Abstract
The thiol oxidoreductase endoplasmic reticulum (ER)p57 interacts with newly synthesized glycoproteins through ternary complexes with the chaperones/lectins calnexin or calreticulin. On proteasomal inhibition calnexin and calreticulin concentrate in the pericentriolar endoplasmic reticulum-derived quality control compartment that we recently described. Surprisingly, ERp57 remained in an endoplasmic reticulum pattern. Using asialoglycoprotein receptor H2a and H2b as models, we determined in pulse-chase experiments that both glycoproteins initially bind to calnexin and ERp57. However, H2b, which will exit to the Golgi, dissociated from calnexin and remained bound for a longer period to ERp57, whereas the opposite was true for the endoplasmic reticulum-associated degradation substrate H2a that will go to the endoplasmic reticulum-derived quality control compartment. At 15°C, ERp57 colocalized with H2b adjacent to an endoplasmic reticulum-Golgi intermediate compartment marker. Posttranslational inhibition of glucose excision prolonged association of H2a precursor to calnexin but not to ERp57. Preincubation with a low concentration (15 μg/ml) of the glucosidase inhibitor castanospermine prevented the association of H2a to ERp57 but not to calnexin. This low concentration of castanospermine accelerated the degradation of H2a, suggesting that ERp57 protects the glycoprotein from degradation and not calnexin. Our results suggest an early chaperone-mediated sorting event with calnexin being involved in the quality control retention of molecules bound for endoplasmic reticulum-associated degradation and ERp57 giving initial protection from degradation and later assisting the maturation of molecules that will exit to the Golgi.
INTRODUCTION
After trimming of two glucose residues from the precursor N-linked oligosaccharide Glc3Man9GlcNAc2, the endoplasmic reticulum (ER) chaperones/lectins calnexin (CNX) and calreticulin (CRT) bind to the resulting monoglucosylated sugar chains of glycoproteins and assist in their folding (Helenius and Aebi, 2001; Schrag et al., 2003). ERp57 is a thiol oxidoreductase that associates with those chaperones and promotes disulfide bonding of the substrate glycoprotein (Oliver et al., 1997; Zapun et al., 1998). In this process, ERp57 can produce transient intermolecular disulfide bonds with the glycoprotein (Molinari and Helenius, 1999). CNX and CRT were shown to participate in quality control retention of defective glycoproteins by prolonged association. This is achieved by cycles of deglucosylation of the substrate by glucosidase II, release from the chaperone, reglucosylation by the folding sensor enzyme UDP-Glc:glycoprotein glucosyltransferase (GT), and reassociation with CNX and CRT (Parodi, 2000). Correct folding allows escape of the glycoproteins from these cycles because they are no longer a substrate for GT. Release from the reglucosylation cycles of glycoproteins that cannot be rescued involves trimming of mannose residues, leading to loss of the mannose residue that is the acceptor for GT (Frenkel et al., 2003) and association to the putative mannose lectin EDEM (Wang and Hebert, 2003). The glycoproteins are then dislocated to the cytosol for proteasomal degradation. We recently described a pericentriolar quality control compartment derived from the ER (ERQC) where CNX, CRT, and endoplasmic reticulum-associated degradation (ERAD) substrates accumulate upon proteasomal inhibition (Kamhi-Nesher et al., 2001). In contrast, BiP, protein disulfide isomerase (PDI), and GT remain in an ER pattern. The fact that GT did not concentrate in the ERQC suggested that the reglucosylation-CNX/CRT binding cycles might involve spatial cycling of the substrates and chaperones. Consistent with these findings, GT was recently found in a high-molecular-weight ER complex together with BiP, GRP94, and other chaperones that excluded CNX, CRT, and ERp57 (Meunier et al., 2002). A similar compartment seems to exist in Saccharomyces cerevisiae (Huyer et al., 2003), although its role may be different than in higher eukaryotes as GT and the reglucosylation cycle are absent in S. cerevisiae (Parodi, 2000).
There is abundant evidence for a role of CNX and CRT in quality control retention and degradation of misfolded or misprocessed glycoproteins. In contrast, the role of ERp57 in quality control is less clear. Here, we have addressed this issue in live cells. We have used human asialoglycoprotein receptor (ASGPR) H2a and H2b as model substrates to study interactions with ERp57 and CNX. The precursor of ASGPR H2a contains a juxtamembrane luminal pentapeptide (EGHRG) that is responsible for its complete retention in the ER (Shenkman et al., 1997). Cleavage of the ectodomain of H2a releases a soluble secreted form of the protein, sH2a (Tolchinsky et al., 1996). In contrast, H2b, the alternatively spliced variant not possessing the luminal pentapeptide, assembles with the ASGPR H1 subunit and exits to the Golgi, reaching the cell surface. H2b exits the ER even when not assembled, with an efficiency of ∼30% (Lederkremer and Lodish, 1991).
Treatment of cells with glucosidase inhibitors such as castanospermine (CST) prevents the initial glucose (Glc) trimming of N-glycans from the Glc3Man9GlcNAc2 precursor to Glc1Man9GlcNAc2 and thus abrogates association to CNX and CRT. Incubation with CST also accelerates the degradation of glycoproteins that are ERAD substrates. Using the model of H2a and H2b, we had shown that this accelerated degradation upon inhibition of Glc trimming cannot be attributed to dissociation of CNX because, among other reasons, low concentrations of CST that do not affect CNX association still accelerated the degradation (Ayalon-Soffer et al., 1999b). We have now found that the low concentrations of CST block association of ERp57. We have also revealed differences in the subcellular routing of ERp57 and CNX upon proteasomal inhibition and in the kinetics of their dissociation from H2a and H2b. These differences suggest a differential participation of the chaperones in the mechanisms of glycoprotein quality control and maturation.
MATERIALS AND METHODS
MATERIALS
Rainbow 14C-labeled methylated protein standards were obtain from Amersham Biosciences (Piscataway, NJ). Promix cell labeling mix ([35S]Met plus [35S]Cys), >1000 Ci/mmol, was from PerkinElmer Life Sciences (Boston, MA). Protein A-Sepharose was from Repligen (Needham, MA). CST was from Genzyme (Boston, MA). Lactacystin (Lac) and N-carbobenzoxyl-leucinyl-leucinyl-leucinal (MG-132) were from Calbiochem (San Diego, CA, CA). 3,3′-Dithiobis sulfosuccimidylpropionate (DSP) was from Pierce Chemical (Rockford, IL). Other common reagents were from Sigma-Aldrich (St. Louis, MO).
Construct
H2a-dsRed was constructed by polymerase chain reaction amplification of H2a G78R, a mutant of H2a that it is not cleaved but is retained and degraded similarly to wild-type (wt) H2a (Yuk and Lodish, 1993) by using primers TCATCGAATTCCATGGCCAAGGACTTTCA and GATGGATCCGAGCTGCCGCCGCCGCCGCTGGCCACCTCGCCGGT. This eliminated the stop codon and added restriction sites (EcoRI at 5′ and BamHI at 3′) used for insertion of the cDNA in frame into a vector (pdsRed1-N1; BD Biosciences Clontech, Palo Alto, CA) encoding the dsRed protein. The fusion protein thus contains H2a G78R in its N terminus followed by a short linker (SGGGGS) and dsRed in the C terminus.
