire based on Gray’s reinforcement sensitivity theory (Gray, 1970). The BIS subscale (seven items) primarily assesses worry and anxiety, whereas the BAS subscale (13 items) measures sensitivity to anticipated/acquired rewards, motivation to achieve desired goals and willingness to approach new appetitive stimuli.
fMRI task
While in the scanner, participants completed a task involving the passive gain or loss of money. Each trial consisted of three phases (Supplementary Figure S1). The first phase (3 s) was a cue stimulus (one of five white shapes on a black background). The second phase (3 s) was a slot machine (one of six colored slot machines). The third phase (2 s) was an indication of monetary outcome (win $1, win $0 or lose $1). A fixation cross was shown during the inter-trial intervals (mean duration 4 s, range 2–6 s). A total of 76 trials were divided into two runs of 38 trials each. Each cue was associated with a fixed probability of being followed by each slot machine, and each slot machine was associated with a fixed probability of winning, losing or breaking even. Three of the slot machines delivered monetary gains (66% chance of winning $1), two of the slot machines always yielded $0 and one slot machine delivered monetary loss (66% chance of losing $1). All participants received the same predetermined order of cues, slot machines and monetary outcomes. To keep participants engaged during the task and allow us to monitor participants’ attention to the task, participants were instructed to indicate by a button press during the presentation of the cue which slot machine they thought was most likely to follow. Subjects who failed to respond to at least a third of all trials (within the 3-s window) were excluded from the final analysis. In the final subject sample, there was no significant between-group difference in the number of button responses (t = 0.50, P = 0.63). To heighten the psychological impact of gaining and losing money, the monetary outcome of one trial chosen randomly from the task was added or subtracted to the subject’s compensation for participating in the study.
MRI data collection
All MRI data were acquired using the Mind Research Network’s mobile Siemens 1.5 T Avanto MRI System on correctional facility grounds. Gradient echo T2*-weighted echoplanar images (EPIs) were acquired with the following parameters: TR = 2800 ms, TE = 39 ms, flip angle = 75°, FOV = 24 × 24 cm2, matrix = 64 × 64, slice thickness = 4.0 mm, gap = 1 mm, voxel size = 3.8 × 3.8 × 4.0 mm3, 38 interleaved axial oblique slices per volume and total of 240 volumes. A high-resolution T1-weighted structural image was acquired for each subject using a four-echo magnetization-prepared rapid gradient-echo sequence (TR = 2530 ms; TE = 1.64, 3.5, 5.36 and 7.22 ms; flip angle = 7°, FOV = 256 × 256 mm2, matrix = 128 × 128, slice thickness = 1.33 mm, no gap, voxel size = 1 × 1 × 1.33 mm3 and 128 interleaved sagittal slices). All four echoes were averaged into a single high-resolution image.
MRI data analysis
fMRI data analysis
All fMRI data analyses were performed using AFNI (Cox, 1996). EPI volumes were slice-time corrected using the fourth slice of the first session as a reference (interleaved ascending, Fourier interpolation) and motion corrected by rigid body alignment to the fourth EPI acquisition. The data were spatially smoothed with a 4-mm full-width at half-maximum Gaussian kernel. The averaged T1-weighted images were processed using FreeSurfer v5.0, as previously described (Fischl, 2012). EPI time series data and high-resolution T1 images skull-stripped in FreeSurfer were normalized to the MNI coordinate system using a 12-parameter linear warp. The time series of both runs were scaled and concatenated before being modeled with canonical gamma variate hemodynamic response functions time-locked to the onsets of monetary outcome stimuli, as well as to the onsets of the cue and slot stimuli. In addition to modeling these stimuli onsets as regressors of interest, residual head motion after volume correction was also entered into the model as a covariate of no interest. The resulting statistical maps were resampled to 3 mm cubic voxels and registered to the same coordinate space as the normalized T1 images for subsequent analyses.
To compare responses related to gains and losses, we performed a linear contrast between gain (+$1), loss (−$1) and neutral ($0) trials. Group differences were considered significant at a corrected P < 0.05 (cluster size >41 voxels at uncorrected P < 0.005). Cluster extents were computed using Monte Carlo simulations implemented in the 3dClustSim program (AFNI).
