Abstract
Emotional reactivity and the ability to modulate an emotional state, which are important factors for psychological well-being, are often dysregulated in psychiatric disorders. Neural correlates of emotional states have mostly been studied at the group level, thereby neglecting individual differences in the intensity of emotional experience. This study investigates the relationship between brain activity and interindividual variation in subjective affect ratings. A standardized mood induction (MI) procedure, using positive facial expression and autobiographical memories, was applied to 54 healthy participants (28 female), who rated their subjective affective state before and after the MI. We performed a regression analysis with brain activation during MI and changes in subjective affect ratings. An increase in positive affective ratings correlated with activity in the amygdala, hippocampus and the fusiform gyrus (FFG), whereas reduced positive affect correlated with activity of the subgenual anterior cingulate cortex. Activations in the amygdala, hippocampus and FFG are possibly linked to strategies adopted by the participants to achieve mood changes. Subgenual cingulate cortex activation has been previously shown to relate to rumination. This finding is in line with previous observations of the subgenual cingulate’s role in emotion regulation and its clinical relevance to therapy and prognosis of mood disorders.
Keywords: fMRI, mood induction, subgenual ACC, amygdale, MACM
INTRODUCTION
Functional neuroimaging can yield insights into neural correlates of healthy and dysregulated mood states and emotional reactivity. The terms mood and emotion may reflect different concepts (e.g. Gross, 1998), which require appropriate definition. In this article, we follow the classification proposed by Scherer (1984) in which ‘affect’ describes different ‘emotional’ phenomena. Thus, a distinction can be drawn between emotion and mood (in addition to other subcategories of affect). Emotions can be viewed as stimulus-related responses or reactions, while mood reflects a slow moving ‘background’ state (Rosenberg, 1998; Bylsma et al., 2008). In other words, ‘emotional reactions are like storms, whereas moods are like seasonal climate change’ (Rottenberg and Gross, 2007).
Studies on emotion and mood can be particularly relevant to mood disorders such as bipolar disorder or major depression (Bylsma et al., 2008). In this study, we focus on mood regulation in particular. Mood induction procedures (MIPs) have been widely applied to study neural correlates of mood states. Due to the great variety of stimulus materials and the lack of reliability or validity of some methods, direct comparisons of such procedures are limited (Habel et al., 2005; Falkenberg et al., 2012). This dilemma reflects the heterogeneity of conceptual approaches in the study of emotion (e.g. Gross, 1998). Various forms of MIPs have been reported in the literature [see Martin (1990) and Westermann et al. (1996) for discussion], with the ‘Velten’ procedure (Velten, 1968; Gerrards-Hesse et al., 1994) being the most widely used (e.g. Gerrards-Hesse et al., 1994; Westermann et al., 1996). In this procedure, statements about positive or negative self-evaluations or somatic states are presented to the participant and he/she is explicitly instructed to try to feel the mood described by the statement. In general, one can distinguish between MIPs ranging from purely automatic mood (like music) induction to highly cognitive, strategic induction procedures (like imagination) and different combinations of these MIPs with differing effectiveness and validity (for meta-analysis, see Gerrards-Hesse et al., 1994; Westermann et al., 1996). MIPs rely on the ability of an individual to change mood effectively and voluntarily. This ability might show considerable individual differences related to habitual emotion regulation strategy, personality or gender (Larsen and Ketelaar, 1989; Westermann et al., 1996). In an investigation of the effectiveness of MIPs, the authors used the percentage of participants who were able to change their mood during different MIPs as a dependent variable, which showed considerable variance across MIPs (Westermann et al., 1996).
Neural correlates of mood induction (MI) have been studied with different approaches, some directly instructing participants to change their mood voluntarily (e.g. Pelletier et al., 2003; Habel et al., 2005; Hofer et al., 2006; Reske et al., 2007), whereas others have induced mood changes more passively (e.g. Goldin et al., 2005; Mitterschiffthaler et al., 2007), by recall of autobiographic memories (e.g. Damasio et al., 2000), unstructured methods (e.g. Koepp et al., 2009) or false feedback (Schneider et al., 1996; Habel et al., 2001).
Both positive and negative mood states elicit activity in the amygdala–hippocampal area, the prefrontal and temporal areas, the anterior cingulate cortex (ACC) and the precuneus (Pelletier et al., 2003; Habel et al., 2005). Indeed, there is some debate about whether or not the functional neuroanatomy of separate emotion categories (such as fear, sadness and happiness) is reliably discernible using conventional approaches (e.g. Phan et al., 2002; Murphy et al., 2003; Pelletier et al., 2003; Wager et al., 2003, Habel et al., 2005, for discussion see Wager et al., 2008). The debate can be extended to neural correlates of mood states.
Most studies on MI focus on negative affective states, because sustained negative mood reflects a prominent symptom in various psychiatric disorders. A brain structure highly relevant in this context is the subgenual ACC (sgACC). Hyperactive in major depression (e.g. Drevets et al., 2008), it has often been activated in studies of sad mood states (Mayberg et al., 1999; Phan et al., 2002) and has been purported to play an important role in sustained negative affect in mood disorders (Drevets et al., 2008; Fontanelle et al., 2012). The functionality of this structure is also predictive of treatment response as well as being a focus of modern neurosurgical approaches to mood disorders (Mayberg et al., 2000, 2005; Seminowicz et al., 2004; Keedwell et al., 2005; Greicius et al., 2007; Kennedy et al., 2007; Mayberg, 2009).
The majority of studies on positive MI have found activation in the ACC and the posterior cingulate gyrus (PCC), the precuneus, the amygdala hippocampus complex and the inferior frontal gyrus (Pelletier et al., 2003; Goldin et al., 2005; Habel et al., 2005; Hofer et al., 2006; Mitterschiffthaler et al., 2007; Reske et al., 2007). There is also evidence of activation in the amygdala and the hippocampus during the experience of positive mood states. Apart from fMRI results, Koepp et al. (2009) found endogenous opioid release in the amygdala during positive MI, which further supports the prominent role of the amygdala in positive mood states.
In addition to sustained negative affect, anhedonia, the inability to experience pleasure, is a characteristic feature of major depression, but also of schizoaffective disorder and schizophrenia (e.g. Germans and Kring, 2001; Horan et al., 2008; Ritsner et al., 2011). Thus, the investigation of effective vs ineffective positive MI has the potential to inform our understanding of anhedonia in psychopathology. In a recent study, our group was able to show significant differences in expressive, subjective experiential and psychophysiological measures in reaction to different positive MIPs in depressed patients, with all three measures contributing significantly to the differences between depressed patients and healthy controls (Falkenberg et al., 2012). This led us to the conclusion that a combination of different measures is highly beneficial to a comprehensive characterization of emotional dysfunctions in depression.