Cells, Culture, and Transfections
NIH 3T3 fibroblasts stably expressing H2a (2–18 cells) or H2b (2-C cells) (Lederkremer and Lodish, 1991) were grown in DMEM plus 10% newborn calf serum under 5% CO2 at 37°C. COS-7 and HeLa cells were grown similarly but with 10% fetal calf serum. Plasmid p53cmyc containing human ERGIC53 with a myc tag at the carboxy terminus (gift of H.-P. Hauri, University of Basel, Basel, Switzerland) or pcDNA1 containing H2b were transfected into COS-7 cells on coverslips using the FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the provided protocol. A similar procedure was used to transiently transfect pdsRed1 containing H2a-dsRed into NIH 3T3 cells.
Antibodies
Polyclonal anti-C-terminal antibody against H2 (αH2) was the one used in earlier studies (Lederkremer and Lodish, 1991). Rabbit polyclonal anti-ERp57 was a kind gift of T. Wileman (Institute for Animal Health, Woking, Surrey, United Kingdom). Mouse monoclonal anti-human ERGIC53 antibody G1/93 was a kind gift of H.-P. Hauri. Rabbit polyclonal anti-CNX and mouse monoclonal anti-ERp57 were from Stressgen Biotechnologies (Victoria, BC, Canada), and mouse monoclonal anti-β-COP was from Sigma-Aldrich. Goat anti-rabbit antibody conjugated to Cy3 (indocarbocyanine), goat anti-rabbit, and goat anti-mouse antibodies conjugated to fluorescein isothiocyanate (FITC) were from Jackson ImmunoResearch Laboratories (West Grove, PA).
[35S]Cys Metabolic Labeling and Immunoprecipitation
Subconfluent (90%) cell monolayers in 60-mm dishes were labeled with [35S]Cys, lysed, and immunoprecipitation (IP) was done as described previously with anti-H2 antibody (Tolchinsky et al., 1996; Shenkman et al., 1997). CST was added at the indicated concentrations to the preincubation, starvation, labeling, and chase media except where indicated. The proteasome inhibitors MG-132 (20 μM) or Lac (10 μM) were added to the chase medium. Analysis of associations to CNX was done as described previously (Ayalon-Soffer et al., 1999b). To analyze associations to ERp57, before lysis cells were incubated with 1 mM DSP in phosphate-buffered saline (PBS) (from 50 mM stock in dimethyl sulfoxide) for 60 min at 4°C followed by 20 min with 50 mM glycine. Cells were then rinsed, lysed, and IP was done as for H2a (Kamhi-Nesher et al., 2001) but using polyclonal anti-ERp57. Immunoprecipitates were then boiled with 1% SDS and 2 mM dithiothreitol (DTT) in PBS for 5 min followed by dilution with 10 volumes of 1% Triton X-100 and 0.5% sodium deoxycholate plus 2 mM oxidized glutathione and subjected to a second IP with anti-H2. To test for disulfide bonding to ERp57 a similar procedure was used except that cells were incubated in the presence or absence of 5 mM dithiothreitol for 5 min at 37°C and then with 100 mM iodoacetamide in PBS for 5 min at 4°C, followed by 2% dimethyl sulfoxide in PBS (instead of DSP) for 60 min at 4°C, proceeding as described above.
Gel Electrophoresis, Fluorography, and Quantitation
Reducing SDS-PAGE was performed on 10% Laemmli gels. The gels were analyzed by fluorography by using 20% 2,5-diphenyloxazole and were exposed to Biomax MS film by using a transcreen-LE from Kodak (Vancouver, BC, Canada). Quantitation was performed in a Fuji BAS 2000 PhosphorImager (Tokyo, Japan).
Immunofluorescence Microscopy
The procedures used were as described previously (Shenkman et al., 1997; Kamhi-Nesher et al., 2001). For treatments with Lac (10 μM), cells on coverslips were incubated with medium containing the drug at 37°C in a CO2 incubator for 3–5 h. Incubation of cells at 15°C was for 3 h in a water bath with addition of 20 mM HEPES (pH 7.4). Digital photography was done on a Leica DMRBE fluorescence microscope.
RESULTS
On Proteasomal Inhibition, Calnexin Concentrates in the Quality Control Compartment; ERp57 Does Not
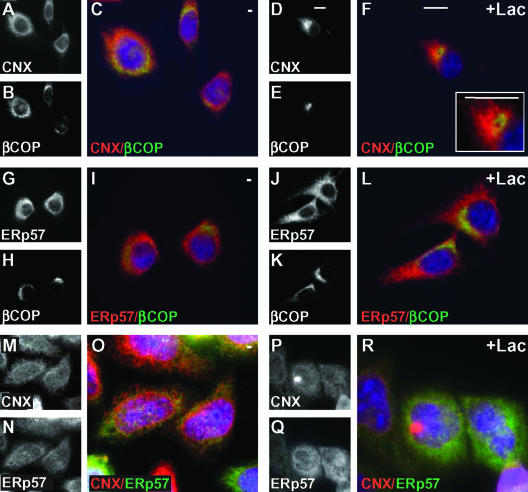
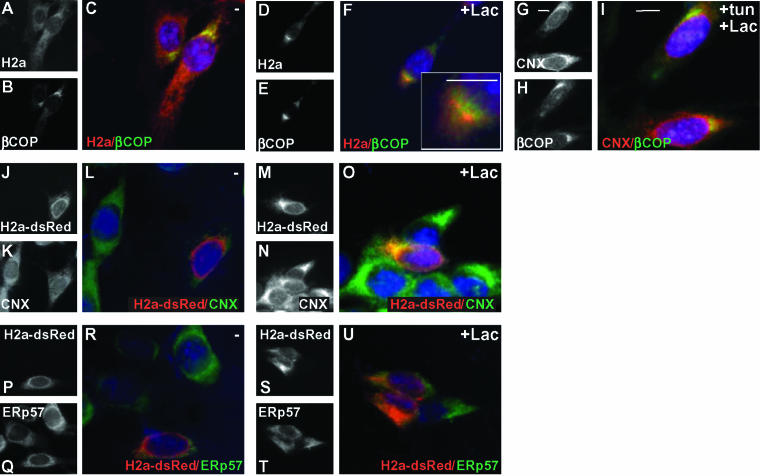
Proteasomal inhibition causes CNX and CRT to concentrate in a pericentriolar compartment derived from the ER, the ERQC (Kamhi-Nesher et al., 2001). Because ERp57 is found in ternary complexes with these chaperones and glycoprotein substrates, we expected that it would also concentrate in the ERQC. NIH 3T3 cells expressing ASGPR H2a were treated for 3 h with the proteasomal inhibitor Lac followed by fixation and immunofluorescence with anti-CNX or anti-ERp57 antibodies together with anti-β-COP as a marker of ERGIC and Golgi. Whereas CNX concentrated, as we had seen before, to a region close to but not overlapping with β-COP, surprisingly, ERp57 remained in an ER pattern similar to that in untreated cells (Figure 1, A–L). To be able to costain CNX and ERp57, we used a mouse anti-ERp57 antibody. HeLa cells were used in Figure 1, M–R, because the mouse anti-ERp57 recognizes only the human or primate proteins. In untreated cells, both CNX and ERp57 formed a disperse ER pattern. However, much of the fluorescence did not overlap, with CNX being more prominent in a perinuclear rim (Figure 1, M–O).
Figure 1.