Measurement of striatal volumes
The averaged T1-weighted images were processed using FreeSurfer (Fischl, 2012). The FreeSurfer tissue segmentation includes volume measurements (in mm3) for four striatal subregions in each hemisphere. We computed correlations between PCL-R scores and volumes of the following striatal subregions: left and right putamen, left and right caudate, left and right accumbens area and left and right pallidum. The accumbens area most closely corresponds to the task-defined VS region-of-interest (ROI).
RESULTS
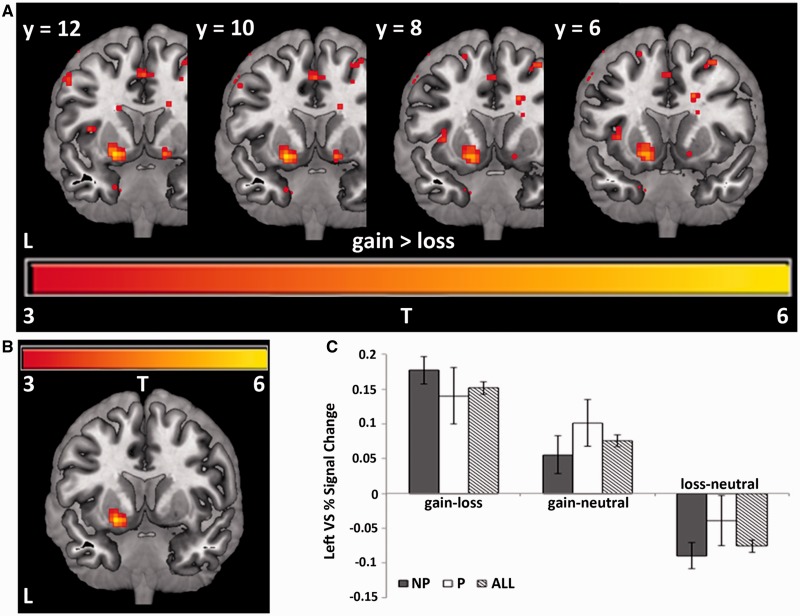
Here we address each of the two main study questions in turn. The first question is whether psychopaths exhibit significantly altered sensitivity to reward or loss in VS. To address this question, we examined BOLD activity in response to stimuli indicating monetary gain, loss and no change (neutral). Across the entire group, we observed greater activation bilaterally in the VS for gain relative to loss trials at P < 0.005 uncorrected (Figure 1A). Activation in the left VS remained significant after whole-brain correction for multiple comparisons (P < 0.05 corrected, t = 5.94, 48 voxels; Figure 1B and Supplementary Table S1). Activity in the left VS was greater for gain relative to neutral but lower for loss relative to neutral (Figure 1C). An outlier test (Grubbs’ test) revealed that one non-psychopathic subject was an outlier for the gain–loss contrast, and that a separate non-psychopathic subject was an outlier for the loss–neutral contrast. After the exclusion of these two subjects, we observed no significant difference in VS response magnitude between psychopaths and non-psychopaths for the gain–neutral contrast (t = 1.08, P = 0.29), loss–neutral contrast (t = 1.20, P = 0.20) or gain–loss contrast (t = 0.85, P = 0.40). These results indicate that psychopaths and non-psychopaths do not exhibit significant overall differences in reward- or loss-related activity in VS.
Fig. 1.
(A) Bilateral activation in VS in response to gains relative to losses, displayed at P < 0.005 uncorrected. (B) Activation in left VS in response to gains relative to losses, corrected for multiple comparisons. Peak activation at MNI coordinates: x = −21, y = 9, z = −9; 48 voxels, P < 0.005 uncorrected, α = 0.05. (C) Bar graph showing the average percent signal change across all 48 voxels of the task-activated region of left VS for non-psychopaths (NP), psychopaths (P) and across all subjects (All). Error bars indicate S.E.M.
