Abstract
Although several paradigms have shown that threatening faces are processed preferentially, no study to date has investigated whether this preferential processing can be manipulated by value associations. Using schematic faces, this study was divided into three phases in order to investigate the effects of associating high values with happy faces and low values with angry faces. The baseline phase, in which elicited a shorter RT and a larger N2pc for angry faces than for happy faces, demonstrated that the preferential processing of angry faces could be obtained in the discrimination task. After the training phase, which established associations between different face targets and their respective values, the anger superiority effect remained absent in a subsequent test phase despite the fact that participants clearly understood that no reward (gain) or punishment (loss) would be provided. Our investigation shows that the ‘anger superiority effect’ can be modified by value associations and that the value effect, rather than the impact of endogenous attention, played a more crucial role in manipulating the preferential processing of angry faces.
Keywords: preferential processing, angry faces, discrimination task, value associations, N2pc
INTRODUCTION
To survive in a world full of various stimuli, individuals need to process threatening ones as quickly as possible. Individuals must also respond to stimuli which can give rise to beneficial results (e.g. food). Although rapid responses to threatening stimuli serve an evolutionary survival function (Öhman and Mineka, 2001), the approach behavior to valued stimuli also has clear adaptive benefits and plays a fundamental role in human cognition (Hickey et al., 2010).
Investigations traditionally use angry faces as threat-relevant stimuli. The preferential processing of angry faces, also known as the ‘anger superiority effect’, has been demonstrated by many paradigms, including visual search (Hansen and Hansen, 1988; Öhman et al., 2009), attentional blink (Maratos et al., 2008) and visual-probe tasks (Holmes et al., 2009). From an evolutionary perspective, this preferential processing can be explained by the rapid attentional allocation required to facilitate appropriate responses to threatening stimuli (Öhman and Mineka, 2001; Holmes et al., 2009; but see Becker et al., 2011; Purcell and Stewart, 2010, for a perceptual confounds explanation).
Experiments have widely used N2pc component to investigate this attentional bias (e.g. Eimer and Kiss, 2007; Fenker et al., 2010; Feldmann-Wüstefeld et al., 2011; Weymar et al., 2011). The N2pc component is an effective electrophysiological marker of spatial selective attention (Luck and Hillyard, 1994; Eimer, 1996; Woodman and Luck, 1999; Kiss et al., 2008). It is an enhanced negativity, typically elicited at posterior electrodes contralateral to the target presented among distractors between 180 and 300 ms after the onset of stimuli display (Luck and Hillyard, 1994; Eimer, 1996; Woodman and Luck, 1999). Its amplitude indicates the amount of attention deployed to task-relevant items (Luck, 2005) or to other salient but task-irrelevant stimuli (Hickey et al., 2006). This component has been linked to the suppression of task-irrelevant stimuli (Luck and Hillyard, 1994; Luck et al., 1997; Luck, 2005) or to the spatially selective attentional processing of task-relevant stimuli in visual searches (Eimer, 1996; Kiss et al., 2008; Mazza et al., 2009). Previous studies using N2pc have provided direct evidence that the preferential processing of threatening faces may be due to more and earlier attentional allocation to these threat-related stimuli (Feldmann-Wüstefeld et al., 2011; Weymar et al., 2011).
Dysfunction in the neural mechanisms controlling threat-related attentional bias, however, can have a negative impact on individuals—processing dysfunction, for instance, may be implicated in causing and maintaining symptoms of anxious individuals (Mogg and Bradley, 1998; Bar-Haim et al., 2007; Holmes et al., 2009). This can be demonstrated by the finding that trait anxiety observers showed an attentional bias to angry faces which modulated by trait anxiety levels (Fox et al., 2008; Eldar et al., 2010). However, it still remains unclear that whether this preferential processing of threatening stimuli is ‘hard-wired’ in our brain or is plastic and can be changed by past experience. If it is plastic and can be modified by past experience, this study may suggest the possibility of the therapeutic intervention for individuals suffering from specific attentional bias. In light of these considerations, the purpose of this study is to examine the effects of value associations on this preferential processing by associating high values with happy faces and low values with angry faces.
The value associations acquired from past experience, such as reward (gain) or punishment (loss) associations, have been demonstrated to have important impact on cognitive processing. This claim has received support from findings such as the stronger negative priming effect after a high-magnitude reward (Della Libera and Chelazzi, 2006), and the substantially enhanced recognition and boosted visual processing efficiency of high-valued stimuli after associations between stimuli and high values (gains or losses) were established (Raymond and O’Brien, 2009; O’Brien and Raymond, 2012). They have also received support from investigations using N2pc, which found larger and earlier N2pc for targets associated with high-magnitude reward (Kiss et al., 2009), and a distractor-elicited N2pc for task-irrelevant stimuli associated with a high value (gain or loss; Hickey et al., 2010).
Using a discrimination task, which was able to establish explicit associations between different values and faces, the effect of value associations on the anger superiority effect was examined. We hypothesize that if different value levels can manipulate preferential processing, then the N2pc elicited by angry faces will be similar to (or even smaller than) the N2pc elicited by happy faces; otherwise, the preferential processing should still exist and be reflected by a stronger N2pc for angry faces.