Concentration of CNX and not ERp57 in the juxtanuclear ERQC upon proteasomal inhibition. Double-label immunofluorescence was done on fixed and permeabilized NIH 3T3 cells (A–L) or HeLa cells (M–R), preincubated for 3 h in the absence or in the presence of 10 μM Lac as indicated. Rabbit polyclonal anti-CNX was used as primary antibody with Cy3-conjugated goat anti-rabbit IgG as the secondary in A–F and M–R. Rabbit polyclonal anti-ERp57 with Cy3-conjugated secondary was used in G–L. Double labeling was done with mouse monoclonal anti-β-COP in A–L or with mouse monoclonal anti-ERp57 in M-R with FITC-conjugated goat anti-mouse IgG. Each colored panel is a merged image corresponding to the red and green channels in the panels to their left and includes 4,6-diamidino-2-phenylindole staining of nuclei. Inserted in F is an enlargement of the same image. Bars, 10 μm.
Although, upon proteasomal inhibition, fewer cells showed a tight concentration of CNX in HeLa cells compared with NIH 3T3 cells, there was no concentration of ERp57 (Figure 1, P–R). This suggests that after initial colocalization in the ER the two chaperones and their associated glycoprotein substrates may have divergent pathways.
ERp57 Dissociates Faster than CNX from ASGPR H2a
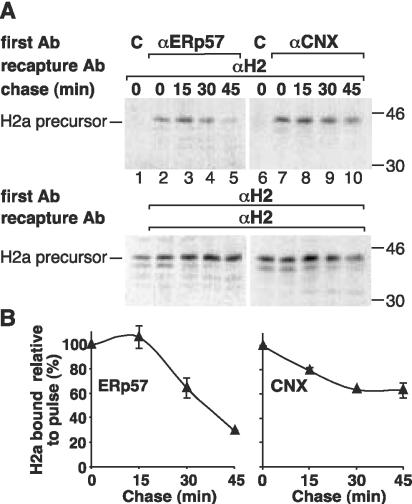
The membrane-bound precursor of ASGPR H2a possesses a luminal pentapeptide that when transplanted to another type II membrane protein (ASGPR H1) caused its ER retention and prolonged association to CNX (Shenkman et al., 1997). To compare the interactions of H2a with ERp57 and CNX, NIH 3T3 cells expressing H2a were metabolically labeled with [35S]Cys, lysed, and IP of cell lysates was done first with anti-ERp57 or anti-CNX antibodies followed by elution and reIP (recapture) with anti-H2 antibody. In the case of ERp57, a cross-linking step was included with the reversible cross-linker DSP to stabilize its weak associations. After pulse labeling, H2a precursor interacted with both CNX and ERp57 (Figure 2 A, top, lanes 2 and 7). No nonspecific interactions were seen when the first IP was done with a control antibody (lanes 1 and 6). Cell samples were chased for short periods of up to 45 min, before degradation starts (there is a lag of ∼1 h before the onset of degradation). We can see a clear difference in the kinetics of dissociation of ERp57 and CNX from H2a. Only 30% of H2a molecules remained bound to ERp57 after 45-min chase compared with those bound after the pulse, whereas 64% were still bound to CNX (Figure 2B). This result suggests that although ERp57 binds to H2a in a ternary complex with CNX, it can dissociate from this complex leaving CNX bound.
Figure 2.
Different kinetics of dissociation of ERp57 and CNX from ASGPR H2a. (A) NIH 3T3 cells stably expressing H2a were metabolically labeled with [35S]Cys for 20 min and chased for the indicated times. Top, IP of cell lysates with control (C), anti-ERp57 (αERp57), or anti-CNX (αCNX) antibodies was followed by boiling in SDS and reimmunoprecipitation (reIP) (recapture) with anti-H2 carboxy-terminal antibody (αH2) as explained in MATERIALS AND METHODS. In the case of ERp57, cells were incubated with the cross-linker DSP before lysis. Bottom, supernatants from the first IP were immunoprecipitated with αH2 followed by boiling in SDS and reIP with αH2 to analyze total H2a. On the right are indicated molecular masses in kilodaltons. The fluorographies in the top panels were exposed 4 times longer than those in the bottom panels. (B) PhosphorImager quantitations were done of three experiments similar to the one in A, and average values were plotted for the ratio of H2a bound to ERp57 or to CNX divided by total H2a and then normalized to the ratio after the pulse (= 100).
Different Kinetics of Dissociation of ERp57 and CNX from H2a and H2b
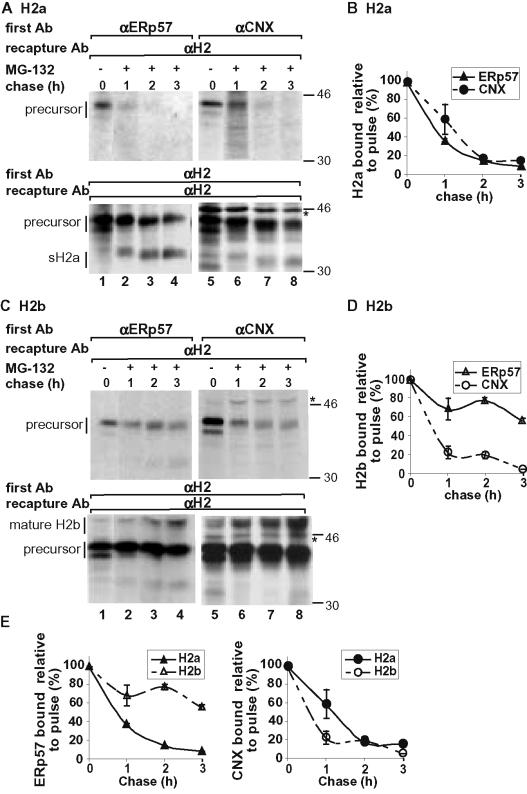
We then compared the interactions of ERp57 and CNX with the precursors of ASGPR H2a (ER retained) and H2b (that exits to the Golgi). H2a and H2b are identical except for the absence in H2b of the luminal pentapeptide already mentioned. Both variants have three N-linked sugar chains and eight luminal cysteines forming four disulfide bonds (Yuk and Lodish, 1995). NIH 3T3 cells expressing H2a (Figure 3, A and B) or H2b (Figure 3, C and D) were pulse labeled and chased for longer chase periods (up to 3h). This experiment was done in the presence of the proteasome inhibitor MG-132 to prevent degradation after the longer chase periods. As seen in Figure 3C, H2b also interacted after pulse labeling with CNX and ERp57. After 3 h of chase, only a small percentage of H2a remained bound to ERp57 or to CNX (Figure 3B). After this chase time, most H2a molecules would have been degraded in the absence of a proteasomal inhibitor (Kamhi-Nesher et al., 2001). Thus, even when degradation is prevented, H2a dissociates from the chaperones. In the case of H2b, after 3 h of chase, a surprisingly large fraction of its precursor remained associated to ERp57 (57% of the initial binding seen after the pulse), whereas only 6% remained associated to CNX (Figure 3, C and D). In Figure 3E, the same data were replotted to reflect more clearly the differences between H2a and H2b. It can be seen that H2a dissociates very quickly from ERp57, whereas H2b remains bound. In contrast, dissociation of CNX was appreciably faster from H2b than from H2a (Figure 3E, right).
Figure 3.