Associations between the changes in subjective affective ratings and brain activation have been seldom analysed to understand the correlates of effective mood change. Goldin et al. (2005) found that subject-specific analysis might yield higher sensitivity in the detection of relevant brain activation, especially in positively valenced affect, thereby demonstrating the advantage of using subjective ratings to analyse subject-specific differences in affect processing. Elucidating the neural correlates of effective and ineffective generation of a positive mood in healthy participants can afford valuable insight into the functional disturbances in psychiatric disorders (e.g. anhedonia), leading to potential prognostic or therapeutic applications. To that end, we applied a modification of a well-validated MIP during fMRI measurements, which in similar form has been used among different clinical and non-clinical populations. The procedure involves presenting smiling faces to the participant. Additionally, the participant is instructed to try to get into a happy mood and is encouraged to use autobiographical material as support.
We hypothesized that for effective MI, positive mood would be correlated with increased activation in the amygdala and the nucleus accumbens (NAcc) because of their involvement in reward processing (Cromwell and Schultz, 2003; McClure et al., 2004), placebo effects in pain (Scott et al., 2007) and approach behaviour (Wager et al., 2007). In addition to the regions engaged in processing facial expressions, such as the fusiform gyrus (FFG), this area has been shown to be more strongly activated by emotional compared with neutral faces (Fusar-Poli et al., 2009). Several studies have demonstrated that the FFG is not only responsive to facial stimuli but also shows an increased response attributed to emotional salience (e.g. Habel et al., 2005; Reske et al., 2007; Kohn et al., 2011).
For ineffective MI, we hypothesized that activation in the sgACC would increase with decreasing subjective effectiveness of the positive MIP. As outlined above, the sgACC is associated to negative affect and its pathological persistence in mood disorders (e.g. Drevets et al., 2008). Nevertheless, there is evidence that elevated sgACC activity could be related to anhedonia (e.g. positive affect; Dunn et al., 2002), (subclinical) anhedonia is associated with impaired white matter integrity in the sgACC in women at risk for depression (Keedwell et al., 2012) and it has been proposed that dysfunctionality in the subgenual prefrontal cortex in melancholia may be related to blunted hedonic response (Pizzagalli et al., 2004). Furthermore, this structure shows increased activity in relation to rumination (Cooney et al., 2010), which has been previously described as a dysfunctional emotion regulation strategy and decreased positive affect (Nolen-Hoeksma et al., 2008).
MATERIALS AND METHODS
Subjects
We studied 54 right-handed healthy individuals (mean age: 29.9; s.d. 8.2; 26 male and 28 female). All participants were screened for neurological, psychiatric or other medical illnesses with impact on brain functioning. Specifically, we conducted a semi-structured interview in which the participants were asked whether they had a history of drug or alcohol abuse, an illness affecting kidney, lung, heart, pancreas or thyroid gland, surgical operation on heart, head or neck and additional surgical operations (which led to exclusion only if MR safety was endangered or brain functioning possibly altered). Furthermore, a history of neurological diseases (such as epilepsy, stroke, traumatic brain injury, and meningitis) or consultation with a psychiatrist or psychotherapist was an exclusion criterion. All participants were additionally screened by an experienced psychologist (N.K.) for psychiatric diseases with the structured clinical interview for DSM-IV (Wittchen et al., 1997). A detailed description of the study protocol was provided and all participants gave written informed consent. The study protocol was approved by the Institutional Review Board of the Medical Faculty, RWTH Aachen University. fMRI results of an unrelated second paradigm, measured in a subsample of this sample, were published recently (Kohn et al., 2011).
Stimulus presentation
For the experimental paradigm, we used Presentation software package 10 (Neurobehavioral Systems, San Francisco, CA, USA) on a desktop computer. Participants watched the pictures via video goggles (VisuaStim XGA; Resonance Technology Inc., Los Angeles, CA, USA) and gave feedback by button press with their right hand on an input device placed comfortably under their right hand.
Standardized MI
The method for MI has been previously described in detail and used in several studies to investigate emotional processing in healthy controls and different clinical groups (Erwin et al., 1992; Gur et al., 1992, 1994; Heimberg et al., 1992; Schneider et al., 1994a, 1995; Weiss et al., 1999; Habel et al., 2005, 2004; Reske et al., 2007). The same stimuli were applied in this study and generated as follows. Caucasian professional actors were instructed to display the emotions while their pictures were taken. The models were draped in black fabric and photographed against a black backdrop to eliminate all clothing and ambient distractors. This set of photographs was reviewed by six raters for asymmetry and ambiguity of expressed emotion. Only unitary and genuine facial expressions were retained (Erwin et al., 1992). The method demonstrated small intraindividual variability and high retest reliability behaviourally (Schneider et al., 1994b). Presenting happy facial expressions, the task instructions were as follows: ‘During this task, I would like you to try to become happy. To help you do that, I will be showing you slides with faces expressing happiness. Look at each face and use it to help you feel happy. For example, you can imagine what would make the person on the slide express that emotion, or you can think of a personal event or memory that made you feel emotions like those expressed by the person in the picture. When you feel happy and are no longer interested in the slide, push the button and the next slide will appear. Don't rush, look at each slide, and try to feel the same emotion expressed by the person in the picture’ (Schneider et al., 1992).
We also applied a neutral MI condition, which is identical to the happy condition except that subjects were asked to remain neutral. This condition has been used as comparison in previous studies (Falkenberg et al., 2012). We opted against including this condition in the regression analyses as using a contrast to the neutral condition would lead to relatively lower contrast estimates and implies an additive nature of brain function, which might not reflect neural activity and also reduce power. Results from standard block-wise analyses of the neutral and happy condition are listed in Supplementary Material.
Subjects viewed the stimuli self-paced. We used a block design in which the MI condition (happy) consisted of 132 EPI measurements (TR). Stimulus presentation was triggered by the fMRI scanner. The first three volumes were discarded to allow for equilibration of the scanner. The MI consisted of three activation (e.g. happy faces, duration 60 s) and two baseline [fixation cross, mean duration 54.4 s (0.7)] blocks (Figure 1).
Fig. 1.