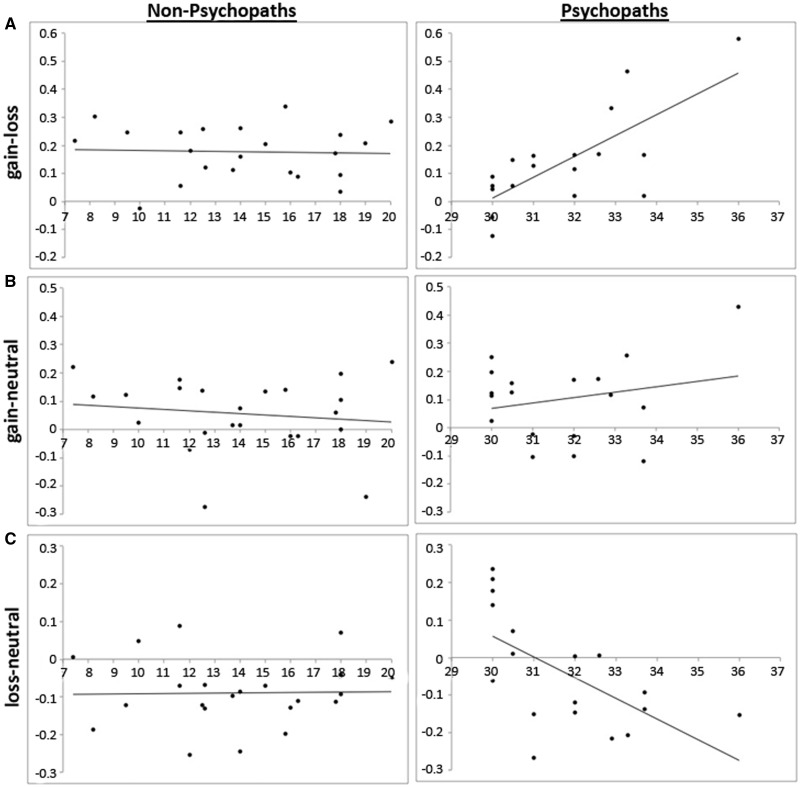
The second question is whether the relationship between psychopathy severity and VS reward/loss sensitivity is consistent across the entire spectrum of psychopathy severity, or if the relationship differs depending on whether one exhibits low or high levels of psychopathic traits. To address this question, we calculated the correlation between overall psychopathy severity (total PCL-R score) and reward-related VS activity (gain-loss in left VS), separately for the psychopathic and non-psychopathic groups. Non-psychopaths exhibited no significant correlation between left VS activation for gain–loss and total PCL-R score (r = −0.04, P = 0.85). In contrast, psychopaths exhibited a strong and significant positive correlation between left VS gain–loss activity and total PCL-R score (r = 0.74, P = 0.0004; Figure 2A). A direct test comparing the total PCL-R score/VS gain–loss activity correlations indicates a highly significant difference between non-psychopathic and psychopathic groups (Z = −2.87, P = 0.004). These results indicate that the relationship between reward/loss-related VS activity and psychopathy severity is significantly different for psychopaths and non-psychopaths.
Fig. 2.
Correlation of percent signal change across all 48 voxels of the task-activated region (left VS; see Figure 1B) and PCL-R scores for (A) gain–loss: activity in left VS had no correlation with PCL-R score for non-psychopaths (r = −0.04, P = 0.85) but positive correlation with PCL-R score for psychopaths (r = 0.74, P = 0.0004); (B) gain–neutral: activity in left VS had no correlation with PCL-R for non-psychopaths (r = −0.14, P = 0.54) but slight positive correlation with PCL-R score for psychopaths (r = 0.23, P = 0.36); (C) loss–neutral: activity in left VS had no correlation with PCL-R score for non-psychopaths (r = 0.02, P = 0.93) but negative correlation with PCL-R score for psychopaths (r = −0.61, P = 0.007).