H2a and H2b show different kinetics of dissociation from ERp57 and CNX. (A) Similar to Figure 2A, except that cells were chased for longer times (as indicated) with complete medium in the presence of 20 μM MG-132. The asterisk indicates a nonspecific band. Indicated on the left are H2a precursor and sH2a, the ectodomain fragment. (B) Average of PhosphorImager quantitations of three experiments similar to the one in A were plotted as in Figure 2B. (C and D) Similar to A and B but with NIH 3T3 cells stably expressing H2b. Indicated on the left are H2b precursor and mature, Golgi-processed H2b. (E) Same values as in B and D were replotted for better comparison of the differences between H2a and H2b in their binding to ERp57 or CNX.
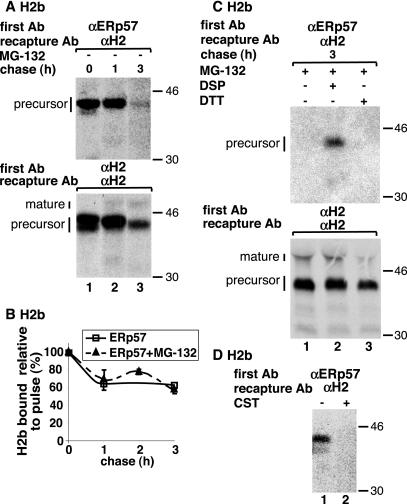
To determine whether H2b that remains bound to ERp57 is on the way to degradation or en route to the Golgi, we also followed the dissociation in the absence of proteasomal inhibition. Although much of H2b was degraded after 3-h chase, we obtained a very similar dissociation curve to remaining H2b precursor as in the presence of the proteasomal inhibitor (Figure 4, A and B). It is interesting to note that the fraction of H2b precursor bound to ERp57 remains very constant after 1-h chase, suggesting that the dissociation is a late event before exit of H2b to the Golgi, where processing to the mature form proceeds quickly.
Figure 4.
H2b binding to ERp57 depends on initial glucose trimming, persists on molecules that survive degradation, and does not involve intermolecular disulfide bonds. (A) Similar to Figure 3C, except that chases were done in the absence of MG-132. Top, overexposed image to be able to see H2b bound to ERp57 after 3-h chase in the absence of MG-132. (B) Values from a PhosphorImager quantitation of the experiment in A were plotted as in Figure 3D and compared with the values obtained in the presence of MG-132 from Figure 3 (dashed curve). (C) Similar to A but coIP of ERp57 with H2b was in this case done after 3-h chase in the presence of 20 μM MG-132 and included incubation without (lanes 1 and 3) or with DSP (lane 2) as indicated in MATERIALS AND METHODS. Top, image was overexposed to try to detect weak signals in the lanes without DSP. (D) Similar to A but with cells after pulse labeling for 40 min in the absence (lane 1) or presence (lane 2) of 100 μg/ml CST (CST was present during preincubation and labeling periods as indicated in MATERIALS AND METHODS).
H2b Binds Noncovalently to ERp57 and Needs Initial Trimming of Glucoses for the Association
The extended association of H2b to ERp57 raised the possibility that intermolecular disulfide bonds are formed. These intermolecular disulfides should allow the coprecipitation of the two proteins in the absence of a cross-linker and should be susceptible to reduction with DTT applied to the cells. We therefore performed a pulse-chase experiment followed by IP, elution, and recapture as done before but in the absence of the cross-linker. Figure 4C shows no coimmunoprecipitation (coIP) in the absence of cross-linker and no difference in the presence or absence of DTT, suggesting that intermolecular disulfide bonds are not present.
The interaction of H2b with ERp57 after dissociation of CNX (Figure 3, C–E) suggested that there may also be direct initial association to ERp57 independent of CNX. To test this possibility, we incubated cells with 100 μg/ml CST to inhibit glucose trimming and thus inhibit association of H2b to CNX as we had shown previously (Ayalon-Soffer et al., 1999b). In these conditions, we completely abrogated association of H2b to ERp57 indicating that glucose trimming and in all likelihood CNX binding is needed for the initial interaction (Figure 4D).
H2a Is Transported to the ERQC with CNX, whereas H2b Colocalizes with ERp57 Close to the ERGIC
We studied the subcellular distribution of CNX and ERp57 in relation to that of H2a and H2b. As we had seen previously, H2a formed in an ER pattern and upon proteasomal inhibition accumulated in the ERQC, next to but not overlapping with the Golgi and ERGIC, labeled with anti-β-COP (Figure 5, A–F). We analyzed whether the relocalization of CNX depends on its binding to the glycoprotein substrates by inhibiting N-glycosylation with tunicamycin before adding Lac. This treatment did not change the pattern of CNX, which still concentrated in the ERQC (Figure 5, G–I). To be able to detect colocalization of CNX with H2a, because both antibodies in our possession are rabbit polyclonals, we used a fusion protein of H2a with dsRed that shows the same retention and degradation rate as H2a (Kondratyev and Lederkremer, unpublished data). On proteasomal inhibition H2a-dsRed transiently transfected into NIH 3T3 cells showed concentration in the ERQC and colocalization with CNX, whereas there was no concentration or colocalization of ERp57 with the ERAD substrate (Figure 5, J–U).
Figure 5.
Like CNX, upon proteasomal inhibition H2a is transported to the ERQC. CNX relocalization is independent of the substrates. (A–F) Similar to Figure 1 but with NIH 3T3 cells stably expressing H2a incubated for 5 h with or without 10 μM Lac as indicated. The antibodies used were rabbit polyclonal anti-H2 carboxy-terminal with Cy3-conjugated goat anti-rabbit IgG and mouse monoclonal anti-β-COP with FITC-conjugated goat anti-mouse IgG. (G–I) The same cells as in A–F were incubated with 10 μg/ml tunicamycin for 3 h after which 10 μM Lac was added for an additional 3 h. Cells were then fixed and reacted with rabbit polyclonal anti-CNX and Cy3-conjugated secondary and mouse monoclonal anti-β-COP with FITC-conjugated secondary. (J–U) NIH 3T3 cells were transiently transfected with a plasmid carrying H2a-dsRed, and after 48 h they were incubated for 3 h with or without 10 μM Lac, fixed, and reacted with rabbit anti-CNX or anti-ERp57 antibodies as indicated using FITC-conjugated goat anti-rabbit IgG as secondary. Bars, 10 μm.
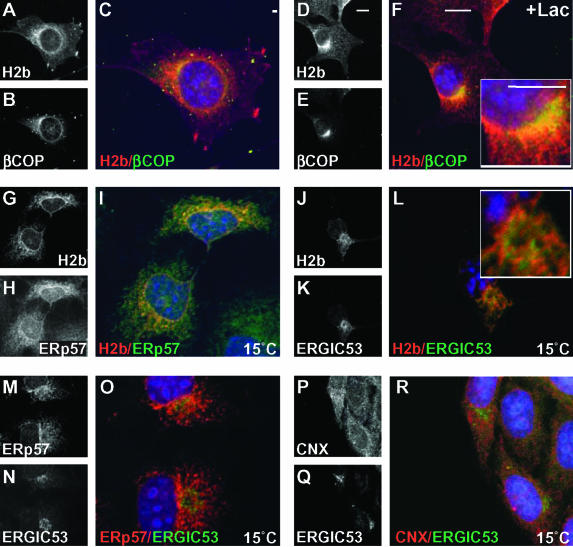
In contrast to H2a, H2b occurred in the ER, Golgi, and cell surface both in the presence or absence of Lac (Figure 6, A–F). However, in the presence of Lac, a portion of H2b staining seemed concentrated next to β-COP but not overlapping, which may indicate traffic of part of the molecules to the ERQC (Figure 6, D–F). This differential distribution of H2a and H2b is consistent with the kinetics of their dissociation from CNX and ERp57. H2a precursor would travel together with CNX to the ERQC and then separate before degradation. ERp57 would dissociate earlier, remaining in an ER pattern. H2b precursor matures very slowly to a Golgi-processed form (Figure 3C, bottom); it remains for a long time associated with ERp57 in the ER or ERGIC before separating from this chaperone and trafficking to the Golgi. Only a small fraction of H2b would travel to the ERQC with CNX, consistent with a fast dissociation of most H2b molecules from this chaperone.