BASELINE PHASE
Although the anger superiority effect has been demonstrated by numerous paradigms, it has not been investigated on the basis of discrimination tasks. The aim of the baseline phase was to determine whether it existed in a discrimination task.
Methods
Participants
Eighteen volunteers from the Southwest University at Chongqing, China, participated in the experiment (ages 19–24 years, mean age 21.7 years, nine female). Informed consent was obtained before the baseline phase. Three participants were excluded due to an excessive rate of ocular artifacts (see data analysis). All participants were right-handed, exhibited normal or corrected-to-normal visual acuity and color vision, with no reported history of neurological disorder. All of the experimental procedures were approved by the Ethics Board of the Southwest University.
Stimuli and procedure
Participants sat in a dimly lit room and faced a computer screen at a distance of 80 cm. The baseline phase consisted of four blocks of 96 trials, preceded by one practice block of 36 trials.
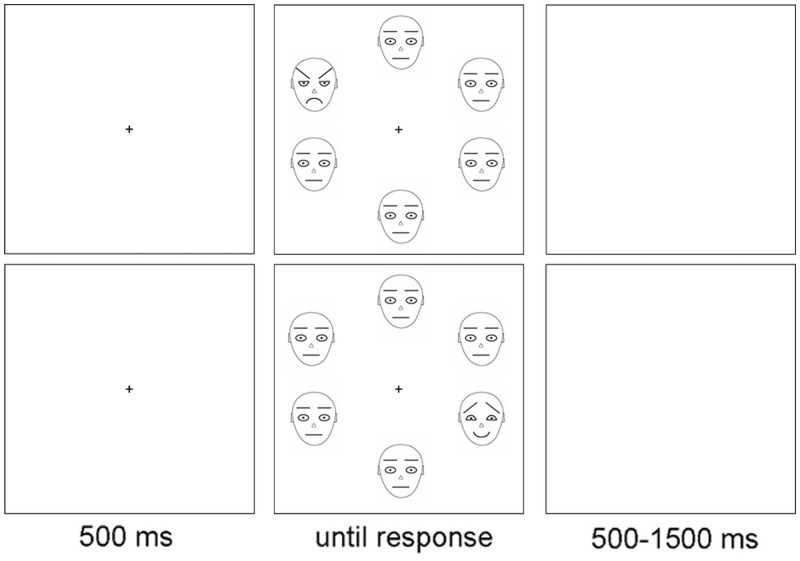
In order to control physical features of faces and minimize drawbacks caused by idiosyncrasies and instantiations of particular faces, schematic faces were used in this study. The use of schematic faces, however, may lead to an attenuation of ecological validity (Öhman et al., 2001). The schematic faces were drawn in black at a white background and represented angry, happy and neutral expressions (Öhman et al., 2001). The outline of the face and nose was drawn with 1-pixel lines, whereas the remaining parts were drawn with 2-pixel lines (Weymar et al., 2011). A fixation point was presented 500 ms at the center of the screen, followed by the stimuli display which was presented until response. The interval between trials varied from 500 to 1500 ms (Figure 1).
Fig. 1.
Illustration of the sequence of events. The stimulus display contained one angry face and five neural faces (top) or one happy face and five neural faces (bottom). Target faces appeared at each position randomly.
The stimulus display consisted of six faces (1.37° width × 1.68° height) displayed in a circle. Half of the trials contained one angry face and five neutral faces, whereas the remaining trials contained one happy face and five neutral faces. Participants were instructed to discriminate the target face by pressing corresponding keys on a standard keyboard: the ‘F’ (or ‘J’) key for angry faces and the ‘J’ (or ‘F’) key for happy faces. Response keys were counterbalanced across participants. Targets appeared 32 times at each position randomly. Participants were instructed to keep their eyes on the fixation point and to minimize blinking or eyes movements during the active parts of the experiment and to respond as accurately and quickly as possible.
Apparatus and data analysis
Brain electrical activity was recorded at 64 scalp sites using tin electrodes mounted in an elastic cap (Brain Products, Munich, Germany), with references on the left and right mastoids, and a ground electrode on the medial frontal aspect. The vertical electro-oculograms (EOGs) were recorded supra- and infra-orbitally at the right eye. The horizontal EOG was recorded from the left vs right orbital rim. All electrode impedance was <5 kΩ. The EEG and EOG were amplified using a 0.05–100 Hz bandpass and continuously digitized at 500 Hz/channel for offline analysis. Eye movement artifacts (blinking and eye movements) were excluded offline. Trials with EOG artifacts (exceeding ±30 µV) and those contaminated with artifacts due to amplifier clipping and peak-to-peak deflection exceeding ±80 µV from averaging were excluded from further analysis. Three participants were excluded as their residual horizontal EOG exceeded ±4 µV in at least one experimental condition. Approximately 13% of the trials were excluded from averaging because of ocular and movements artifacts. Only trials with correct responses were analyzed.