We next examined whether the observed group difference in correlation between VS gain–loss activity and PCL-R score could be due primarily to either the VS response to gain or loss, individually, or if it is due to the combination of the two. To address this question, we computed separate within-group correlations between PCL-R score and VS activity for the gain–neutral and loss–neutral contrasts, respectively (Figure 2B and C). Among non-psychopaths, there was no significant correlation between PCL-R score and VS activity for either contrast (gain–neutral: r = −0.14, P = 0.54; loss–neutral: r = 0.02, P = 0.93). Among psychopaths, there was a non-significant correlation for gain–neutral (r = 0.23, P = 0.36) and a significantly negative correlation for loss–neutral (r = −0.61; P = 0.007). A direct test comparing the total PCL-R score/VS reward-activity correlations shows no significant difference between non-psychopathic and psychopathic groups for the gain–neutral contrast (Z = −1.09, P = 0.28) and a marginally significant difference between groups for the loss–neutral contrast (Z = −1.99; P = 0.05). In neither the gain–neutral nor loss–neutral contrast was the correlation with PCL-R score among psychopaths as strong as in the gain–loss contrast (r = 0.72, P = 0.0004); hence, the significant positive correlation between PCL-R score and gain–loss VS activity can be viewed as a combination of positive correlation with gain–neutral activity and negative correlation with loss–neutral activity. However, given that the loss–neutral correlation was much stronger than the gain–neutral correlation, this finding appears to be driven primarily by the loss–neutral VS activity.
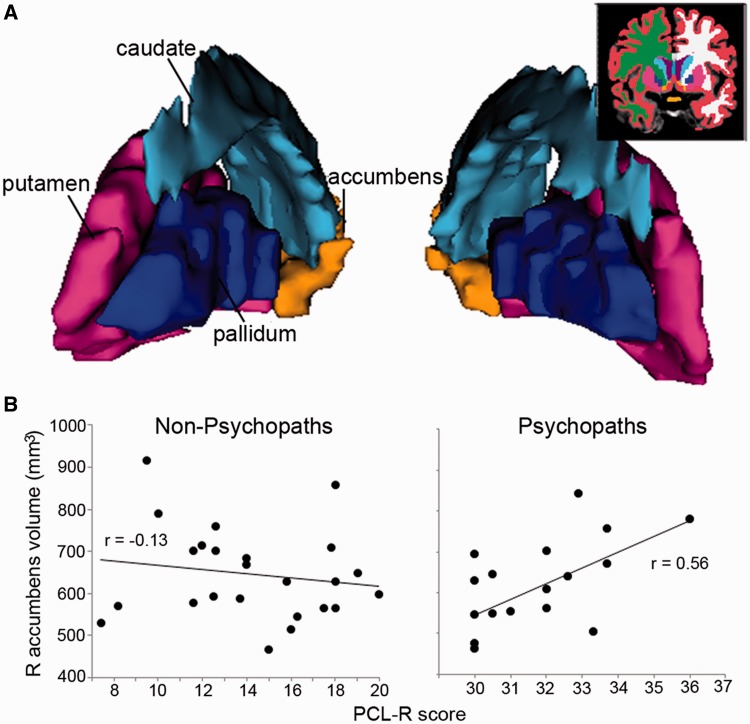
As a follow-up to the VS activity finding, we examined structural characteristics of VS to determine whether we could observe similar differences between psychopaths and non-psychopaths. Specifically, we computed volumes of striatal subregions for each group (Supplementary Table S2). There were no significant between-group differences in mean volume for any of the subregions (all P > 0.28). However, like the VS activity results described previously, there was a significant difference between groups in the correlation between VS volume and PCL-R score (Figure 3). Volume of the right accumbens area was not significantly correlated with PCL-R score among non-psychopaths (r = −0.13, P = 0.55) but significantly positively correlated with PCL-R score among psychopaths (r = 0.56, P = 0.02). Again these within-group correlations were significantly different between psychopaths and non-psychopaths (Z = 2.24, P = 0.03).
Fig. 3.
(A) Three-dimensional-rendered striatal subregions of a representative subject. Inset: coronal slice illustrates the segmentation. (B) Correlation of right accumbens area volume (mm3) and PCL-R score. Volume in this region of VS had no correlation with PCL-R score for non-psychopaths (r = −0.13, P = 0.55) but positive correlation with PCL-R score for psychopaths (r = 0.56, P = 0.02). See Supplementary Table S2 for group mean volumes.