Figure 6.
H2b traffics to the Golgi. At 15°C, it colocalizes with ERp57 near the ERGIC. (A–F) Similar to Figure 5, A–F, but with NIH 3T3 cells stably expressing H2b incubated for 5 h with or without 10 μM Lac as indicated. (G–R) COS cells were transiently transfected with plasmids carrying H2b and human ERGIC53 and after 48 h, they were incubated for 3 h at 15°C to arrest traffic beyond the ERGIC. Rabbit anti-H2 antibodies were used as described above in double labeling with mouse monoclonal anti-ERp57 (G–I) or mouse monoclonal anti-ERGIC53 (J–L) with FITC-conjugated goat anti-mouse IgG as secondary. In M–R, mouse anti-ERGIC53 was used as described above in double labeling with rabbit anti-ERp57 (M–O) or anti-CNX (P–R) with Cy3-conjugated secondary. Bars, 10 μm.
To test the hypothesis that H2b travels to the ERGIC with ERp57, we incubated COS cells transiently transfected with plasmids carrying H2b and ERGIC53 at 15°C to arrest the traffic of H2b. A portion of H2b and ERp57 colocalized in these conditions in a punctate juxtanuclear pattern (Figure 6, G–I). Both occurred close to although mostly not overlapping with ERGIC53 (Figure 6, J–O). In contrast to ERp57, CNX did not change its ER pattern at 15°C (Figure 6, P–R).
ERp57 Is a Candidate for Initial Protection of Molecules from Degradation; CNX Is Not
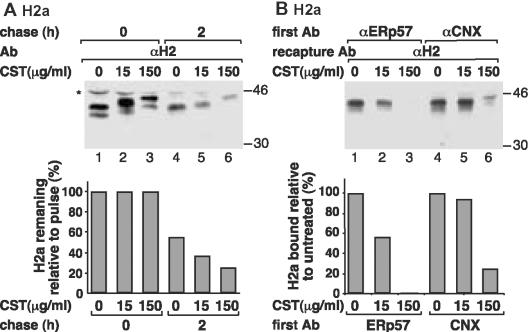
Inhibition of Glc trimming causes accelerated ERAD of glycoproteins (Moore and Spiro, 1993; Keller et al., 1998; Ayalon-Soffer et al., 1999b). It was suggested that this effect reflects a role of CNX in initial protection of glycoprotein precursors from degradation, giving time for the molecules to achieve correct folding and processing. However, we had shown that low concentrations of the inhibitor CST still caused accelerated degradation without affecting CNX binding (Ayalon-Soffer et al., 1999b). Therefore, we analyzed the binding of ERp57 to H2a upon incubation of cells with different concentrations of CST. Incubation with 150 μg/ml CST caused a shift in the mobility of H2a due to the Glc residues that cannot be removed. The drug also gave a 2.1-fold increase in the amount of H2a degraded after metabolic labeling and 2-h chase (Figure 7A). This concentration of CST almost abolished binding of ERp57 and reduced that of CNX to 25% of an untreated sample (Figure 7B). Incubation with 15 μg/ml CST caused a smaller shift in the migration of H2a due to partial inhibition of Glc trimming and still gave a significant (1.6-fold) increase in the amount of H2a degraded (Figure 7A, lane 5). However, incubation with this low concentration caused almost no effect on the binding of CNX. In contrast, binding of ERp57 was reduced to 57% of an untreated sample (Figure 7B). Therefore, inhibition of the binding of ERp57 and not of CNX correlates with accelerated degradation. The implication is that ERp57 and not CNX has a role in the initial protection of H2a precursor from ERAD.
Figure 7.
Partial and complete inhibition of Glc trimming result in accelerated degradation of H2a and inhibit its association to ERp57 but not to CNX. (A) Cells stably expressing H2a were metabolically labeled as in Figure 2 and chased for 2 h in the absence or in the presence of CST at the indicated concentrations (CST was present during preincubation, labeling, and chase as indicated in MATERIALS AND METHODS). H2a was immunoprecipitated from cell lysates and analyzed by SDS-PAGE followed by fluorography. The asterisk indicates a nonspecific band. Values obtained from PhosphorImager quantitation are plotted for H2a relative to pulse after normalization of the values of pulse labeling (= 100). (B) CoIP of H2a with ERp57 or CNX as in Figure 2, except that cells were metabolically labeled for 40 min in the presence or absence of CST as in A. Values from PhosphorImager quantitation are plotted for H2a bound to ERp57 or CNX relative to the samples that were not treated with CST (= 100). Values were normalized for total H2a as in A. Shown in A and B are experiments representative of three repeat experiments.
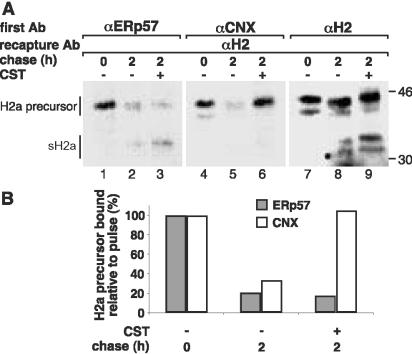
Inhibition of the Excision of the Last Glc Residue Leads to Prolonged Association of H2a Precursor to CNX but Not to ERp57
Posttranslational incubation with Glc trimming inhibitors, after excision of the original Glc residues from the precursor oligosaccharide Glc3Man9GlcNAc2, leads to accumulation of Glc1Man9–7GlcNAc2 on newly synthesized glycoprotein molecules. The stabilized monoglucosylated sugar chains are the result of transfer of a Glc residue by GT, which cannot be removed due to inhibition of glucosidase II (Hebert et al., 1996). Using this principle, we analyzed the association of ERp57 and CNX to H2a upon incubation of cells with CST only during the chase period. Whereas in untreated cells both chaperones dissociated from H2a to a similar extent after 2 h of chase, treatment with CST led to complete recovery of the binding to CNX to a level similar to that seen after the pulse (Figure 8A, middle, and B). In contrast, there was no change in the dissociation of ERp57 from H2a precursor. This suggests that dissociation of CNX is driven by deglucosylation by glucosidase II, whereas ERp57 dissociates from the H2a/CNX complex with time, regardless of the glucosylation and binding status of CNX to H2a. We could observe a very small amount of sH2a (the cleaved 35-kDa H2a ectodomain fragment) associated to ERp57 in untreated cells. The percentage of sH2a associated to ERp57 increased significantly after treatment with CST during the chase period (1.4-fold) (Figure 8A, lane 3). No association to sH2a could be seen for CNX in untreated or treated cells. This suggests that similar to the association with H2b, ERp57 remains associated after processing (cleavage) of H2a to some sH2a molecules likely on their way to secretion.