The averaged epoch was 500 ms, including 100 ms pre-stimulus and 400 ms post-stimulus. Separate averages were computed for each face type (angry or happy) and each contralaterality (electrode ipsilateral or contralateral to the target location). The ipsilateral waveform was computed as the average of the left-sided electrode to the left-sided targets and the right-sided electrode to the right-sided targets, whereas the contralateral waveform was computed as the average of the left-sided electrode to the right-sided targets and the right-sided electrode to the left-sided targets. Analyses focused on PO7 and PO8, where N2pc were maximal (Brisson and Jolicœur, 2008; McDonald et al., 2009). Only trials with targets appearing at lateral locations were included in analyses. The N2pc were quantified as the mean amplitudes voltage within an early (150–190 ms) and a later (230–320 ms) post-stimulus time window. For all analyses, Greenhouse-Geisser adjustments to the degrees of freedom were used where appropriate.
Results
Behavioral results
For reaction times (RTs), trials with RTs exceeding mean ± 3 s.d. for each participant were excluded from analyses. The mean RT for angry faces was shorter than for happy faces (737 ms vs 805 ms), t(14) = 5.34, P < 0.001. For response accuracy, the difference between angry and happy faces was not significant (96.3% vs 96.5%), t(14) = 0.298, P = 0.77.
ERP results
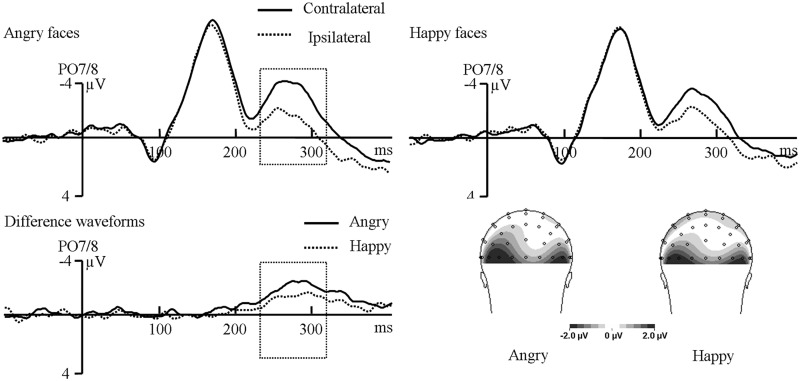
Figure 2 illustrates the event-related potentials (ERPs) elicited by face targets at PO7/8 electrodes in the baseline phase. A repeated-measures analysis of variance (ANOVA) on N2pc values with contralaterality (contralateral to the targets vs ipsilateral to the targets) and face types (angry vs happy) as factors was conducted. In the early time window, only the interaction between contralaterality and face types was significant, F(1,14) = 6.72, P = 0.021, suggesting a larger early N2pc for angry faces than for happy faces. This was confirmed by the simple effect test that the early N2pc only presented in response to angry faces, P = 0.033. In the later time window, there was a significant main effect of contralaterality, F(1,14) = 43.66, P < 0.001, demonstrating the presence of the later N2pc. Importantly, there was a significant interaction between contralaterality and face type, F(1,14) = 6.75, P = 0.021, showing that the later N2pc was larger for angry faces than for happy faces. The simple effect test confirmed the presence of later N2pc (Md = Mcontra − Mipsi) in response to angry faces (Md = −1.91 µV), P < 0.001 and happy faces (Md = −1.23 µV), P < 0.001. For the latencies of N2pc, the difference between angry and happy faces (282 ms vs 286 ms) was not significant, t(14) = 0.77, P = 0.45.
Fig. 2.
Grand-averaged ERPs and scalp topographic maps elicited by angry faces and happy faces at PO7/8 electrodes in the baseline phase.
Discussion
The behavioral results suggested that angry faces were discriminated more quickly than happy faces, which was consistent with previous studies that found a detection advantage for real (e.g. Horstmann and Bauland, 2006; Pinkham et al., 2010; Schmidt-Daffy, 2011) as well as schematic angry faces (e.g. Öhman et al., 2001; Lundqvist and Öhman, 2005). The larger N2pc for angry faces than for happy faces in both the early and later time window suggested that more attention was deployed to angry faces than to happy faces. These results showed that the anger superiority effect indeed existed in discrimination tasks.
TRAINING PHASE
The main goal of the training phase was to establish associations between different face targets and their respective values.
Methods
Participants, materials and methods during this phase were the same as the baseline phase, except for the new value manipulation. Correct responses to happy faces were followed by ‘+10’ feedbacks (gain) and incorrect responses were followed by ‘−10’ feedbacks (loss). Correct responses to angry faces were followed by ‘+1’ feedbacks (gain) and incorrect responses were followed by ‘−1’ feedbacks (loss). Each point was worth ∼1.42 RMB cents, and there was an accumulated reward feedback after each block. The maximum pay was 30 RMB yuan, and no participant earned <28 RMB yuan. Participants were told clearly that their pay would correspond to the total points earned during the training phase. This phase started when participants reported they had rested sufficiently (mean = 7.07 min, s.d. = 1.03) after the baseline phase.
Results
Behavioral results
In contrast to the baseline phase, the difference between angry and happy faces (691 ms vs 715 ms) was not significant, t(14) = 1.6, P = 0.132. For response accuracy, there was also no significant difference between angry and happy faces (98.3% vs 98.6%), t(14) = 0.745, P = 0.469.