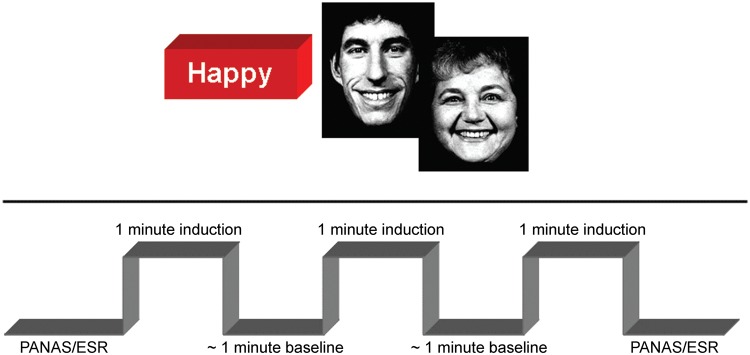
In the upper half of the figure two sample images of the facial stimuli used for support in the mood induction are displayed. The lower half illustrates one mood induction run. Before the mood induction subjects had to rate their subjective affective state using PANAS and ESR (Emotional self rating, Schneider et al., 1994a), followed by mood induction blocks (60s) and baseline (mean: 54.4s).
Dependent measure for quantifying the subjective MI effect was the Positive and Negative Affect Scale (PANAS; Watson et al., 1988), a 5-point unipolar intensity scale that includes 20 items with referenced emotional descriptors for orthogonal positive and negative dimensions. The scale required ratings of ‘How did you actually feel during the last few minutes?’. The ratings were administered before and after the MI. Furthermore, the emotional self-rating (ESR) scale (Schneider et al., 1994a) was used to assess the specificity of the emotion (on a 5-point unipolar intensity scale, i.e. whether subjects felt happy during the MIP).
In this MI, recruitment of autobiographical material was encouraged and participants were afterwards asked whether they imagined used autobiographical material to get into the mood specified. All participants referred to positive autobiographical memories in order to get into a positive mood.
Behavioural data analysis
All behavioural analyses were performed with SPSS 15 (SPSS Inc., Chicago, IL, USA). The dependent measure for quantifying the MI effect was derived from the ratings of positive and negative affect scores of the PANAS, consisting of the sum of 10 positive/negative item ratings divided by 10 (Davidson, 1998; similar to Habel et al., 2004, 2005). From the mean of 10 positive/negative item ratings, a difference score was computed by subtracting the mean positive (and negative) PANAS score after the MI from the mean positive (or negative) PANAS score acquired before the MI. Thus, we received two difference scores, one for positive ratings after MI minus positive ratings before MI (at baseline), and one for negative ratings after MI minus negative ratings before MI (at baseline). Thus, an increase in positive affect is reflected by positive values and an increase in negative affect by negative values. A 2 × 2 repeated measures ANOVA, with gender as group factor and valence (positive and negative PANAS scores) as repeated measure, was conducted to analyse gender differences.
fMRI data acquisition and analysis
Functional imaging was performed on a 3-T Trio MR scanner (Siemens Medical Systems, Erlangen, Germany) using echo-planar imaging sensitive to blood oxygen level dependant contrast (T2*, voxel size 3.5 × 3.5 × 3.5 mm3, 64 × 64 matrix, FoV 224 mm2, 36 slices, gap 0.35 mm, TR 2.2 s, TE 30 ms, α = 90°).
Analyses of functional images were performed with SPM5 (Wellcome Department of Cognitive Neurology, London, UK). Slice time correction, realignment, stereotaxic normalization (3.5 × 3.5 × 3.5 mm), smoothing (8 mm FWHM Gaussian kernel) and high-pass filtering (7.81 mHz) were applied.
For single subject analyses, the MI blocks (three happy blocks) were defined. Statistical parametric maps were calculated independently for each subject by using a boxcar convolved with a hemodynamic response function (HRF). Maps of the happy condition were taken to the second level for further analysis. For the analysis of effective and ineffective MI, we entered the difference scores from the positive PANAS scores and the contrast estimates of the HRF of happy MI blocks into a simple regression in SPM. We performed regression analyses with changes of affect ratings, as this approach is superior in power to a median split of change in affect ratings (e.g. Royston et al., 2006).
A positive correlation of PANAS difference scores with brain activation during happy MI reflects increased brain activation with larger positive difference scores, which reflect greater positive mood changes. This association identifies structures that underlie effective MI. A negative correlation, on the other hand, reflects brain areas modulating ineffective MI. The negative correlation reflects increasing brain activation with decreasing changes in positive affect scores.
For descriptive purposes, we extracted beta estimates from the significant clusters (compare Figures 2–4). Additionally, we calculated a 2 × 5 repeated measures ANOVA with mean beta estimates from these significant clusters as repeated measure and gender as group variables.
Fig. 2.
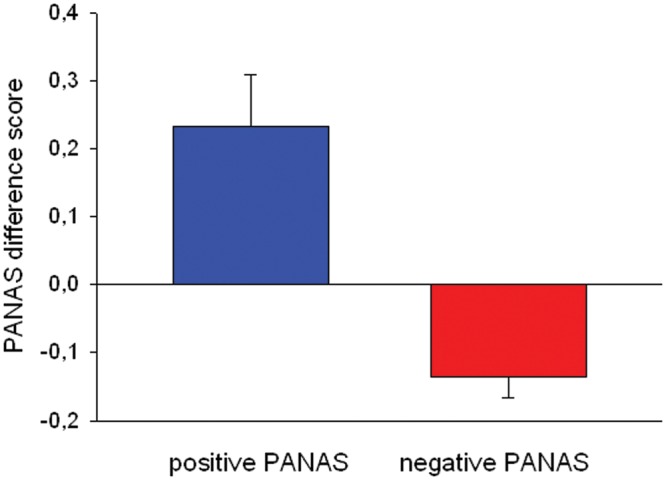
Displayed are PANAS difference score. The score reflects the change in subjective affect ratings. The positive and negative PANAS scores before and after the positive mood induction are substracted (and multiplied by −1 for ease of understanding). The blue bar displays change of positive PANAS and the red bar displays change in negative PANAS scores.
Fig. 3.