Figure 8.
Inhibition of the removal of the last Glc extends the association of H2a precursor to CNX and of cleaved sH2a to ERp57. (A) Cells expressing H2a were metabolically labeled for 40 min and chased for the indicated times in the absence or in the presence of 150 μg/ml CST. In this experiment, CST was present only during the chase period. Cell lysates were immunoprecipitated as in Figure 2. The fluorographies in the left and middle panels were exposed 4 times longer than that in the right panel. The experiment is representative of three repeat experiments. (B) Values from PhosphorImager quantitation of the experiment in A are plotted for H2a bound to ERp57 or CNX relative to the pulse (= 100).
DISCUSSION
It is well established that ERp57 associates with glycoproteins during their biosynthesis in ternary complexes with CNX or CRT (Oliver et al., 1999; Helenius and Aebi, 2001; Schrag et al., 2003). However, the need for a ternary complex could be only an initial requirement for ERp57 association to the glycoprotein. We have seen that the precursor of ASGPR H2b remains associated to ERp57 long after dissociation of CNX (Figure 3). Nevertheless, the initial association is dependent on glucose trimming, seemingly through CNX binding (Figure 4D). The interaction of H2b with ERp57 after dissociation of CNX is noncovalent, it is not stabilized by intermolecular disulfide bonds (Figure 4C). Mixed disulfide bonds were seen between ERp57 and Semliki Forest virus proteins (Molinari and Helenius, 1999) and with unassembled MHC class I molecules (Lindquist et al., 2001). However, these covalent intermediates represented a small fraction of the total protein and thus, even in those cases one cannot exclude the existence of noncovalently bound molecules in addition to the covalent binding. The difference between the fast dissociation of ERp57 from H2a and the prolonged association with H2b is striking, given that the only difference between the two variants is a pentapeptide next to the membrane domain. During their syntheses, both H2a and H2b remain in a monomeric state and only a minor portion forms disulfide-linked dimers and small oligomers, and they do not aggregate (Ayalon-Soffer et al., 1999a; Kamhi-Nesher et al., 2001). The differential dissociation of ERp57 from H2a and H2b must relate to the different routing of the two variants, uncleaved H2a precursor to the ERQC, and mature H2b to the Golgi. The prolonged association of ERp57 to H2b is consistent with the trafficking of both proteins to a region adjacent to the ERGIC before H2b is transported to the Golgi (Figure 6). Oxidation or isomerization of disulfide bonds of H2b and its exit to the Golgi are slow (Ayalon-Soffer et al., 1999a; Kamhi-Nesher et al., 2001), which suggests a function for ERp57 in its prolonged interaction with the protein. The degree of association of ERp57 to H2b was similar in the presence or absence of a proteasomal inhibitor (Figure 4B). This is consistent with the fact that proteasomal inhibition rescues a major portion of H2b molecules that can then mature and exit to the Golgi (Kamhi-Nesher et al., 2001).
On proteasomal inhibition, ERp57 is not transported to the ERQC, in contrast to CNX (Figure 1). This is consistent with the fast dissociation of ERp57 from H2a, implying that they separate before transport of the ERAD substrate to the ERQC. Our results are summarized in the model depicted in Figure 9. CNX and CRT are relocalized to the ERQC upon proteasomal inhibition (Kamhi-Nesher et al., 2001). Consistently, CNX was found to segregate from ER exit sites and the ERGIC (Spiliotis et al., 2002). CNX and CRT were not found in a large complex of chaperones bound to BiP (Meunier et al., 2002). Interestingly, ERp57 was not included in the BiP complex either, suggesting that it may shuttle independently between different ER subdomains. CNX concentrated in the ERQC even after cell treatment with tunicamycin, which blocks N-glycosylation and thus inhibits CNX binding to its substrates (Figure 5, G–I). This suggests that CNX does not need to interact with the substrates for its relocalization. We speculate that CNX is normally the carrier of the substrates to the ERQC. This is suggested by a recent report, where overexpression of CNX was shown to slow down the retrotranslocation of mutant CFTR and its deposition in cytosolic aggresomes. Instead, overexpressed CNX and CFTR colocalized in a compartment that according to all its characteristics is the ERQC (Okiyoneda et al., 2003). An interesting question to address in future studies is what signals the concentration of CNX in the ERQC.
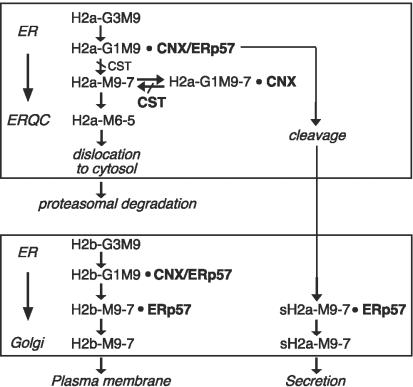
Figure 9.
Working model for differential interactions of H2a and H2b with ERp57 and CNX leading to productive maturation or ERAD. Transfer of the precursor oligosaccharide Glc3Man9GlcNAc2 (G3M9) to the protein takes place in the ER followed by removal of two Glc residues. The glycoproteins then bind CNX and ERp57. Transport of H2a precursor to the ERQC involves rescue attempts with cycles of reglucosylation, CNX binding and deglucosylation. Molecules that cannot be rescued are further trimmed to yield species bearing Man6GlcNAc2 and Man5GlcNAc2 (Frenkel et al., 2003), dislocated to the cytosol and degraded by the proteasomes. Release from CNX can come about by correct processing (cleavage) to sH2a, a portion of which remains bound to ERp57 until it exits the ER to the Golgi on the way to secretion. A significant portion of H2b molecules undergo correct processing (folding) and release from CNX, but remain bound to ERp57 until they exit the ER toward the Golgi and the plasma membrane. Posttranslational inhibition of Glc trimming by CST leads to stable association to CNX; for a minority of the molecules, it leads to stabilization of the initial H2a/CNX/ERp57 complex followed by fast cleavage.