ERP results
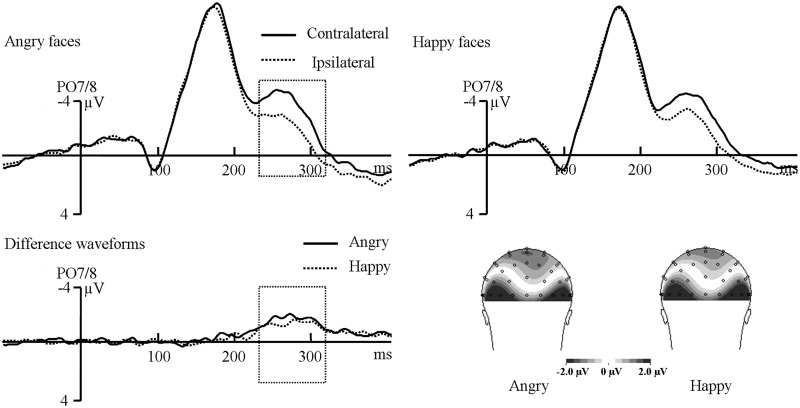
Figure 3 illustrates the ERPs obtained in the training phase. A repeated-measures ANOVA on N2pc values with contralaterality and face type as factors revealed no significant effect in the early time window, all Ps > 0.12. In the later time window, a significant main effect of contralaterality was observed, F(1,14) = 20.09, P = 0.001, demonstrating the presence of N2pc for both face targets. Importantly, there was no significant interaction between contralaterality and face type, F(1,14) = 2.54, P = 0.133, indicating that the N2pc triggered by angry faces was similar to happy faces. For the latencies of N2pc, the difference (279 ms vs 274 ms) was not significant, t(14) = 0.80, P = 0.44.
Fig. 3.
Grand-averaged ERPs and scalp topographic maps elicited by angry faces and happy faces at PO7/8 electrodes in the training phase.
Discussion
We found, as reflected by both the behavioral and ERP data, that the anger superiority effect disappeared. This demonstrated that the immediate outcome feedback could impact the processing of emotional faces and that the stimulus–value association had been established.
TEST PHASE
The test phase was designed to investigate whether the anger superiority effect could be changed after having already established the value associations. In this phase, participants were informed quite clearly that no reward or punishment would be given. As hypothesized, if previously established value associations can change the preferential processing, the N2pc elicited by angry faces should be similar to (or even smaller than) happy faces; otherwise, an enhanced N2pc to angry faces should still be observed.
Methods
Participants, materials and methods were the same as the baseline phase. The test phase started when participants reported they had enough rest (mean = 6.51 min, s.d. = 1.18) after the training phase.
Results
Behavioral results
Consistent with the training phase, the RT difference between angry and happy faces (674 ms vs 692 ms) was not significant, t(14) = 1.64, P = 0.124. For response accuracy, there was also no significant difference between face targets (96.5% vs 96.4%), t(14) = 0.242, P = 0.812.
Additionally, we performed a repeated-measures ANOVA on RTs and accuracy to targets with phase (the baseline phase vs the training phase vs the test phase) and face type as factors. For RTs, both the phase and face type showed a significant main effect, F(2,28) = 29.55, P < 0.001, and F(1,14) = 8.96, P = 0.01, suggesting different RTs across phases and between face types. The interaction between phase and face type was also significant, F(2,28) = 23.64, P < 0.001, showing that the RT differences between angry and happy faces varied across phases with the introduction of value associations. The simple effect test confirmed that the RT difference between angry and happy faces was only significant in the baseline phase (P < 0.001) and was eliminated by the value manipulation in the training phase (P = 0.132) and the test phase (P = 0.124), which was consistent with the results of separate analyses in the three phases. Furthermore, both the mean RT to angry faces and happy faces were shorter in the training phase (Ps < 0.005) and the test phase (Ps < 0.001) than in the baseline phase, but the differences between the training phase and the test phase were not significant, Ps > 0.10. For accuracy, only the main effect of phase was significant, F(2,28) = 12.34, P = 0.001. Follow-up tests revealed a higher accuracy in the training phase (98.5%) than in baseline phase (96.4%), P < 0.001, and in test phase (96.5%), P = 0.006, indicating that the immediate outcome feedback could improve participants’ task performance.
ERP results
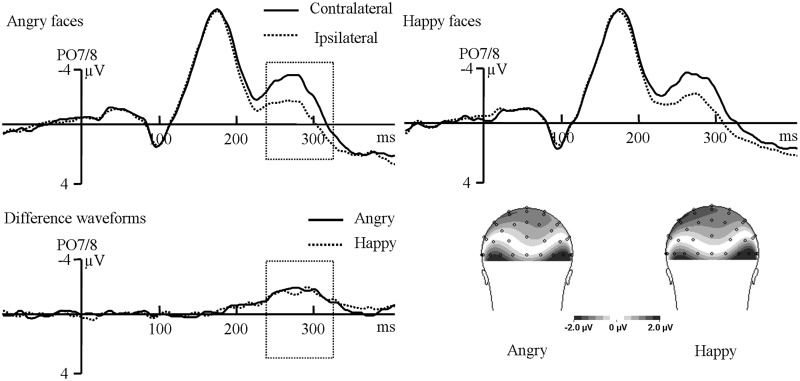
Figure 4 illustrates the ERPs elicited by face targets in the test phase. No significant effect was observed in the early time window, all Ps > 0.14. In the later time window, the main effect of contralaterality was significant, F(1,14) = 28.34, P < 0.001, reflecting the presence of the N2pc elicited by face targets. Importantly, there was no significant interaction between contralaterality and face type, F(1,14) = 0.095, P = 0.76, suggesting similar N2pc patterns triggered by angry and happy faces. For the latencies of N2pc, although the N2pc to happy faces was numerically earlier than to angry faces (279 ms vs 268 ms), the difference was not significant, t(14) = 1.65, P = 0.12.