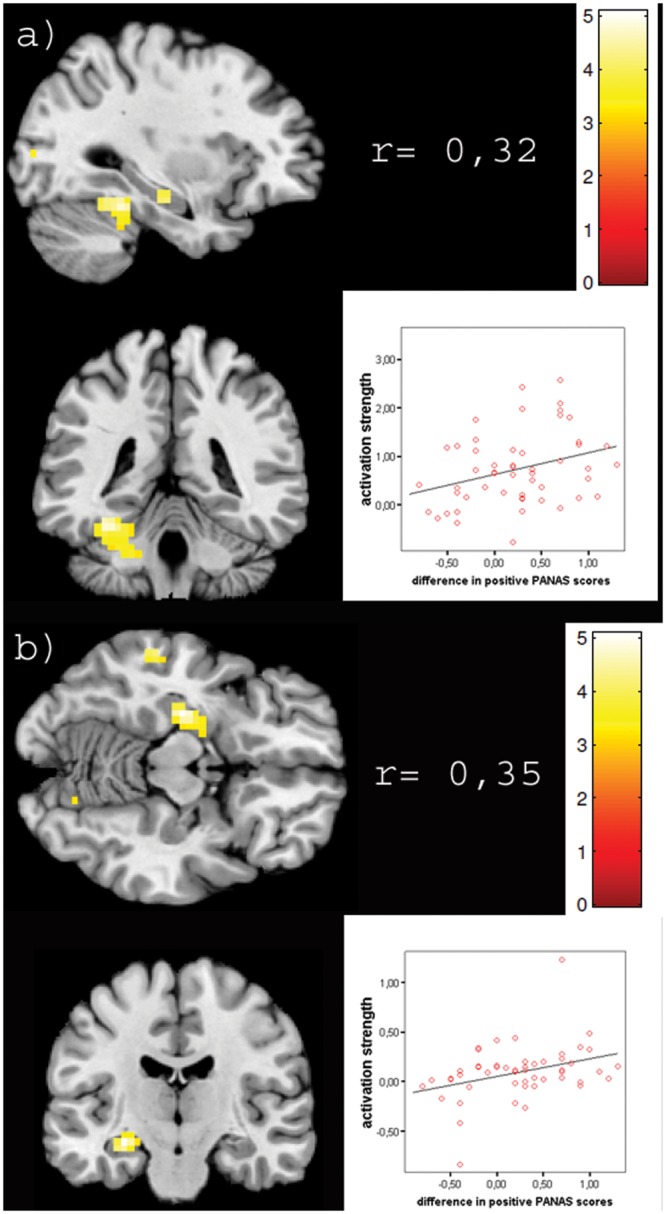
Displayed are sections of significant clusters from the positive correlation of brain activation with subjective affect ratings on the left side, for the fusiform gyrus (a) and the amygdale and hippocampus cluster (b). Illustrated on the right side of the image is the correlation index of mean beta values from the respective cluster with subjective rating, the scatter plot of these values and colour coding of beta values in the image. All Correlations are significant (p < 0.01).
Fig. 4.
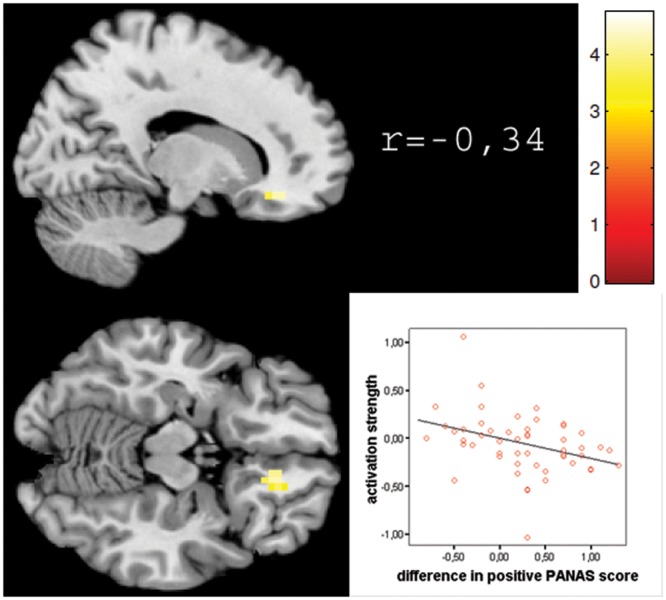
Displayed are sections of significant clusters from the negative correlation of brain activation with subjective affect ratings on the left side for the subgenual anterior cortex. Illustrated on the right side of the image is the correlation index of mean beta values from the cluster with subjective rating (p<0.01), the scatter plot of these values and colour coding of beta values in the image.
In order to correct for multiple comparisons within a volume, we applied a cluster extent threshold determined by Monte Carlo simulations (Slotnick et al., 2003). For a threshold at the voxel level of P = 0.001, and spatial properties as presented in this study, 10 000 simulations resulted in an extent threshold of nine resampled voxels. This procedure prevents a false discovery rate >5% due to multiple testing and was applied to the simple regression.
For the anatomical localization, we referred to the probabilistic cytoarchitectonic maps (Eickhoff et al., 2005).
Corollary analysis
Number of faces
We conducted an analysis of the number of faces seen per participant and block in order to determine the interindividual variance and investigate correlations with brain activation clusters identified by the regression results. Therefore, we report mean values, standard deviation as well as range for the number of faces viewed. The reported values are mean values from the three blocks. The range was determined by the absolute highest/lowest number of faces viewed per block.
Additionally, we conducted correlations (Spearman ρ) with all activation clusters determined by the regression analysis and positive affect and negative affect difference scores. Bonferroni-correction (BC) for multiple comparisons was conducted for this set of comparisons.
Controlling for baseline differences in positive affect
In order to control whether results from the regression analyses were largely dependant on baseline PANAS scores, we included the PANAS scores as nuisance variable in an additional design. This approach basically should capture the variance which is explained by baseline scores and thus ensures that associations with brain activation are driven by change scores. Nevertheless, as change score and baseline score are intercorrelated, inclusion of the baseline PANAS scores captures variance, which is also explained by difference scores.
Meta-analytic connectivity modeling (MACM) and functional characterization (FC) analyses were performed in order to functionally characterize activation clusters that did not wholly conform to our hypotheses to ensure formal reverse inference and systematically characterize the activation cluster (see Supplementary Materials for details).
RESULTS
Behavioural data
Both PANAS difference scores differed significantly from zero (one sample t-test; for negative PANAS difference scores: t = 4.32; P < 0.001; for positive PANAS scores: t = −3,09; P = 0.003, compare Figure 2). ESR results are reported in Supplementary Material.
Repeated measures ANOVA with gender as group factor and valence (positive and negative PANAS scores) as repeated measure did not yield significant results for the group factor (F(1,52) = 0.404, P = 0.528, 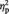 = 0.008;
= 0.008; 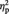 = effect size; partial eta-squared; Cohen, 1973). Post hoc t-tests for the two measures were also insignificant (P between 0.713 and 0.583).