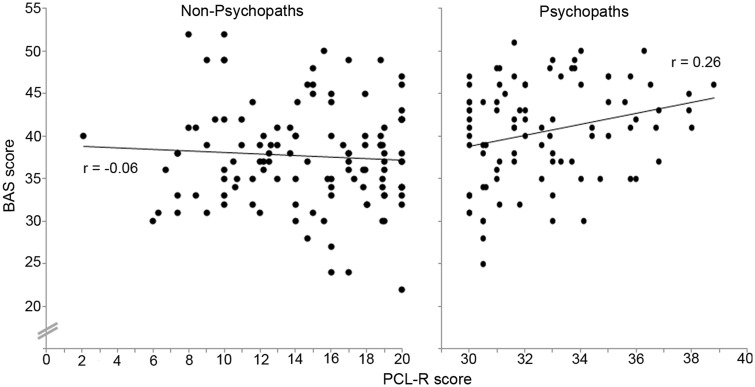
As another follow-up, we examined the relationship between psychopathy severity and a widely used self-report measure of behavioral motivation (BIS/BAS) in a much larger sample of inmates (n = 93 psychopaths and n = 117 non-psychopaths). BIS scores indicate anxiety and behavioral inhibition, whereas BAS scores indicate sensitivity to appetitive stimuli and reward. The BAS results closely mirrored the VS functional and structural imaging findings. Among non-psychopaths, there was no significant correlation between PCL-R score and BAS score (r = −0.06, P = 0.51), but among psychopaths, there was a significant positive correlation between PCL-R score and BAS score (r = 0.26, P = 0.01; Figure 4). These correlations were significantly different between groups (Z = 2.31, P = 0.02). We observed no such group difference for BIS scores; both psychopaths and non-psychopaths exhibited no significant correlation between BIS score and PCL-R score (r = −0.19 and r = −0.05, respectively; Z = 1.01, P = 0.31).
Fig. 4.
BAS scores and PCL-R scores had no correlation for non-psychopaths (r = −0.06, P = 0.51) but positive correlation for psychopaths (r = 0.26, P = 0.01).
Finally, we examined the relationship between the main dependent measures related to reward processing (VS activity, accumbens volume and BAS score) and the two factor scores of the PCL-R (Supplementary Table S3). For both groups, the correlational relationships for each factor score were similar to the correlational relationships for total PCL-R score.
DISCUSSION
The aim of this study was to evaluate the neural substrates of reward and loss sensitivity in criminal psychopaths. For decades, psychopathy researchers have theorized that deficits in processing reward and punishment may underlie the impulsive and remorseless behavior of criminal psychopaths (Cleckley, 1941; Lykken, 1957; Fowles, 1980; Gorenstein and Newman, 1980; Blair, 2008). Although we found no overall differences in the mean level of VS activity in response to reward or loss between psychopaths and non-psychopaths, we did observe a marked difference in the relationship between VS activity to reward vs loss and psychopathy severity between the two groups, with non-psychopaths exhibiting no significant correlation and psychopaths exhibiting a strong positive correlation. This positive correlation with gain–loss VS activity in psychopaths appeared to be driven primarily by a negative correlation with loss–neutral VS activity. Volume of the accumbens area of right VS also correlated positively with psychopathy severity among psychopaths, but not non-psychopaths. Moreover, in an analysis of self-reported reward sensitivity and appetitive motivation, we again observed a similar pattern (i.e. no significant correlation with psychopathy severity among non-psychopaths, but a strong positive correlation among psychopaths). These convergent neurofunctional, neurostructural and psychological results provide novel evidence that reward and loss processing may play a key role in psychopathic behavior.
We believe that these results indicate a potentially important interaction between psychopathy severity and sensitivity to rewards relative to losses. Among non-psychopaths, neither reward nor loss sensitivity (as measured by VS response and BAS self-report) had a significant relationship with psychopathy severity. We propose that this is because greater levels of reward sensitivity in non-psychopaths may be adequately tempered by intact behavioral control mechanisms, ultimately yielding no significant relationship between reward/loss sensitivity and overtly reckless behavior. Psychopaths, on the other hand, notoriously lack such behavioral restraints. Thus, greater differences in VS activity to rewards relative to losses and greater levels of appetitive motivation in psychopaths may directly correspond to greater levels of impulsive, careless and irresponsible (‘psychopathic’) behavior.