Fig. 4.
Grand-averaged ERPs and scalp topographic maps elicited by angry faces and happy faces at PO7/8 electrodes in the test phase.
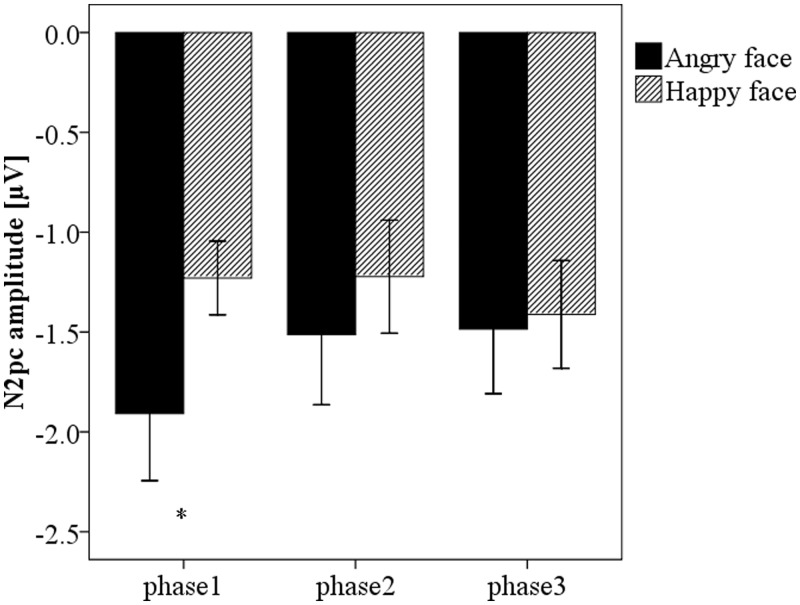
Because the early N2pc seems increased in the training phase, we conducted a repeated-measures ANOVA on the early N2pc values with phase, face type and contralaterality as factors. There was only a significant main effect of phase, F(2,28) = 60.74, P < 0.001. Follow-up tests showed that the early N2pc in the training phase was larger than in the baseline phase and the test phase, Ps < 0.001, while the difference between the baseline phase and the test phase was not significant, P > 0.99. In the later time window, we performed a repeated-measures ANOVA on N2pc amplitude (Md = Mcontra − Mipsi) and latencies across phases. For the amplitude, the main effect of phase (F < 1) and face type (F(1,14) = 3.26, P = 0.093) was not significant. Crucially, the interaction between phase and face type was significant, F(2,28) = 3.9, P = 0.032, suggesting that the anger superiority effect had been eliminated. The simple effect test showed that a larger N2pc for angry faces than for happy faces was only obtained in the baseline phase (P = 0.021), whereas similar N2pc patterns were found in the training phase (P = 0.182) and the test phase (P = 0.241), which was consistent with the results of separate analyses in the three phases (Figure 5). Moreover, the N2pc for angry faces was marginally smaller in the training phase (P = 0.067) and significantly smaller in the test phase (P = 0.03) than in the baseline phase, but the difference between the training phase and the test phase was not significant, P = 0.88. However, there was no significant difference of N2pc for happy faces across phases, all Ps > 0.30, indicating that the N2pc to happy faces remained relatively stable across the three phases. For the latencies, there was no significant effect, all Ps > 0.14.
Fig. 5.
Magnitude of the N2pc amplitude (contralateral minus ipsilateral) for angry and happy faces across all phases. Asterisks indicate significant differences (P < 0.05).
Discussion
The test phase replicated the results of the training phase, although participants clearly knew that no reward or punishment provided in this phase. The continued absence of the anger superiority effect from the training phase to the test phase suggested that, by associating target faces with values (gain or loss) at an earlier time (the training phase), the anger superiority effect could be eliminated. However, as reflected by the shorter RTs to both face targets in the training phase and the test phase than in the baseline phase, there may be a practice effect facilitating responses to face targets that may contaminate the value effect.
CONTROL EXPERIMENT
The aim of the control experiment was to examine whether the practice effect could potentially contaminate the current findings. Participants might have become extremely practiced after completing three successive phases. If the absence of the anger superiority effect in the test phase was indeed the result of value associations, we should observe it throughout the control experiment. If it was due to the practice effect, however, then it should disappear in the last phase of the control experiment.