= effect size; partial eta-squared; Cohen, 1973). Post hoc t-tests for the two measures were also insignificant (P between 0.713 and 0.583).
fMRI data
For the successful MI (positive correlation with PANAS difference scores), we observed significant activation clusters in the left FFG (peak voxel MNI: −32/−46/−18) extending to the left cerebellum (lobule IV and V), the right cerebellum (lobule VI; peak voxel MNI: 18/−80/−18) extending into the right FFG; the left hippocampus extending into the amygdala (peak voxel MNI: −28/−21/−10); the left parietal operculum (OP 3; peak voxel MNI: −38/−4/21) and the left middle occipital gyrus (peak voxel MNI: −28/−91/10) (Table 1 and Figure 3).
Table 1.
The table shows brain regions displaying significant positive correlations with positive affect change (effective mood change) and negative correlations with positive mood change (ineffective mood change)
| Efficient mood change | ||||
|---|---|---|---|---|
| Brain region | Hemisphere | MNI coordinates | Cluster size | Peak voxel t-value |
| Fusiform gyrus and cerebellum | L | −32, −46, −18 | 90 | 4.68 |
| R | 18, −80, −18 | 33 | 4.41 | |
| Amygdala–hippocampus complex | L | −28, −21, −10 | 29 | 5.08 |
| Parietal operculum | L | −38, −4, 21 | 9 | 4.24 |
| Middle occipital gyrus | L | −28, −91, 10 | 9 | 3.44 |
| Middle temporal gyrus | L | −60, −35, −10 | 9 | 4.12 |
| Inefficient mood change | ||||
| Subgenual ACC | R | 18, 35, −14 | 18 | 4.73 |
| Mammilary bodies/brainstem | L | −4, −7, −18 | 12 | 4.48 |
Cluster labelling has been performed using SPM Anatomy toolbox which relies on cytoarchitectonic maps. Hemisphere, peak voxel MNI-coordinates, cluster size and peak voxel t-values are given for each region.
For ineffective MI, we observed significant associations between brain activation and mood change in the right sgACC (peak voxel MNI: 18/35/−15) and the mammillary bodies/brain stem (peak voxel MNI: −4/−7/−18; see Table 1 and Figure 4).
Localization of clusters for both contrasts was computed with the SPM anatomy toolbox, which is based on probabilistic cytoarchitectonic maps.
Gender as a group variable did not yield significant results for any of the five regions (F(1,52) = 0.447, P = 0.507, 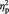 = 0.009; post hoc t-test P-values between 0.412 and 0.829).
= 0.009; post hoc t-test P-values between 0.412 and 0.829).
Number of faces
Our participants viewed a mean of 22.5 faces per block, with a s.d. 12.4. The overall range of faces viewed was from 5 to 51. The correlation analysis revealed a significant correlation between the right FFG cluster and number of faces viewed (ρ = 0.376, P = 0.0065). All other correlations did not reach significance. The correlation between right FFG and number of perceived faces survived BC for multiple comparisons (PBC = 0.007).
Controlling for baseline differences in positive affect
Significant negative associations with positive affect are observable in the mamillary bodies and (when lowering the threshold to 0.01, uncorr.) negative associations of positive affect scores with sgACC-vmPFC activation can be seen (compare Figures S1 and S2 in Supplementary Material).
MACM analysis
As the sgACC cluster extends into the orbitofrontal regions and only partially lies in the sgACC, we conducted a MACM analysis with this cluster as seed region. The MACM analysis revealed significant co-activation in the seed region (sgACC–vmPFC) in the right sgACC, right rectal gyrus, right caudate nucleus, left putamen, right amygdala and the left inferior frontal gyrus (see Figure S3 in Supplementary Material for details).
Functional characterization
The seed region in the sgACC–vmPFC is over-proportionally involved in the behavioural domains of cognition, emotion, perception–gustation, happiness, sexual interoception and action inhibition (action inhibition does not survive correction for multiple comparisons).
DISCUSSION
Affect regulation and the ability to experience an emotion is an important feature of intact affective functioning. Emotion experience and mood are also of clinical relevance since it is disturbed in many psychiatric disorders. The neural correlates have been identified in a number of studies, but the subjective experience has not been sufficiently taken into account in most analyses. Since there is no objective way to determine the strength and quality of affect, we rely on subjective reports concerning the experienced affect. Hence, the degree of effectiveness of MIP can vary between participants. We focused on the question of which neural correlates modulate effective and ineffective positive mood change. Thus, regression analyses were computed with difference scores of positive affect ratings and brain activation during happy MI.
The effectiveness of the applied MIP was demonstrated by subjective ratings for the whole group. Thus, the happy MI led to significant changes in the subjectively felt emotion at the group level, while interindividual variance in subjective ratings is still sufficient for a regression. Consistent with our hypotheses, the results show a correlation with affective ratings in the amygdala and hippocampus, the FFG and cerebellum. These regions seem to modulate successful MI. The involvement of the sgACC seems to be less substantiated by our data, as at least a part of the associated cluster cannot be localized solely within the sgACC. Nevertheless, we show that activation in this cluster corresponds to dysfunctional mood regulation strategies, such as rumination, and thus underlies ineffective MI.
Neural correlates of effective MI
We identified several activation clusters that show increased activation with larger changes in subjective positive affect. Congruent with our hypothesis, activation in the amygdala and hippocampus was closely related to subjective mood ratings. There is extensive evidence of the involvement of this area in positive MIPs (Schneider et al., 1997; Pelletier et al., 2003; Habel et al., 2005; Hofer et al., 2006; Mitterschiffthaler et al., 2007; Reske et al., 2007; Koepp et al., 2009). Endogenous opioid release in the amygdala has been observed during a happy MI (Koepp et al., 2009), thus corroborating a correlation between neural activity and the subjective degree of positive affect. Additionally, the MIP applied in this study relied on the recruitment of positive autobiographical material and visual stimulation with a strong contagion potential. All participants indicated having used autobiographical material to induce a mood change. As the hippocampus and, in part, the amygdala are also involved in the evaluation of positive emotional salient material (e.g. Vandekerckhove et al., 2005), the activation observed in our study could result from better memories of positive episodes or better imaginative abilities in some of our participants. Amygdala involvement could reflect its role in the generation of positive affect, the maintenance thereof, and, especially, the modulation of emotionally salient information. Anatomically, it is in the position to modulate cortical neuronal activation thresholds and information processing via projections from the central nucleus of the amygdala to cholinergic neurons (Whalen, 1998; Davis and Whalen, 2001).