This study features several methodological strengths. This is the first fMRI study of reward and loss processing in a group of stringently classified psychopaths (PCL-R ≥30). Moreover, the group of n = 18 criminal psychopaths is the largest such sample ever collected for a task-related fMRI study. In addition, the combination of functional and structural MRI analyses in a study of criminal psychopathy is unique to our research group (Motzkin et al., 2011; Ly et al., 2012) and offers convergent support for our conclusions.
One potential limitation worth considering is the range of PCL-R scores of participants in the MRI correlation analyses, particularly for the psychopathic inmates. For the VS fMRI and volumetric data (Figures 1–3), the psychopathic group had PCL-R scores that ranged from 30 to 36 (out of a maximum possible range of 30–40). Although this relatively narrow range of psychopathic PCL-R scores yielded highly significant P-values (e.g. P = 0.0007 for correlation with VS gain–loss fMRI data and P = 0.02 for correlation with VS volumetric data), we were nonetheless concerned that the observed relationship may not hold for larger numbers of psychopathic inmates with a greater range of psychopathy severity. To address this concern, we analyzed previously collected BAS data from a much larger group of inmates (Newman et al., 2005; Wallace et al., 2009). The BAS scale is a widely used measure of reward sensitivity in psychological research (Bijttebier et al., 2009). We reasoned that if greater differences in VS activation in response to monetary gains relative to monetary losses are related to approach-related motivation at the psychological level, then we should observe a similar relationship between BAS score and PCL-R score. Indeed, this is what we found: no significant correlation among non-psychopaths but a significant positive correlation among psychopaths (Figure 4). Importantly, the n = 93 psychopaths in this follow-up study spanned nearly the full range of PCL-R scores (30–39). Hence, we believe that the fMRI and BAS data provide convergent support for our interpretation.
At first glance, our results may not appear to be entirely consistent with previous fMRI studies correlating psychopathic traits with increased reward-related VS activity (Buckholtz et al., 2010; Bjork et al., 2012). We see two possible reasons for this apparent discrepancy. One is the difference in task paradigms. The previous studies examined VS responses during the anticipation of monetary gain (relative to no gain), whereas our study examined VS responses to the receipt of monetary gain, monetary loss and no gain. A second important difference is the participant sample. The previous studies used community samples, which were likely composed entirely of non-psychopaths, as the prevalence of psychopathy in the general U.S. adult population is believed to be <1% (Hare, 2003), and likely even lower among subjects screened for substance use history. In fact, the severity of ‘psychopathy’ in these non-incarcerated community samples is almost certain to be dramatically lower than even our sample of non-psychopathic criminal offenders, who had PCL-R scores ranging from 7 to 20 (mean of 14.1; Table 1). Given these considerable differences between study designs and subject populations, we believe that our study has generated unique standalone data on the neural substrates of reward and loss processing in actual psychopaths.
The results of this study may inform a broader discourse on categorical (i.e. qualitatively distinct type of individual) vs dimensional (i.e. quantitatively greater degree of certain traits or characteristics) perspectives on psychiatric disorders (Chabernaud et al., 2012). Despite ample empirical support for the dimensional conception of psychopathy (Marcus et al., 2004; Edens et al., 2006; Walters et al., 2007, 2008), there are at least two previous studies indicating categorical effects (Patrick et al., 1993; Young et al., 2012). The present neurobiological data join these previous psychophysiological and behavioral findings in demonstrating categorically distinct features of psychopathy. To address this issue more definitively from a neuroscientific standpoint, future brain imaging studies using larger samples that include individuals spanning the entire range of psychopathy severity will be necessary to determine whether there are indeed certain neurobiological correlates of psychopathy that appear to be non-dimensional in nature.