Methods
Sixteen new participants participated in the control experiment (ages 20–24 years, mean age 22.5 years, eight females), none of whom participated in the previous three phases. The control experiment also consisted of three phases. The material and methods in these three phases were the same as the previous ones, except that no value was involved and no brain electrical activity was recorded.
Results
The repeated-measures ANOVA on RTs with phase (control phase 1 vs control phase 2 vs control phase 3) and face type (angry vs happy) as factors showed no significant interaction between phase and face type, F < 1. The significant main effect of face type, F(1,15) = 36.43, P < 0.001, indicating faster responses to anger faces than to happy faces throughout the three phases (750 ms vs 795 ms, 698 ms vs 734 ms and 682 ms vs 718 ms for control phase 1, 2 and 3, respectively). The main effect of phase was also significant, F(2,30) = 31.04, P < 0.001. Follow-up tests revealed that the mean RT in the control phase 1 was longer than the mean RT in the control phase 2 and phase 3 (Ps < 0.001), whereas the difference between the control phases 2 and 3 was not significant, P = 0.114, which demonstrated that there may be a practice effect with the continuous performing on the tasks from control phase 1 to phase 2. For response accuracy, there was only a significant main effect of phase, F(2,30) = 7.24, P = 0.003. Follow-up tests showed that the accuracy in control phase 3 was higher than in control phase 1, P = 0.013.
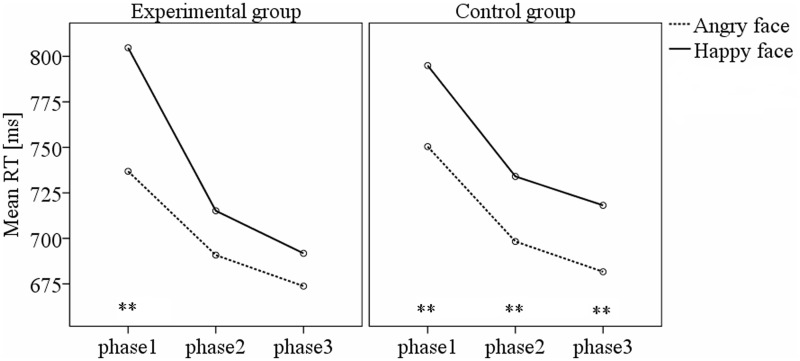
Furthermore, we also conducted a repeated-measures ANOVA on RTs with phase and face type as within-subjects factors, and group (experimental vs control) as between-subjects factors. There was a significant main effect phase, F(2,58) = 60.47, P < 0.001, with accelerated responses from the first phases to subsequent phases of the experimental group and control group. The main effect of face type was also significant, F(1,29) = 30.87, P < 0.001, reflecting an overall faster responses to angry faces than to happy faces. Moreover, the interaction between phase and face type was significant, F(2,58) = 17.54, P < 0.001, suggesting that the difference between face targets varied across phases. Importantly, there was a significant interaction across the three factors, F(2,58) = 8.61, P = 0.001, indicating that the RT patterns to different face targets across phases were different between different groups. This could be confirmed by the separate ANOVAs conducted in experimental and control group, respectively, which revealed that the anger superiority effect only existed in the baseline phase in the experimental group while it persisted throughout the three phases in the control group (Figure 6).
Fig. 6.
Mean RT for angry faces and happy faces in experimental group (left panel) and control group (right panel). Asterisks indicate significant differences (P < 0.001).
Discussion
Participants responded faster to angry faces than to happy faces, demonstrating the existence of the anger superiority effect throughout the control experiment. Admittedly, as reflected by the main effect of phase, although there was indeed a practice effect with the progression of the experiment, this practice effect only indiscriminatingly accelerated responses to both face targets instead of eliminating the preferential processing of angry faces. These results, particularly the three-way interaction, seemed to suggest that the absence of the anger superiority effect in the test phase was indeed due to the value effect rather than the practice effect.
GENERAL DISCUSSION
In this study, three key phases were conducted to investigate whether the preferential processing of angry faces could be manipulated by value associations. The baseline phase provided evidence that the anger superiority effect is obtainable in the discrimination task. The training phase established associations between face targets and values. The results of analyses within and across phases and in the control experiment demonstrated that the anger superiority effect can indeed be modified by value associations.
The N2pc component reflects spatial selective attention (Luck and Hillyard, 1994; Eimer, 1996; Woodman and Luck, 1999; Kiss et al., 2008). The larger N2pc for angry faces in the baseline phase suggests that more selective attention was deployed to angry faces. This result is consistent with previous studies (e.g. Eimer and Kiss, 2007; Feldmann-Wüstefeld et al., 2011; Weymar et al., 2011). Results based on our discrimination task extend previous results based on detection tasks (Hansen and Hansen, 1988; Öhman et al., 2009), attentional blink (Maratos et al., 2008) and visual-probe tasks (Holmes et al., 2009).
The presence of anger superiority effect in the discrimination task prompted us to associate different values with different face targets in the training phase. Similar RT and N2pc patterns for angry and happy faces demonstrated that stimulus–value associations had been established.