Furthermore, we observed covarying activation in bilateral FFG extending into the cerebellum, increasing with higher positive affect during the happy MI. As the FFG is strongly involved in the processing of (emotional) faces (e.g. Vuilleumier and Pourtois, 2007; Loughead et al., 2008), activation in this area might arise from a stronger stimulation and contagion through emotional facial stimuli contrasted to neutral. The possibility of greater attention to socially relevant emotional stimuli is in line with the observed activation in the occipital gyrus. Lane et al. (1999) demonstrated that emotional arousal can modulate activity in the visual processing stream, which was corroborated by later studies (Gundel et al., 2003; Reske et al., 2009; Kohn et al., 2011).
Furthermore, the applied paradigm allowed for substantial interindividual differences in the strategy used to change the mood, as it combines mood change via contagion processes by valenced facial expression and conscious mood change through autobiographic memory. The variance in the number of faces viewed by participants possibly reflects these differences in individual strategy, which is further supported by a significant correlation between the number of faces viewed and activity in the right FFG, as it is likely that participants would choose to view a larger number of faces only if the faces were part of their strategy. Nevertheless, elevated activity is possibly related primarily to a stronger bottom-up input from more stimuli, while the reason why the participants chose to view more faces might be related to strategy.
We hypothesized the involvement of reward-related structures in effective MI, which, however, we did not observe. A possible explanation might be that differential involvement of these structures is not adequately reflected by interindividual differences in subjective affect ratings. Additionally, activation in the striatal regions subserving reward processing can be seen as a consequence of successful MI, whereas amygdala, hippocampus and FFG activation most probably reflect individual differences in the strength of the positive mood experience.
We did not find significant associations between nucleus accumbens activation and subjective mood change. This may be due to ventral striatal activation as a consequence of a happy mood state as opposed to a linear increase in activation with subjective mood change as modelled by our regression or it may reflect a deactivation in the NAcc during a happy mood state as proposed by Carlezon and Thomas (2009).
Neural correlates of ineffective mood change
We also identified structures associated with ineffective happy mood changes. Consistent with our hypotheses, the sgACC was the largest cluster identified to be negatively correlated with a positive mood change.
The sgACC was implicated in a variety of tasks related to emotional processing. It was activated during induced sadness and happiness (George et al., 1995; Habel et al., 2005), while selecting sad or happy targets in an emotional go-no-go task (Elliott et al., 2000) and during extinction learning and responding to fear-conditioned stimuli (Phelps et al., 2004). In general, the sgACC has been proposed to play an important role in emotion regulation (Drevets et al., 2008), generation and regulation of motivationally relevant or reward-related behaviour (Ochsner, 2008) and internally generated mood (Reiman et al., 1997; Habel et al., 2005; Phan et al., 2005). In our study, sgACC activation inversely correlated with voluntary positive mood changes and was thus perhaps reflective of a failure to engage in positive memories or respond to emotional facial expressions with an equivalent emotion. As an abnormally elevated activation in the sgACC in affective disorders is associated with abnormal emotional reactivity and impaired generation of affective states (e.g. anhedonia), a negative relationship between efficacy of a positive MI and sgACC activation seems highly plausible, which in relation to our data also includes a sgACC deactivation in effective mood change. However, sgACC activation has also been found in group analysis of positive emotional reactivity, which does not fit the proposed interpretation. A possible explanation of this dissociation might be that other studies on affective reactivity also, presumably, included subjects who were not able to effectively change their mood, although a significant group mean difference in behavioural ratings could be observed. Thus, sufficiently liberal thresholds for brain imaging data would lead to significant brain activation in the sgACC during positive mood, which in fact may not correlate to positive mood experience but rather reflect a functional dysregulation of this structure. This would explain why sgACC activation is not consistently found in positive affective reactivity studies, as activation in this area would be a function of group selection.
Vogt (2009) points out that sgACC activity is most often found related to sadness, whereas activity elicited by happiness is located further anterior in the pregenual part of the ACC, which according to the author should be distinguished since these areas differ in cytoarchitecture and structural connectivity. However, the sgACC, in contrast to the pregenual ACC, has selective direct connections to the amygdala, lateral hypothalamus and parabrachial nucleus. Thus, the sgACC can be seen as an ‘autonomic control center’ (Vogt, 2009). However, Vogt (2009) claims the sgACC to be functionally involved in storage of emotionally valenced autobiographical memories, thus sgACC activation might be related to ‘activated’ negative autobiographical events, which might impair generation of a positive affective state. This account also fits nicely to results proffered by Cooney et al. (2007), who observed stronger sgACC activity when contrasting recruitment of positive autobiographical memories after induction of sadness to recruitment of positive autobiographical memories prior to this MI. The induction of sadness might have triggered negative autobiographical memories and thus led to stronger involvement of the sgACC in recall of autobiographic memories, since the sgACC would be activated due to persistent negative autobiographical memories. As persistent negative autobiographical memories essentially reflect rumination, and a positive correlation between sgACC activity and rumination in depression has been previously described (Cooney et al., 2010), its involvement in ineffective mood change might be a marker of dysfunctional mood regulation strategies, such as rumination (Nolen-Hoeksma et al., 2008).
Since the sgACC cluster extends into the orbitofrontal regions, the cluster may not solely reflect sgACC reactivity, although recent evidence points towards an extensive functional overlap of subgenual and adjacent cortical regions (e.g. area 10 and gyrus rectus; Price and Drevets, 2012). MACM analysis and functional characterization were performed in order to reliably classify the functional profile of this cluster. The analysis revealed significant co-activations in the right amygdala, the right putamen, sgACC, rectal gyrus and caudate nucleus. Additionally, the functional characterization strongly points towards an involvement of this area in emotion processing, specifically in tasks relating to happiness, sexual interoception and gustation, as well as action inhibition. These analyses further support the notion that this area is involved in emotion processing, and its involvement in action inhibition may be interpreted as a regulatory function in emotion processing, thus corroborating its involvement in sexual interoception as this mental process is likely accompanied by self-regulation due to shamefulness (see review by Georgiadis et al., 2009).