Many studies have addressed the roles of CNX, CRT, and ERp57 in folding and disulfide bond formation. Special attention has been granted to their role in MHC class I folding and assembly (Farmery et al., 2000; Harris et al., 2001; reviewed in Cresswell et al., 1999; Bouvier, 2003). CNX is also a known participant in ER quality control retention. The involvement of ERp57 is however less clear. In ERAD there is a typical lag of about an hour before the onset of degradation. This is also true for H2a (Lederkremer and Lodish, 1991). Therefore, after 45 min of chase, degradation has not yet started and there is already considerable dissociation of ERp57, which suggests that it is unlikely to be involved in the ER retention of the substrate (Figure 2). In contrast, dissociation from CNX is accelerated only after the start of degradation (Figure 3). CNX may thus participate in the retention and routing of H2a to the ERQC, releasing the glycoprotein before degradation takes place. On the other hand, ERp57 could fulfill a different but important role in ER quality control: protection of the substrate from degradation during the initial folding attempts, coincident with the lag before the start of degradation. This role cannot be ascribed to CNX, as suggested by our previous evidence and that of other laboratories (Bennett et al., 1998; Ayalon-Soffer et al., 1999b; Chillaron et al., 2000). Our results show a concentration-dependent correlation between the effect of inhibition of Glc trimming by CST in increasing degradation of H2a and its effect in inhibiting the association of ERp57 (Figure 7). There is no such correlation for the association of CNX. The inhibition of ERp57 binding by a concentration of CST lower than that needed to dissociate CNX suggests the involvement of another lectin, possibly CRT. However, we could not detect binding of CRT to H2a or H2b in coIP experiments (data not shown). There could be several explanations for this failure to detect CRT binding: 1) a technical difficulty due to low-affinity binding, 2) the involvement of another unidentified lectin, or 3) divalent binding of ERp57 to CNX. To test the first possibility, we tried a variety of conditions for coprecipitation with CRT (e.g., mild detergents, cross-linking, and ATP depletion), in all cases with negative results (our unpublished data). The third alternative would imply that conditions that lead to dissociation of 50% of CNX molecules could cause dissociation of 100% of ERp57. Moreover, one could consider the following scenario: if all three sugar chains of H2a bind CNX, there could be a preferred dissociation from one of them, e.g., the middle sugar chain, which may leave two CNX molecules positioned in a way that does not elicit divalent binding of ERp57. Therefore, a small inhibition of CNX binding could lead to a much larger inhibition of ERp57 association. Several recent studies determined the structure of CRT and of the luminal domain of CNX and their sites of interaction with ERp57 (Ellgaard et al., 2001; Schrag et al., 2001; Frickel et al., 2002; Leach et al., 2002). A unique binding site in CNX and CRT suggests monovalent molecules. However, in vivo they may sometimes behave as divalent (Branza-Nichita et al., 2000). For ERp57 cross-linking and isothermal titration microcalorimetry studies of its association to CRT in vitro suggest 1:1 complexes (Frickel et al., 2002). However, the situation could be different in cells, where cross-linking showed 1:1 complexes but higher order associations could not be discarded (Oliver et al., 1999). In a study of unassembled CD1b molecules compared with unassembled class I major histocompatibility complex (MHC) heavy chains, inhibition of Glc trimming led to the increased degradation of both proteins (Hottinger et al., 1999). This was despite the fact that unassembled CD1b bound CNX and CRT, whereas unassembled class I MHC binds only CNX. It could be that like in the case of H2a, it is actually ERp57 who is in charge of the protection of these proteins from degradation. This protection could be accomplished by a role of ERp57 in preventing early improper disulfide bond formation that could lead to aggregation and targeting of the glycoproteins to degradation (Daniels et al., 2003).
Posttranslational inhibition of Glc trimming caused a sharp decrease in the dissociation of CNX due to inhibition of the excision of the last Glc residue (Figure 8). In contrast, the dissociation of ERp57 from H2a precursor was not affected by the persistence or not of the last Glc residue. However, there was an increase in the association of ERp57 to the cleaved fragment sH2a. This fragment encompasses the entire ectodomain and conserves all the sugar chains and cysteines present in the membrane bound precursor. The increased association of ERp57 to sH2a may be the result of inhibition of the removal of the Glc from the small number of molecules that still conserve the initial CNX/ERp57 complex. This complex would then be stabilized, leading after cleavage to an increased association of ERp57 to sH2a molecules presumably on their way to secretion (Figure 9), although we cannot rule out that they might end up degraded. Dissociation of ERp57 from H2a is clearly independent of the dissociation of CNX. CNX remains associated to H2a precursor but dissociates after cleavage, whereas the opposite is true for ERp57. CRT and ERp57 associate with a very low affinity and fast dissociation rate and this may also hold true for the association of CNX and ERp57 (Frickel et al., 2002). Therefore, it is reasonable to think of an independent stabilization of the link between the glycoprotein substrates and the lectins by glucosylation of the sugar chains, whereas their association to ERp57 can be stabilized by a given folding state or by transient disulfide bonds. This behavior fits well our working model depicted in Figure 9: CNX would remain bound to ERAD substrates (uncleaved H2a precursor molecules); traffic to the ERQC; and after trimming of mannose residues, it would release the substrates before degradation takes place (Frenkel et al., 2003). In contrast, ERp57 would remain bound to molecules undergoing productive maturation (cleaved sH2a and H2b membrane-bound precursor) and release them upon exit to the Golgi. Our results are consistent with those obtained with class I MHC where CNX associates with retained unassembled heavy chains and ERp57 (together with CRT) has an extended association with maturing assembled complexes (Farmery et al., 2000; Diedrich et al., 2001; Harris et al., 2001; Paulsson et al., 2001). Future work with other ERAD substrates compared with glycoproteins undergoing productive maturation should explore the generality of our model for the differential roles of CNX and ERp57.
Acknowledgments
We are grateful to T. Wileman for an anti-ERp57 antibody and to H-P. Hauri for the p53myc plasmid and anti-ERGIC53 antibody. This work was supported by a grant from the US-Israel Binational Science Foundation and a grant from the Israel Science Foundation, founded by the Israeli Academy of Sciences.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0899. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–12–0899.
Abbreviations used: ASGPR, asialoglycoprotein receptor; CNX, calnexin; CRT, calreticulin; CST, castanospermine; DSP, 3,3′ dithiobis sulfosuccimidylpropionate; ERAD, endoplasmic reticulum-associated degradation; ERGIC, endoplasmic reticulum-Golgi intermediate compartment; ERQC, endoplasmic reticulum-derived quality control compartment Glc, glucose; IP, immunoprecipitation; Lac, lactacystin; MG-132, N-carbobenzoxyl-leucinyl-leucinyl-leucinal.