Crucially, the elimination of anger superiority effect observed in the training phase persisted in the test phase, despite the explicit instruction that none of the face targets would yield any reward or punishment. Similar findings of the continuous value effect were also obtained in earlier studies, which found facilitatory visual processing of items associated with a high value after value associations (Della Libera and Chelazzi, 2009; O’Brien and Raymond, 2012). Furthermore, because participants clearly knew that no reward or punishment was provided in the test phase, different outcomes could no longer be expected based on different faces. Hence, there should be no different motivational significance or endogenous bias caused by different expected outcomes, therefore, leaving the value effect mainly responsible for the absence of anger superiority effect. However, we could not completely exclude the contribution of attentional set in processing of target faces, since there was no particular motivation for participants to switch their attentional set during the test phase, leading to the possibility of an attentional perseveration effect. Thus, the disappearance of anger superiority effect in the test phase could reflect, to some extent, the effect of residual attentional set that has been established during the training phase.
To further clarify the effect of value associations on the elimination of the anger superiority effect, we excluded the practice effect which may contaminate the value effect with a control experiment and revealed an interaction between phase and face type. The results seemed to suggest that the effect of value associations were responsible for modifying the anger superiority effect. Specifically, we compared ERPs with face targets across phases to examine the specific details which mainly contributed to the disappearance of the anger superiority effect. The early N2pc amplitude increased in the training phase compared with other phases (Figures 2–4), which may have been due to higher activation levels caused by the immediate reward or punishment feedbacks. Importantly, the results also showed that the N2pc for angry faces became smaller with the introduction of value manipulation, whereas it remained stable across phases for happy faces. That is to say, the value effect (including both the reward effect and punishment threat) diminished the salience of the angry faces and extinguished the ability of these stimuli to capture more attention than happy faces as during the baseline phase. This result was reflected by the smaller N2pc for angry faces in the training phase and particular in the test phase compared with in the baseline phase, which directly caused similar N2pc patterns for angry and happy faces in the training phase and the test phase. Consequently, angry faces showed no preferential processing. However, the value effect did not directly and significantly enhance the attentional processing of happy faces, as reflected by the stable N2pc across phases.
In contrast to previous studies which demonstrated facilitated processing of stimuli associated with high values (Hickey et al., 2010; O’Brien and Raymond, 2012), the value effect eliminated the anger superiority effect by weakening the saliency of angry faces and making them less attention-drawing, rather than directly enhancing the attentional processing of happy faces associated with high values. This difference may be attributable to elimination of the anger superiority effect involved in both the processing of reward-related and threatening stimuli, which can be modulated by the activation of the mesencephalic dopamine system and amygdala (Anderson and Phelps, 2001; Schultz, 2002; Öhman, 2005; Murray, 2007), while in previous studies using stimuli without emotional valences, only the processing of reward-related stimuli was implicated. Nevertheless, this study using the ERP technique could not shed light on the exact mechanisms underlying the elimination of the anger superiority effect. Further studies using fMRI technique are necessary to investigate the exact role of them and other areas that may be involved.
It should be noted that the accuracy in the training phase, which was crucial to establish the value associations, was remarkably high (98.3% vs 98.6% for angry and happy faces). As a consequence, most of the feedback was reward feedback (only around three loss feedbacks for each face type). The value associations were mainly and repeatedly reinforced by reward feedbacks, therefore, although the punishment threat also imposed strong impact on face processing. Together with previous findings that only reward, and not punishment, could impact visual orienting (Rutherford et al., 2010) and receive facilitated processing in the attention blink task (Raymond and O’Brien, 2009), we tended to believe that reward played a more crucial role in modifying the anger superiority effect, although we cannot completely rule out a contribution of punishment threat.
This study extends the literature on anger superiority effect by revealing potential factors that may impact it. It also demonstrates a stronger value effect on visual processing and attentional control by using stimuli with emotional valance. Importantly, the finding that value associations can change the anger superiority effect in normal individuals suggests possibilities for the therapeutic intervention of individuals suffering from specific attentional bias, such as anxiety and blood phobic (Buodo et al., 2010). Future studies can investigate whether these specific attentional biases could be eliminated or whether the dysfunctional mechanisms that control the specific attentional bias could be recovered by value associations.
In conclusion, this study showed that the preferential processing of angry faces could indeed be modified by associating different values with different face targets. Additionally, the continuous value effect despite participants knowledge that no reward or punishment would be given suggests that it was the value effect, instead of the impact of endogenous attention, which played a more crucial role in changing the preferential processing of angry faces.
Conflict of Interest
None declared.
Acknowledgments
We are grateful to Mathias Weymar for his providing us with the face materials. This research was supported by the Key Discipline Fund of National 211 Project (NSKD11011) and the Fundamental Research Funds for the Central Universities (SWU1209317).