Overall, this study yields unique and novel data on the neurobiology of reward and loss processing and psychopathy. The functional and structural neuroimaging data presented here converge to demonstrate that brain–behavior relationships among criminal psychopaths differ significantly from non-psychopathic offenders.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by a UW-Madison/UW-Milwaukee Intercampus Research Incentive Grant and grants from the National Institutes of Health (MH070539, DA026505, MH086787, MH078980). The authors thank Keith Harenski for his assistance with MRI data collection and Rebecca Ray and Cendri Hutcherson for their assistance in implementing the fMRI task. They also thank many at the Wisconsin Department of Corrections for making this research possible. They are especially indebted to Deputy Warden Tom Nickel and Dr. Kevin Kallas.
REFERENCES
- Arnett PA, Smith SS, Newman JP. Approach and avoidance motivation in psychopathic criminal offenders during passive avoidance. Journal of Personality and Social Psychology. 1997;72:1413–28. doi: 10.1037//0022-3514.72.6.1413. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Wallace JF, Maccoon DG, Curtin JJ, Newman JP. Clarifying the factors that undermine behavioral inhibition system functioning in psychopathy. Personality Disorders. 2010;1:203–17. doi: 10.1037/a0018950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijttebier P, Beck I, Claes L, Vandereycken W. Gray's Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clinical Psychology Review. 2009;29:421–30. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Hommer DW. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biological Psychology. 2012;89:408–15. doi: 10.1016/j.biopsycho.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2008;363:2557–65. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010;13:419–21. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the bis bas scales. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- Chabernaud C, Mennes M, Kelly C, et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;71:434–42. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity. St. Louis, MO: Mosby; 1941. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG., Jr Psychopathic, not psychopath: taxometric evidence for the dimensional structure of psychopathy. Journal of Abnormal Psychology. 2006;115:131–44. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- First MB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: implications of gray's two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychological Review. 1980;87:301–15. [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8:249–66. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. 2nd edn. Toronto, Ontorio, Canada: Multi-Health Systems; 2003. [Google Scholar]
- Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry. 2011;16:792–9. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, et al. Cortical thinning in psychopathy. American Journal of Psychiatry. 2012;169:743–49. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Marcus DK, John SL, Edens JF. A taxometric analysis of psychopathic personality. Journal of Abnormal Psychology. 2004;113:626–35. doi: 10.1037/0021-843X.113.4.626. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. The Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, MacCoon DG, Vaughn LJ, Sadeh N. Validating a distinction between primary and secondary psychopathy with measures of Gray's BIS and BAS constructs. Journal of Abnormal Psychology. 2005;114:319–23. doi: 10.1037/0021-843X.114.2.319. [DOI] [PubMed] [Google Scholar]
- Newman JP, Widom CS, Nathan S. Passive avoidance in syndromes of disinhibition: psychopathy and extraversion. Journal of Personality and Social Psychology. 1985;48:1316–27. doi: 10.1037//0022-3514.48.5.1316. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Schmauk FJ. Punishment, arousal, and avoidance learning in sociopaths. Journal of Abnormal Psychology. 1970;76:325–35. doi: 10.1037/h0030398. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behavioral and Brain Functions. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Smith SS, Newman JP. Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology. 1990;99:430–9. doi: 10.1037//0021-843x.99.4.430. [DOI] [PubMed] [Google Scholar]
- Wallace JF, Malterer MB, Newman JP. Mapping Gray's BIS and BAS constructs onto factor 1 and factor 2 of Hare's Psychopathy Checklist - Revised. Personality and Individual Differences. 2009;47:812–16. doi: 10.1016/j.paid.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters GD, Brinkley CA, Magaletta PR, Diamond PM. Taxometric analysis of the Levenson Self-Report Psychopathy scale. Journal of Personality Assessment. 2008;90:491–8. doi: 10.1080/00223890802248828. [DOI] [PubMed] [Google Scholar]
- Walters GD, Duncan SA, Mitchell-Perez K. The latent structure of psychopathy: a taxometric investigation of the Psychopathy Checklist Revised in a heterogeneous sample of male prison inmates. Assessment. 2007;14:270–8. doi: 10.1177/1073191107299594. [DOI] [PubMed] [Google Scholar]
- Young L, Koenigs M, Kruepke M, Newman JP. Psychopathy increases perceived moral permissibility of accidents. Journal of Abnormal Psychology. 2012;121:659–67. doi: 10.1037/a0027489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.