Emotion regulation vs mood regulation
As stated in the ‘introduction’ section, mood and emotion can be conceptually distinguished from one another. In the terminology first proposed by Scherer (1984), which we adopt for this study, both would be subordinate concepts of ‘affect’. Although mood and emotion can be notionally distinguished, they may interact with each other, as a negative mood state can enhance the probability of experiencing negative emotions (compare Rosenberg, 1998; Falkenberg et al., 2012). This study on MIPs can be referred to as a study of mood regulation [see Gross (1998) for details]. Since mood and emotion are interrelated, a comparison to studies involving neural correlates of emotion regulation might be of interest. Studies on emotion regulation have increased in the recent years, focusing mostly on the neural correlates of structured regulation strategies (reappraisal) pertaining to emotional reactivity induced by valenced visual stimuli (for a review, see Ochsner and Gross, 2007). Most studies have tended to explore the neural correlates of down-regulation of negative emotional reactivity (meta-analysis by Diekhoff et al., 2011). A common finding in the studies that investigated increased negative emotional reactivity (Ochsner et al., 2004; Eippert et al., 2007; Kim and Hamann, 2007; Domes et al., 2010; Schulze et al., 2011; Lang et al., 2012) is elevated amygdala activation. To the best of the authors’ knowledge, there is only one study that involves increased positive emotional reactivity (the ‘emotion analogue’ to the happy MIP used in this study). Kim and Hamann (2007) asked participants to up-regulate their emotional reactivity to positively valenced International affective picture set pictures and observed brain activation in the medial superior frontal gyrus, middle frontal gyrus, supplementary motor area, orbitofrontal gyrus, pregenual anterior cingulate gyrus, inferior frontal gyrus, middle temporal gyrus, amygdala, hippocampus, thalamus, PCC, precuneus, putamen and the caudate. As the authors did not correlate with behavioural affect measures (correlations with arousal are presented) or collect any direct affect ratings (participants were asked how successfully they regulated their emotions after the scanning session), the parametric relation of this widespread activation pattern to regulation success remains unclear. The observed activation pattern partly overlaps with our findings of effective and ineffective mood regulation, but other recent studies on emotion regulation cannot fully elucidate the neural correlates of effective and ineffective increase of a positive mood state. We have, however, been able to show that some areas involved in emotion regulation may also be parametrically engaged in effective induction of mood.
Several limitations in relation to the study design have to be acknowledged. First, and most important, the MIP used in our study is a combined MIP (compare Westermann et al., 1996). It basically combines automatic emotion contagion processes via facial expressions with imagination methods via autobiographical memories. This might lead to highly variable cognitive processes interindividually and within a MI block. A high variability in cognitive processes is disadvantageous to fMRI data analysis. Nevertheless, the MIP used in this study leads in effect to a change in mood, which is its experimental purpose.
A further limitation is that we did not obtain detailed information on the nature and number of autobiographical memories recalled during the MIP, which might have helped in interpreting interindividual differences.
Further studies with different MIPs or mood regulation strategies are needed in order to substantiate the claim that effectiveness of a mood change or mood regulation is coupled with brain activation in specific (modality- or strategy-specific) brain areas. This assumption, if substantiated by further research, might strongly support the development of individualized psychotherapeutic interventions for psychiatric disorders, among which dysregulated mood regulation and anhedonia are prominent. Researchers might be able to uncover a set of brain areas subserving different functional and dysfunctional mood regulation strategies.
As one example, this approach might be the basis for the therapeutic use of neurofeedback, as learning to regulate activity in brain areas relevant to certain mood regulation strategies via feedback might impact on mood regulation itself.
Finally, a comparison of brain areas subserving effective mood vs emotion regulation would be of high theoretical interest as there might exist considerable overlapping functional networks associated with these two processes, which we, regrettably, were not able to compare or differentiate. This might be a worthwhile focus for future research.
CONCLUSION
We identified brain structures underlying ineffective and effective positive change in mood. Among the structures subserving effective mood change were the amygdala, hippocampus and the FFG. Activation in these areas might relate to the strategy applied by the individual and likely reflects the functional correlates of effective mood change by autobiographical material (amygdala–hippocampus) and emotional facial stimuli (FFG).
Our results point to a cluster encompassing the sgACC and vmPFC as reflecting failure to voluntarily create a positive mood. MACM analyses reveal that this cluster is associated to happiness, sexual interoception and gustation, as well as action inhibition. Specifically, the sgACC has been shown to be involved in the modulation of emotion, the encoding of valenced autobiographical events and rumination. Its clinical relevance is contingent on the fact that it is heavily affected in mood disorders and, therefore, is a target of diverse therapeutic interventions.
In summary, the combination of brain imaging and behavioural data enabled us to uncover brain activation patterns underlying effective and ineffective mood changes in healthy participants, with the former largely reflecting strategy-dependent association while the latter resemble dysfunctions found in mood disorders and dysfunctional mood regulation.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of interest
None declared.
Acknowledgments
We thank Maryse Scheller, Anna Giesen, Kristina Mickartz, Ariane Preibsch and Jonas Albers for their assistance, and all our volunteers for their participation. This work was supported by the Faculty of Medicine, RWTH Aachen University (START program 27/07 and 138/09) and by the German Research Foundation (DFG, IRTG 1328, International Research Training Group). N.K. is funded by the RWTH Third Line Excellence Initiative of the DFG: Future Concept, Mobilizing People Measure, Gender Stipend. R.C.G. was supported by grants from the US NIMH (MH-084856, MH-64045 and MH-60722). U.H. is supported by the Initiative and Networking Fund of the Helmholtz Association (Helmholtz Alliance for Mental Health in an Ageing Society). S.B.E. is supported by the Human Brain Project (R01-MH074457-01A1) and the Helmholtz Alliance on Systems Biology (Human Brain Model).