REFERENCES
- Anderson AK, Phelps EA. Lesions of the human amygdale impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker SI, Horstmann G, Remington RW. Perceptual grouping, not emotion, accounts for search asymmetries with schematic faces. Journal of Experimental Psychology: Human Perception and Performance. 2011;37:1739–57. doi: 10.1037/a0024665. [DOI] [PubMed] [Google Scholar]
- Brisson B, Jolicœur P. Express attentional re-engagement but delayed entry into consciousness following invalid spatial cues in visual search. PLoS One. 2008;3:e3967. doi: 10.1371/journal.pone.0003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buodo G, Sarlo M, Munafò M. The neural correlates of attentional bias in blood phobia as revealed by the N2pc. Social Cognitive and Affective Neuroscience. 2010;5:29–38. doi: 10.1093/scan/nsp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychological Science. 2006;17:222–27. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychological Science. 2009;20:778–84. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology. 1996;99:225–34. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology. 2007;74:108–12. doi: 10.1016/j.biopsycho.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2010;85:252–7. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Schmidt-Daffy M, Schubö A. Neural evidence for the threat detection advantage: differential attention allocation to angry and happy faces. Psychophysiology. 2011;48:697–707. doi: 10.1111/j.1469-8986.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Heipertz D, Boehler CN, et al. Mandatory processing of irrelevant fearful face features in visual search. Journal of Cognitive Neuroscience. 2010;22:2926–38. doi: 10.1162/jocn.2009.21340. [DOI] [PubMed] [Google Scholar]
- Fox E, Derakshan N, Shoker L. Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. NeuroReport. 2008;3:259–63. doi: 10.1097/WNR.0b013e3282f53d2a. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Hansen RD. Finding the face in the crowd: an anger superiority effect. Journal of Personality and Social Psychology. 1988;54:917–24. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience. 2010;30:11096–103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, McDonald JJ, Theeuwes J. Electrophysiologic evidence of the capture of visual attention. Journal of Cognitive Neuroscience. 2006;18:604–13. doi: 10.1162/jocn.2006.18.4.604. [DOI] [PubMed] [Google Scholar]
- Holmes A, Bradley BP, Nielsen MK, Mogg K. Attentional selectivity for emotional faces: evidence from human electrophysiology. Psychophysiology. 2009;46:62–8. doi: 10.1111/j.1469-8986.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Horstmann G, Bauland A. Search asymmetries with real faces: testing the anger-superiority effect. Emotion. 2006;6:193–207. doi: 10.1037/1528-3542.6.2.193. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, Eimer M. Reward priority of visual target singletons modulates ERP signatures of attentional selection. Psychological Science. 2009;20:245–51. doi: 10.1111/j.1467-9280.2009.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Van VJ, Eimer M. The N2pc component and its links to attention shifts and spatially selective visual processing. Psychophysiology. 2008;45:240–49. doi: 10.1111/j.1469-8986.2007.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. “The operation of attention—millisecond by millisecond-over the first half second.”. In: Ogmen H, Breitmeyer BG, editors. The First Half Second: The Microgenesis and Temporal Dynamics of Unconscious and Conscious Visual Processes. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:1000–14. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: an ambiguity resolution theory of visual selective attention. Cognitive Psychology. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Öhman A. Emotion regulates attention: the relationship between facial configuration, facial emotion, and visual attention. Visual Cognition. 2005;12:51–84. [Google Scholar]
- Maratos FA, Mogg K, Bradley BP. Identification of angry faces in the attentional blink. Cognitve Emotion. 2008;22:1340–52. doi: 10.1080/02699930701774218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza V, Turatto M, Caramazza A. Attention selection, distractor suppression, and N2pc. Cortex. 2009;45:879–90. doi: 10.1016/j.cortex.2008.10.009. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Hickey C, Green JJ, Whitman JC. Inhibition of return in the covert deployment of attention: evidence from human electrophysiology. Journal of Cognitive Neuroscience. 2009;21:725–33. doi: 10.1162/jocn.2009.21042. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–48. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–97. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, Raymond JE. Learned predictiveness speeds visual processing. Psychological Science. 2012;23:359–63. doi: 10.1177/0956797611429800. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–58. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fear, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Juth J, Lundqvist D. Finding the face in a crowd: relationships between redundancy, target emotion, and target gender. Cognition and Emotion. 2009;24:1216–28. [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80:381–96. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Griffin M, Baron R, Sasson NJ, Gur RC. The face in the crowd effect: anger superiority when using real faces and multiple identities. Emotion. 2010;10:141–46. doi: 10.1037/a0017387. [DOI] [PubMed] [Google Scholar]
- Purcell DG, Stewart AL. Still another confounded face in the crowd. Attention, Perception, & Psychophysics. 2010;72:2115–27. doi: 10.3758/bf03196688. [DOI] [PubMed] [Google Scholar]
- Raymond JE, O’Brien JL. Selective visual attention and motivation: the consequences of value learning in an attentional blink task. Psychological Science. 2009;20:981–88. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, O’Brien JL, Raymond JE. Value associations of irrelevant stimuli modify rapid visual orienting. Psychonomic Bulletin & Review. 2010;17:536–42. doi: 10.3758/PBR.17.4.536. [DOI] [PubMed] [Google Scholar]
- Schmidt-Daffy M. Modeling automatic threat detection: development of a face-in-the-crowd task. Emotion. 2011;11:153–68. doi: 10.1037/a0022018. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Öhman A, Hamm AO. The face is more than its parts—brain dynamics of enhanced spatial attention to schematic threat. NeuroImage. 2011;58:946–54. doi: 10.1016/j.neuroimage.2011.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–9. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]