References
- Ayalon-Soffer, M., Kamhi-Nesher, S., and Lederkremer, G.Z. (1999a). Folding and self-assembly do not prevent ER retention and proteasomal degradation of asialoglycoprotein receptor H2a. FEBS Lett. 460, 112–116. [DOI] [PubMed] [Google Scholar]
- Ayalon-Soffer, M., Shenkman, M., and Lederkremer, G.Z. (1999b). Differential role of mannose and glucose trimming in the ER degradation of asialoglycoprotein receptor subunits. J. Cell Sci. 112, 3309–3318. [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Van, L.J., and Kearse, K.P. (1998). Calnexin association is not sufficient to protect T cell receptor alpha proteins from rapid degradation in CD4(+)CD8(+) thymocytes. J. Biol. Chem. 273, 23674–23680. [DOI] [PubMed] [Google Scholar]
- Bouvier, M. (2003). Accessory proteins and the assembly of human class I MHC molecules: a molecular and structural perspective. Mol. Immunol. 39, 697–706. [DOI] [PubMed] [Google Scholar]
- Branza-Nichita, N., Negroiu, G., Petrescu, A.J., Garman, E.F., Platt, F.M., Wormald, M.R., Dwek, R.A., and Petrescu, S.M. (2000). Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J. Biol. Chem. 275, 8169–8175. [DOI] [PubMed] [Google Scholar]
- Chillaron, J., Adan, C., and Haas, I.G. (2000). Mannosidase action, independent of glucose trimming, is essential for proteasome-mediated degradation of unassembled glycosylated Ig light chains. Biol. Chem. 381, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Cresswell, P., Bangia, N., Dick, T., and Diedrich, G. (1999). The nature of the MHC class I peptide loading complex. Immunol. Rev. 172, 21–28. [DOI] [PubMed] [Google Scholar]
- Daniels, R., Kurowski, B., Johnson, A.E., and Hebert, D.N. (2003). N-Linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol. Cell 11, 79–90. [DOI] [PubMed] [Google Scholar]
- Diedrich, G., Bangia, N., Pan, M., and Cresswell, P. (2001). A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J. Immunol. 166, 1703–1709. [DOI] [PubMed] [Google Scholar]
- Ellgaard, L., Riek, R., Herrmann, T., Guntert, P., Braun, D., Helenius, A., and Wuthrich, K. (2001). NMR structure of the calreticulin P-domain. Proc. Natl. Acad. Sci. USA 98, 3133–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmery, M.R., Allen, S., Allen, A.J., and Bulleid, N.J. (2000). The role of ERp57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. J. Biol. Chem. 275, 14933–14938. [DOI] [PubMed] [Google Scholar]
- Frenkel, Z., Gregory, W., Kornfeld, S., and Lederkremer, G.Z. (2003). Endoplasmic reticulum-associated degradation of mammalian glycoproteins involves sugar chain trimming to Man6–5GlcNAc2. J. Biol. Chem. 278, 34119–34124. [DOI] [PubMed] [Google Scholar]
- Frickel, E.M., Riek, R., Jelesarov, I., Helenius, A., Wuthrich, K., and Ellgaard, L. (2002). TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc. Natl. Acad. Sci. USA 99, 1954–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M.R., Lybarger, L., Yu, Y.Y., Myers, N.B., and Hansen, T.H. (2001). Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. J. Immunol. 166, 6686–6692. [DOI] [PubMed] [Google Scholar]
- Hebert, D.N., Foellmer, B., and Helenius, A. (1996). Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 15, 2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2001). Intracellular functions of N-linked glycans. Science 291, 2364–2369. [DOI] [PubMed] [Google Scholar]
- Hottinger, R., Staffler, G., Majdic, O., and Stockinger, H. (1999). Analysis of the early biogenesis of CD1b: involvement of the chaperones calnexin and calreticulin, the proteasome and beta(2)-microglobulin. Int. Immunol. 11, 1615–1623. [DOI] [PubMed] [Google Scholar]
- Huyer, G., Longsworth, G.L., Mason, D.L., Mallampalli, M.P., McCaffery, J.M., Wright, R.L., and Michaelis, S. (2004). A striking quality control subcompartment in Saccharomyces cerevisiae: the endoplasmic reticulum-associated compartment (ERAC). Mol. Biol. Cell 15, 908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi-Nesher, S., Shenkman, M., Tolchinsky, S., Fromm, S.V., Ehrlich, R., and Lederkremer, G.Z. (2001). A novel quality control compartment derived from the endoplasmic reticulum. Mol. Biol. Cell 12, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S.H., Lindstrom, J., and Taylor, P. (1998). Inhibition of glucose trimming with castanospermine reduces calnexin association and promotes proteasome degradation of the alpha-subunit of the nicotinic acetylcholine receptor. J. Biol. Chem. 273, 17064–17072. [DOI] [PubMed] [Google Scholar]
- Leach, M.R., Cohen-Doyle, M.F., Thomas, D.Y., and Williams, D.B. (2002). Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J. Biol. Chem. 277, 29686–29697. [DOI] [PubMed] [Google Scholar]
- Lederkremer, G.Z., and Lodish, H.F. (1991). An alternatively spliced miniexon alters the subcellular fate of the human asialoglycoprotein receptor H2 subunit. Endoplasmic reticulum retention and degradation or cell surface expression. J. Biol. Chem. 266, 1237–1244. [PubMed] [Google Scholar]
- Lindquist, J.A., Hammerling, G.J., and Trowsdale, J. (2001). ER60/ERp57 forms disulfide-bonded intermediates with MHC class I heavy chain. FASEB J. 15, 1448–1450. [DOI] [PubMed] [Google Scholar]
- Meunier, L., Usherwood, Y.-K., Chung, K.T., and Hendershot, L.M. (2002). A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell 13, 4456–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari, M., and Helenius, A. (1999). Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature 402, 90–93. [DOI] [PubMed] [Google Scholar]
- Moore, S.E., and Spiro, R.G. (1993). Inhibition of glucose trimming by castanospermine results in rapid degradation of unassembled major histocompatibility complex class I molecules. J. Biol. Chem. 268, 3809–3812. [PubMed] [Google Scholar]
- Okiyoneda, T., Harada, K., Takeya, M., Yamahira, K., Wada, I., Shuto, T., Suico, M.A., Hashimoto, Y., and Kai, H. (2004). {Delta}F508 CFTR pool in the ER is increased by calnexin overexpression. Mol. Biol. Cell 15, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J.D., Roderick, H.L., Llewellyn, D.H., and High, S. (1999). ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell 10, 2573–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J.D., vanderWal, F.J., Bulleid, N.J., and High, S. (1997). Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science 275, 86–88. [DOI] [PubMed] [Google Scholar]
- Parodi, A.J. (2000). Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 69, 69–93. [DOI] [PubMed] [Google Scholar]
- Paulsson, K.M., Wang, P., Anderson, P.O., Chen, S., Pettersson, R.F., and Li, S. (2001). Distinct differences in association of MHC class I with endoplasmic reticulum proteins in wild-type, and beta2-microglobulin- and TAP-deficient cell lines. Int. Immunol. 13, 1063–1073. [DOI] [PubMed] [Google Scholar]
- Schrag, J.D., Bergeron, J.J., Li, Y., Borisova, S., Hahn, M., Thomas, D.Y., and Cygler, M. (2001). The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 8, 633–644. [DOI] [PubMed] [Google Scholar]
- Schrag, J.D., Procopio, D.O., Cygler, M., Thomas, D.Y., and Bergeron, J.J.M. (2003). Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem. Sci. 28, 50–58. [DOI] [PubMed] [Google Scholar]
- Shenkman, M., Ayalon, M., and Lederkremer, G.Z. (1997). Endoplasmic reticulum quality control of asialoglycoprotein receptor H2a involves a determinant for retention and not retrieval. Proc. Natl. Acad. Sci. USA 94, 11363–11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis, E.T., Pentcheva, T., and Edidin, M. (2002). Probing for membrane domains in the endoplasmic reticulum: retention and degradation of unassembled MHC class I molecules. Mol. Biol. Cell 13, 1566–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolchinsky, S., Yuk, M.H., Ayalon, M., Lodish, H.F., and Lederkremer, G.Z. (1996). Membrane-bound versus secreted forms of human asialoglycoprotein receptor subunits - role of a juxtamembrane pentapeptide. J. Biol. Chem. 271, 14496–14503. [DOI] [PubMed] [Google Scholar]
- Wang, T., and Hebert, D.N. (2003). EDEM an ER quality control receptor. Nat. Struct. Biol. 10, 319–321. [DOI] [PubMed] [Google Scholar]
- Yuk, M.H., and Lodish, H.F. (1993). Two pathways for the degradation of the H2 subunit of the asialoglycoprotein receptor in the endoplasmic reticulum. J. Cell Biol. 123, 1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk, M.H., and Lodish, H.F. (1995). Enhanced folding and processing of a disulfide mutant of the human asialoglycoprotein receptor H2b subunit. J. Biol. Chem. 270, 20169–20176. [DOI] [PubMed] [Google Scholar]
- Zapun, A., Darby, N.J., Tessier, D.C., Michalak, M., Bergeron, J.J., and Thomas, D.Y. (1998). Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J. Biol. Chem. 273, 6009–6012. [DOI] [PubMed] [Google Scholar]