REFERENCES
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–91. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Carlezon W, Thomas M. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacol. 2009;56:122–32. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement. 1973;33:107–12. [Google Scholar]
- Cooney RE, Joormann J, Atlas LY, Eugène F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–4. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:470–8. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. Journal of Neurophysiology. 2003;89:2823–38. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–30. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, et al. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping. 2010;31:758–69. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RT, Kimbrell TA, Ketter TA, et al. Principal components of the beck depression inventory and regional cerebral metabolism in unipolar and bipolar depression. Biological Psychiatry. 2002;51:387–99. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan K, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–44. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Research. 1992;42:231–40. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Falkenberg I, Kohn N, Schöpker R, Habel U. Mood induction in depressive patients: a comparative multi-modal approach. PLoS One. 2012;7:e30016. doi: 10.1371/journal.pone.0030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. American Journal of Psychiatry. 1995;152:341–51. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Reinders AATS, Paans AMJ, Renken R, Kortekaas R. Men versus women on sexual brain function: prominent differences during tactile genital stimulation, but not during orgasm. Human Brain Mapping. 2009;30:3089–101. doi: 10.1002/hbm.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germans MK, Kring AM. Hedonic deficit in anhedonia: support for the role of approach motivation. Personality and Individual Differences. 2000;28:659–72. [Google Scholar]
- Gerrards-Hesse A, Spies K, Hesse FW. Experimental inductions of emotional states and their effectiveness: a review. British Journal of Psychology. 1994;85:55–78. [Google Scholar]
- Goldin PR, Hutcherson CAC, Ochsner KN, Glover GH, Gabrieli JDE, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2:271–99. [Google Scholar]
- Gundel H, O’Connor MF, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: an FMRI study. American Journal of Psychiatry. 2003;160:1946–53. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Gur RC, Skolnick BE, Gur RE. Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain and Cognition. 1994;25:271–86. doi: 10.1006/brcg.1994.1036. [DOI] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–14. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Habel U, Klein M, Shah NO, et al. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. American Journal of Psychiatry. 2004;161:1806–13. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- Habel U, Wild B, Topka H, Kircher T, Salloum JB, Schneider F. Transcranial magnetic stimulation: no effect on mood with single pulse during learned helplessness. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:497–506. doi: 10.1016/s0278-5846(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Gur RE, Erwin RJ, Shtasel DL, Gur RC. Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psychiatry Research. 1992;42:253–65. doi: 10.1016/0165-1781(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006;32:854–62. doi: 10.1016/j.neuroimage.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin. 2008;34:856–74. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biological Psychiatry. 2012;72(4):296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. American Journal of Psychiatry. 2007;164:778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Hammers A, Lawrence AD, Asselin MC, Grasby PM, Bench CJ. Evidence for endogenous opioid release in the amygdala during positive emotion. Neuroimage. 2009;44:252–6. doi: 10.1016/j.neuroimage.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Kohn N, Kellermann T, Gur RC, Schneider F, Habel U. Gender differences in the neural correlates of humor processing: implications for different processing modes. Neuropsychologia. 2011;49:888–97. doi: 10.1016/j.neuropsychologia.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–97. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang S, Kotchoubey B, Frick C, Spitzer C, Grabe HJ, Barnow S. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2012;59:1727–34. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T. Extraversion, neuroticism and susceptibility to positive and negative mood induction procedures. Personality and Individual Differences. 1989;10:1221–8. [Google Scholar]
- Loughead J, Gur RC, Elliott M, Gur RE. Neural circuitry for accurate identification of facial emotions. Brain Research. 2008;1194:37–44. doi: 10.1016/j.brainres.2007.10.105. [DOI] [PubMed] [Google Scholar]
- Martin M. On the induction of mood. Clinical Psychology Review. 1990;10:669–97. [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. The Journal of Clinical Investigation. 2009;119:717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. The American Journal of Psychiatry. 1999;156(5):675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Fu CH, Dalton JA, Andrew CM, Williams SC. A functional MRI study of happy and sad affective states induced by classical music. Human Brain Mapping. 2007;28:1150–62. doi: 10.1002/hbm.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F, Nimmo-Smith I, Lawrence A. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3(5):400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Bouthillier A, Levesque J, et al. Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport. 2003;14:1111–6. doi: 10.1097/00001756-200306110-00003. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, et al. Neuroanatomical correlates of externally and internally generated human emotion. American Journal of Psychiatry. 1997;154:918–25. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Reske M, Habel U, Kellermann T, et al. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. Journal of Psychiatric Research. 2009;43:592–9. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Reske M, Kellermann T, Habel U, et al. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. Journal of Psychiatric Research. 2007;41:918–27. doi: 10.1016/j.jpsychires.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Arbitman M, Lisker A. Anhedonia is an important factor of health-related quality-of-life deficit in schizophrenia and schizoaffective disorder. Journal of Nervous and Mental Disease. 2011;199:845–53. doi: 10.1097/NMD.0b013e3182349ce6. [DOI] [PubMed] [Google Scholar]
- Rosenberg EL. Levels of analysis and the organization of affect. Review of General Psychiatry. 1998;2:247–70. [Google Scholar]
- Rottenberg J, Gross JJ. Emotion and emotion regulation: a map for psychotherapy researchers. Clinical Psychology: Science and Practice. 2007;14:323–8. [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statistics in Medicine. 2006;25:127–41. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Scherer KR. On the nature and function of emotion: a component process approach. In: Scherer KR, Ekman P, editors. Approaches to Emotion. Hillsdale, NJ: Erlbaum; 1984. pp. 293–317. [Google Scholar]
- Schneider F, Grodd W, Weiss U, et al. Functional MRI reveals left amygdala activation during emotion. Psychiatry Research: Neuroimaging. 1997;76:75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Gur RE, Muenz LR. Standardized mood induction with happy and sad facial expressions. Psychiatry Research. 1994a;51:19–31. doi: 10.1016/0165-1781(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Jaggi JL, Gur RE. Differential effects of mood on cortical cerebral blood flow: a 133xenon clearance study. Psychiatry Research. 1994b;52:215–36. doi: 10.1016/0165-1781(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RE, Alavi A, et al. Cerebral blood flow changes in limbic regions induced by unsolvable anagram tasks. American Journal of Psychiatry. 1996;153:206–12. doi: 10.1176/ajp.153.2.206. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RE, Mozley LH, et al. Mood effects on limbic blood flow correlate with emotional self-rating: a PET study with oxygen-15 labeled water. Psychiatry Research: Neuroimaging. 1995;61:265–83. doi: 10.1016/0925-4927(95)02678-q. [DOI] [PubMed] [Google Scholar]
- Schneider F, Heimann H, Mattes R, Lutzenberger W, Birbaumer N. Self-regulation of slow cortical potentials in psychiatric patients depression. Biofeedback and Self-Regulation. 1992;17:202–14. doi: 10.1007/BF01000403. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Krüger A, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry. 2011;69:564–73. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–36. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Research. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove MM, Markowitsch HJ, Mertens M, Woermann FG. Bi-hemispheric engagement in the retrieval of autobiographical episodes. Behavioural Neurology. 2005;16:203–10. doi: 10.1155/2005/460745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten E. A laboratory task for induction of mood states. Behaviour Research and Therapy. 1968;6:473–82. doi: 10.1016/0005-7967(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Regions and subregions of the cingulate gyrus. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford, UK: Oxford University Press; 2009. pp. 5–30. [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta J-K. Placebo effects on human μ-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–61. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect—the panas scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiss U, Salloum JB, Schneider F. Correspondence of emotional self rating with facial expression. Psychiatry Research. 1999;86:175–84. doi: 10.1016/s0165-1781(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26:557–80. [Google Scholar]
- Whalen PJ. Fear, vigilance and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:411–8. [Google Scholar]
- Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


